RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
RBSE Class 11 Chemistry Chemical Bonding and Molecular Structure InText Questions and Answers
Question 4.1.
Write the Lewis dot structure of CO molecule.
Answer:
The Lewis dot structure of CO molecule can be written in following steps.
Step 1: Count the total number of valence electrons of
carbon (C) and oxygen (O) atoms.
6 C = 2, 4 = 1s2, 2s2 2p2
8 O = 2, 6 = 1s2, 2s2 2p1
Valence electrons in Carbon = 4
Valence electrons in Oxygen = 6
Total valence electrons = 4 + 6 = 10
Step 2: Write the skeletal structure of molecule (CO).
Step 3: Draw a single bond between C and O and complete the octet on O, the remaining two electrons are the lone pairs on carbon.
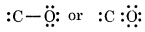
This structure does not complete the octet on carbon and therefore, multiple bonding is shown between C and O atoms. In this case, triple bond must be present between C and O atoms, which satisfies the octet rule condition for both atoms.
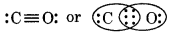

Question 4.2.
Write the Lewis structure of the nitrite ion, NO2.
Answer:
The Lewis structure of the nitrite ion can be written in following stes.
Step 1. Count the total number of valence electrons of the nitrogen atom, the oxygen atom and the additional one negative charge (equal to one electron).
N (2s2 2p3), O (2s2,2p4)
5 + (2 × 6) + 1 = 18e
Step 2. The skeletal structure of NO2 is written as :
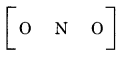
Step 3. Draw a single bond (one shared electron pair) between the nitrogen and each of the oxygen atoms I completing the octet on oxygen atoms. This, however, V does not complete the octet on nitrogen if the remaining two electrons constitute lone pair on it.
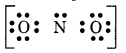
Hence, we have to resort to multiple bonding between nitrogen and one of the oxygen atom (in this case a double bond). This leads to the following Lewis dot Structure
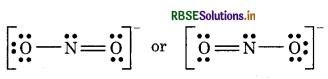
Question 4.3
Explain the structure of CO23- ion in terms of resonance.
Answer:
The Lewis structure of CO2 ion has two single bonds and one double bond between carbon and oxygen atoms. All carbon to oxygen bonds in CO2 are equivalent. So, the carbonate ion is best described as a esonance hybrid of the canonical forms I, II and III as hown below:
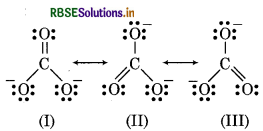
Question 4.4.
Explain the structure of CO2 molecule.
Answer:
C - O bond length in CO2 is 115 pm experimentally.
C = O 121 pm
C = O 110 pm
Since, the Carbon-Oxygen bond length in CO2 (115 pm) ies between the values for CO and CO. So, a single Lewis structure can not represent this position and it ecomes necessary to write more than one Lewis tructures. Hence, the structure of CO2 is best described s a hybrid of the canonical or resonance forms I, II and
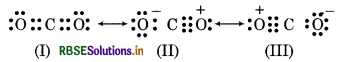
RBSE Class 11 Chemistry Chemical Bonding and Molecular Structure Textbook Questions and Answers
Question 4.1
Explain the formation of a chemical bond.
Answer:
The force of attraction which holds the constituents atoms, ions etc) present in a molecule or species is called hemical bond. It can be formed due to following reasons:
- Lowering of energy of combining atoms
- Octet rule
Lewis postulated that the atoms achieve the stable octet when they are linked by chemical bonds. In the ormation of a molecule, only the outer shell electrons ake part in chemical combination and they are known is valence electrons.
Atoms combine with one another in different ways:
- by transfer of electrons leading to the formation of electrovalent bond.
- by sharing of electrons leading to the formation covalent bond.

Question 4.2.
Write Lewis dot symbols for atoms of th following elements: Mg, Na, B, O, N, Br.
Answer:
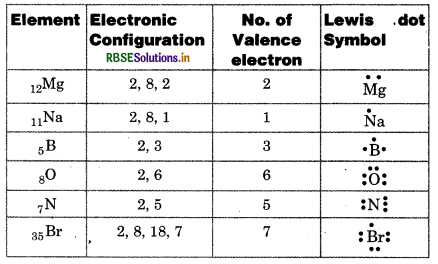
Question 4.3.
Write Lewis symbols for the followin atoms and ions:
S and S2; Al and Al3+; H and H-
Answer:
Lewis symbols for S and S2-
No. of electrons = 16
Electronic configuration = 2, 8, 6
Lewis dot symbol = :S:
S2- No. of electrons
16 + 2 = 18
Electronic configuration = 2, 8, 8
Lewis dot symbol = 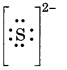
Lewis symbols for Al and Al3+
No. of electrons = 13
Al Electronic configuration = 2, 8, 3
Lewis dot symbol = Al
Al3+ No. of electrons = 13 - 3 = 10
Electronic configuration = 2, 8
Lewis dot symbol = [Al]3+
Lewis Symbols for H and H-
No. of electrons = 1
H Electronic configuration = 1
Lewis dot symbol = H
No. of electrons = 1 + 1 = 2
H- Electronic configuration = 2
Lewis dot symbol = [H]-
Question 4.4.
Draw the Lewis structures following molecules and ions:
H2S, SiCl4, BeF2, CO32- HCOOH
Answer:
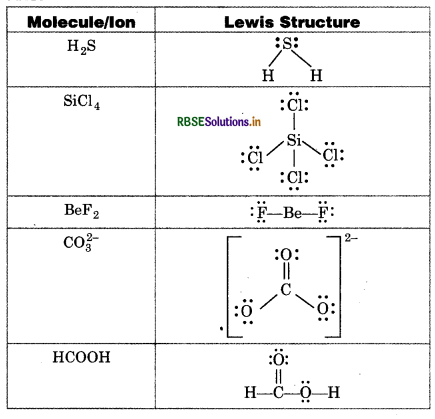
Question 4.5.
Define octet rule. Write its significance and limitations.
Answer:
Octet Rule: According to this rule, "atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells."
Significance of Octet Rule
- This rule helps to explain the combination of different atoms with each other to form a chemical bond.
- It explains the structure of chemical compounds.
Limitations of Octet Rule:
(i) The incomplete octet of the central atom In some compounds, the number of electrons surrounding the central atom is less than eight. e.g., LiCl, BeH2, BCl3 etc.
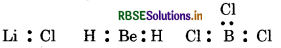
Li, Be and B have 1, 2 and 3 valence electrons.
(ii) Odd electron molecules In molecules having odd number of electrons like NO and NO2, the octet rule is not satisfied for all the atoms.

(iii) The expanded octet In some compounds, there are more than eight valence electrons around the central atom, this is called expanded octet.
e.g. PF5, SF6, H2SO4 etc.
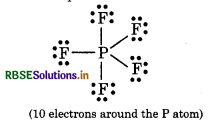

Question 4.6.
Write the favourable factors for the formation of ionic bond.
Answer:
- Low ionization enthalpy of the element forming cation.
- High electron affinity (more negative electron gain enthalpy) of the element forming anion.
- High lattice enthalpy of the ionic compound formed.
Question 4.7.
Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4 AsF5, H2S, PH3
Answer:
According to VSEPR model, the shape of molecule epends upon the number of bond pair of electrons and one pair of electrons on central atom.
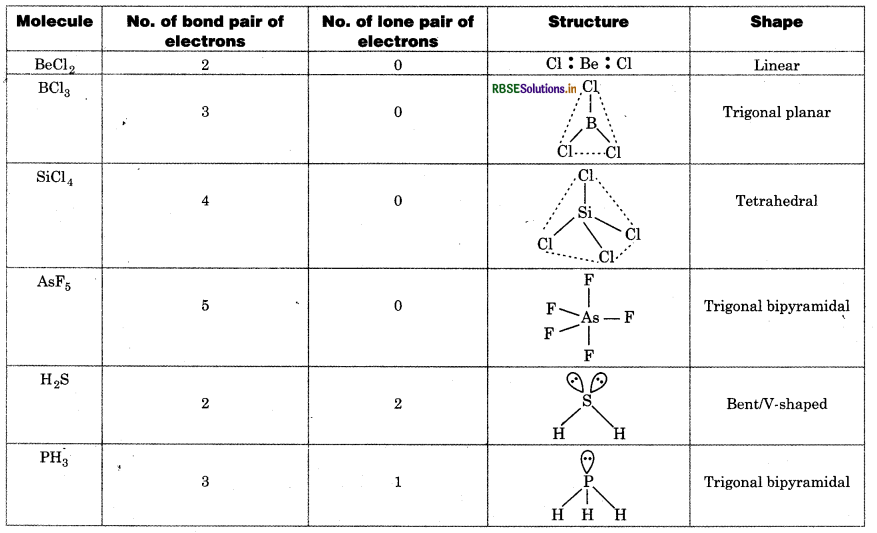
Question 4.8.
Although geometries of NH and H2O molecules are distorted tetrahednal, bond a angle in water is less than that of ammonia. Discuss.
Answer:
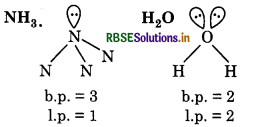
In NH3, only one lone pair is present on N-atom and in b H2O two lone pair of electrons are present on O-atom. 4 Since we know that, according to VSEPR theory, lp-lp repulsion is greater than bp-lp repulsion. So, bond ngle of water (104.5°)is less than that of ammonia 106.5°).
Question 4.9.
How do you express the bond strength in terms of bond order?
Answer:
Bond order is directly proportional to the bond trength of a molecule or ion. Greater the bond order of molecule or ion, greater will be the bond strength.
Question 4.10.
Define the bond length.
Answer:
The equilibrium distance between the nuclei of two onded atoms in a molecule is called bond length.

Question 4.11.
Explain the important aspects of resonance with reference to the CO23- ion.
Answer:
Lewis symbols for S and S2-
No. of electrons = 16
Electronic configuration = 2, 8, 6
Lewis dot symbol = :S:
S2- No. of electrons
16 + 2 = 18
Electronic configuration = 2, 8, 8
Lewis dot symbol = 
Lewis symbols for Al and Al3+
No. of electrons = 13
Al Electronic configuration = 2, 8, 3
Lewis dot symbol = Al
Al3+ No. of electrons = 13 - 3 = 10
Electronic configuration = 2, 8
Lewis dot symbol = [Al]3+
Lewis Symbols for H and H-
No. of electrons = 1
H Electronic configuration = 1
Lewis dot symbol = H
No. of electrons = 1 + 1 = 2
H- Electronic configuration = 2
Lewis dot symbol = [H]-
Question 4.12.
H3PO3 can be represented by structures and 2 shown as below. Can these two structures be taken as the canonical form of the resonance hybrid representing H3PO3 ? If not, give reasons for the same.
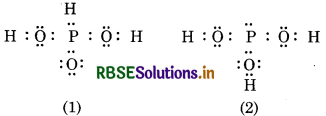
Answer:
These two structures (1 and 2) of H3PO3 can not b taken as canonical forms of the resonance hybri because in these structures, the relative position o hydrogen is changed. Canonical forms should differ only in the position o electrons and not in the position of atoms.
Question 4.13.
Write the resonance structures for SO3 NO2 and NO-3.
Answer:
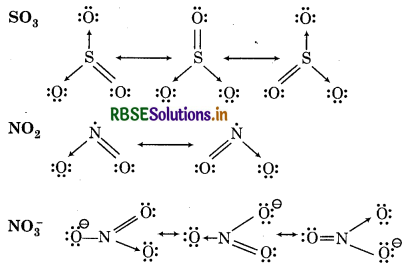
Question 4.14.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
(a) K and S,
(b) Ca and O,
(c) Al and N.
Answer:
(a) K and S
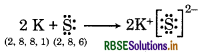
(b) Ca and O
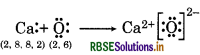
(c) Al and N
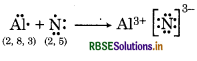
Question 4.15.
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Answer:
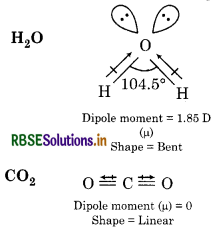
The net dipole moment of H2O molecule is 1.85D which indicates that two O-H dipoles are not in a straight line but inclined to each other at certain angle. So, the shape of H2O molecule is bent. Whereas, the net dipole moment of CO2 is zero so two equal bond dipoles point in opposite directions and cancel the effect of each other. So, the shape of CO2 molecule is linear.
Question 4.16.
Write the significance/applications of dipole moment.
Answer:
Significance of dipole moment
(i) It predicts the polarity of molecule.
(ii) It is useful to determine ionic character of molecule.
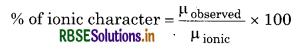
(iii) It is useful to determine geometry of the molecule.
(iv) It helps to differentiate cis and trans isomers. Cis-isomer has higher has higher dipole moment than trans-isomer.
Question 4.17.
Define electronegativity. How does it differ from electron gain enthalpy ?
Answer:
Electronegativity is defined as the tendency of an element to attract the shared pair of electrons towards itself in a covalent bond. While, electron gain enthalpy is defined as the amount of energy released when a neutral isolated gaseous atom accepts an electron to form the gaseous anion.

Question 4.18.
Explain with the help of suitable example, polar covalent bond.
Answer:
When the shared pair of electrons between the two atoms gets displaced more towards electronegative atom in a heteronuclear molecule, then the resultant covalent bond is called polar covalent bond.

Question 4.19.
Arrange the bonds in order of increasing S ionic character in the molecules:
LiF, K2O, N2, SO2 and CIF3
Answer:
According to Fajan's rule, the increasing order of ionic character in the given molecules is.
N2 < SO2 < CIF3 < K2O < LiF
Question 4.20.
The skeletal structure of CH3COOH as ca shown below is correct, but some of the m bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
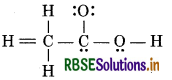
Answer:
The correct Lewis structure for acetic acid is

Question 4.21
Apart from tetrahedral geometry, another di possible geometry for CH4 is square planar hi with the four H-atoms at the corners of the square and the C-atom at its centre. Explain why CH4 is not square planar?
Answer:
Electronic configuration of carbon atom (6C)
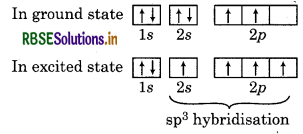
Since, CH4 possesses sp3 hybridisation so its shape is tetrahedral. CH4 is not square planar because dsp2 hybridisation is not possible due to absence of d-orbitals. It also contains four bond pairs of electrons but no lone pair. So, according to VSEPR theory, its geometry is tetrahedral and bond angle is 109°28'. Hence, CH, is not square planar.
Question 4.22.
Explain why BeH2 molecule has a zero dipole moment although the BeH2 bonds are polar?
Answer:
BeH2 is a linear molecule. Its structure is shown as below:
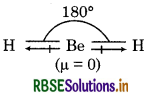
this molecule, the two equal bond dipoles point in posite directions and cancel the effect of each other. , BeH2 molecule has a zero dipole moment (μ = 0), though the Be-H bonds are polar.
Question 4.23.
Which out of NH3 and NF3 has higher dipole moment and why?
Answer:
NH3 possesses higher dipole moment than NF3, though both the molecules are pyramidal in shape. It n be explained with the help of structure of both olecules as follows:
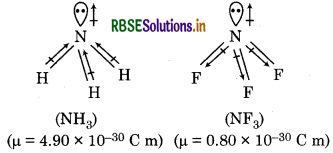
NH3, the orbital dipole due to lone pair is in the same rection as the resultant dipole moment of the three -H bonds, whereas in NF3, the orbital dipole due to ne pair is in the opposite direction to the resultant pole moment of three N-F bonds. So, the NH3 has gher dipole moment than NF3.
Question 4.24.
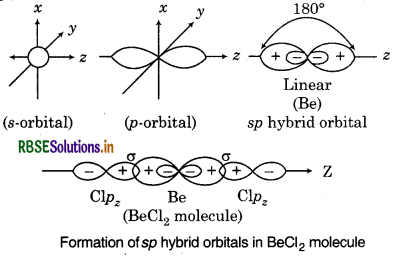
What is meant by hybridization of atomic orbitals ? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Answer:
Hybridization: It can be defined as the process of termixing of the orbitals of slightly different energies as to re-distribute their energies, resulting in the rmation of new set of orbitals of equivalent energies nd shape.
shape of sp Hybrid Orbitals: The mixing of one s and e porbital results in the formation of two equivalent hybrid orbitals. Each sp hybrid orbital has 50% character and 50% p-character. The shape of sp hybrid bitals is linear and bond angle is 180°.
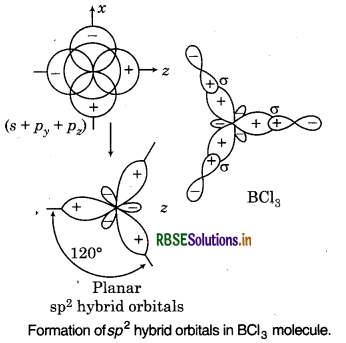
Shape of sp2 Hybrid Orbitals:
The mixing of one s and two p-orbitals results in th formation of three equivalent sp2 hybrid orbitals. Eac sp2 bybrid orbital has 33.3% s-character and 66.79 p-character. The shape of sp2 hybrid orbitals is trigona planar and bond angle is 120°.
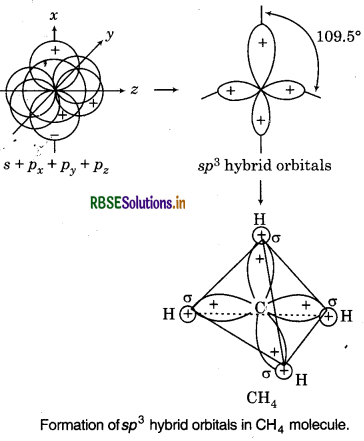
Shape of sp3 Hybrid Orbitals :
The mixing of one s and three p-orbitals results in th formation of four equivalent sp3 hybrid orbitals. Each sp3 hybrid orbital has 25% s-character and 75 p-character. The shape of sp3 hybrid orbitals tetrahedral and bond angle is 109°28′.
Question 4.25.
Describe the change in hybridization (if any) of the Al atom in the following reaction:
AlCl3 + Cl- → AICl4-
Answer:
Electronic configuration of 13Al = 2, 8, 3
= 1s2 2s2 2p6 3s2 3p1
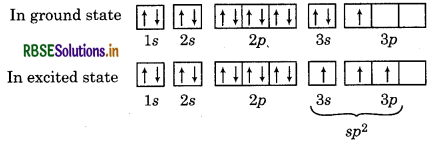
∴ In the formation of AlCl3, Al undergoes sp hybridisation and possesses trigonal planar geometry. While in case of AICI
∴ In the formation of AIC, Al undergoes sp3 hybridisation and possesses tetrahedral geometry.

Question 4.26.
Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
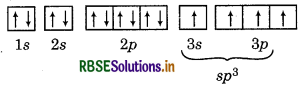
Answer:
BF3 + NH3 → F3B NH3
No. of bond pairs = 3
No. of lone pairs = 0
Hybridization = sp2
No. of bond pairs = 3
No. of lone pairs = 1
Hybridization = sp
When BF3 reacts with NH3 then hybridization of B in BF3 changes from sp2 to sp3 hybridization while hybridization of nitrogen in NH3 remains same because nitrogen shares its lone pair with electron deficient boron.
Question 4.27.
Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Answer:
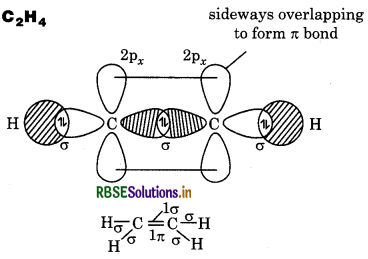
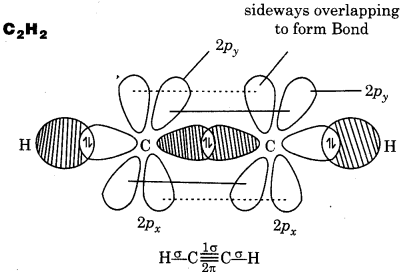
Question 4.28.
What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2
(b) C2H4
Answer:
(a) C2H2
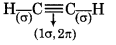
∴ It contains 3σ and 2л bonds.
(b) C2H4
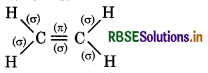
∴ It contains 5σ and 1π bonds.
Question 4.29.
Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
(a) 1s and 18
(b) 1s and 2px
(c) 2p, and 2p,
(e) ls and 2s
Answer:
(c) 2p, and 2p,, because these orbitals overlap sideways resulting in the formation of л-bond.

Question 4.30.
Which hybrid orbitals are used by carbon atoms in the following molecules ?
(a) CH3 - CH3
(b) CHg - CH = CH2
(c) CHg - CH - OH
(d) CH3CHO
(e) CH3 - COOH
Answer:
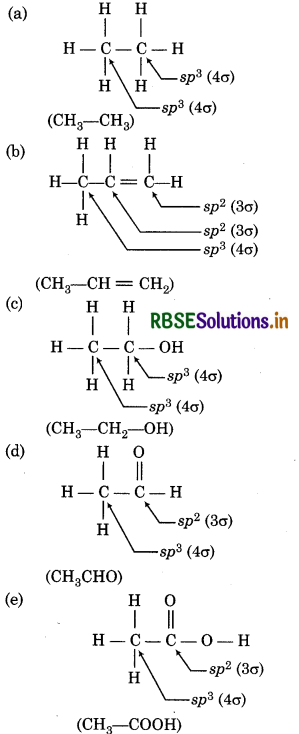
Question 4.31.
What do you understand by bond pairs and lone pairs of electrons ? Illustrate by giving one example of each type.
Answer:
Bond pair of electrons means the shared pair of ectrons present between the bonded atoms. Lone pair electrons means the pair of electrons which does not ke part in bonding.
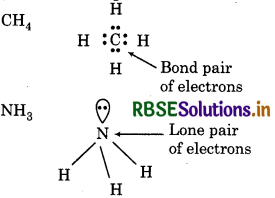
Question 4.32.
Distinguish between a sigma and a pi bond.
Answer:
|
Sigma bond |
Pi bond |
|
It is formed by end-to-end overlapping of atomic orbitals. |
It is formed by sideways overlapping of atomic orbitals. |
|
It is a strong bond. |
It is a weak bond. |
|
It involves s s, s-p and p-p overlapping. |
It involves only p-p overlapping. |
|
It involves free rotation of atoms. |
It restricts free rotation of atoms. |
Question 4.33.
Explain the formation of H2 molecule of the basis of valence bond theory.
Answer:
Consider two hydrogen atoms A and Bhaving on electron in its 1s subshell. These atoms approach eac other having nuclei NA and NB and electrons present i them are represented by eд and eg. When the two atom are at large distance from each other, then there is n interaction between them. As these atoms begin t approach each other, new attractive and repulsive force begin to operate.
- Attractive forces arise between nucleus of A an electron of Band vice-versa i.e., NA - еA, NB - еBM, or NA - еB, NB - еA
- Repulsive forces arise between nuclei of two atoms A and Bi.e., NA − NB.
- Repulsive forces arise between electrons of two atoms A and Bi.e.,еA - еB.
Attractive forces tend to bring the two atoms close t each other while repulsive forces tend to push then apart.
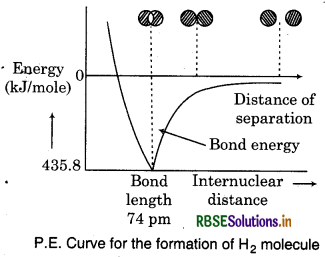
It has been found that the magnitude of new attractive forces is more than the new repulsive forces. As a result two atoms approach each other and potential energy decreases. Further, a stage is reached where the ne force of attraction balances the force of repulsion and system acquires minimum energy. At this stage, the two hydrogen atoms are said to be bonded together to form a stable molecule having the bond length of 74 pm. The energy released in the formation of H2 molecule is called bond enthalpy, which corresponds to the minimum in the curve represented in above figure. 435.8 kJ of energy is required to dissociate one mole of H2 molecule.

Question 4.34.
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer:
There are following important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
- The combining atomic orbitals must have almost equal energies. e.q. 1s-1s, 2s-2s, 2pz-2pz, 2px 2px etc overlapping are possible.
- The combining atomic orbitals must have the same symmetry about the molecular axis. e.g. 2p, orbital can combine with 2p, orbital but not with 2p2 or 2p, orbitals.
- The combining atomic orbitals must overlap to the maximum extent.
Question 4.35.
Use molecular orbital theory to explain why the Be, molecule does not exist.
Answer:
Electronic Configuration:
Be = 2, 2 = 1s2, 2s2
Be = 2, 2 = 1s2, 2s2
Molecular Orbital Configuration :
(ols)2, (σ* 18)2, (02s)2, (σ* 2s)2
Molecular Orbital Diagram:
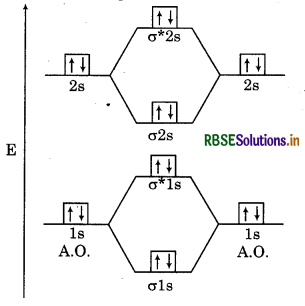
Bond order = \(\frac{N_b-N_a}{2}=\frac{4-4}{2}=\frac{0}{2}=0\)
Since, the bond order of Be, is zero so Be, molecule does not exist.
Question 4.36.
Compare the relative stability of the B following species and indicate their magnetic properties :
O2, O2+ O2- (superoxide), O22- (peroxide).
Answer:
O2 : Electronic configuration
8O = 2,6 = 1S2, 2S2 sp1
Each oxygen atom has 8 electrons.
∴Total no. of electrons = 8 + 8 = 16
Molecular orbital electronic configuration,
(σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
Bond order = \(\frac{N_b-N_a}{2}\)
= \(\frac{10-6}{2}\) = 2
∴ It has two unpaired electrons so it is paramagnetic in nature.
O+2: Total no. of electrons = 16 - 1 = 15
Molecular orbital electronic configuration. (σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-5}{2}=2.5\)
∵ It has one unparied electron so it is paramagnetic in nature.
O-2: Total no. of electrons = 16 + 1 = 17
H
Molecular orbital electronic configuration
(σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
\(\frac{N_b-N_a}{2}=\frac{10-7}{2}=1.5\)
∵ It has one unpaired electron so it is paramagnetic in nature.
Question 4.37.
Write the significance of a plus and a minus sign shown in representing the orbitals.
Answer:
A plus sign representes a positive wave function d a minus sign represents a negative wave function. hen two atomic orbitals having wave functions of milar sign combine with each other, then a bonding olecular orbital is formed. However, when two atomic bitals having wave functions of opposite sign combine ith each other, then an antibonding molecular orbital formed.
Question 4.38.
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to the equatorial bonds?
Answer:
Electronic configuration of 15P = 2, 8, 5
= 1s2, 2s2 sp6, 3s2 3p3
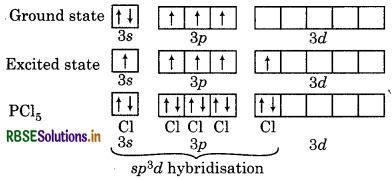
Five orbitals i.e., one s, three p and one d-orbitals are ailable for hybridisation to form five sp3d hybrid bitals in the formation of PCl5. These five sp3d hybrid bitals are directed towards the five corners of trigonal pyramidal.
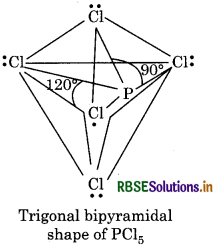
All the bond angles in this structure are not equivalent. PCl5, three P-Cl bonds lie in one plane with bond gle of 120°. These bonds are called equatorial bonds. The remaining two P-Cl bonds, one lying above ar other lying below the equatorial plane form an angle 90° with the plane. These bonds are called axial bonds Axial bonds are longer as compared to the equatori bonds due to great repulsion on axial bond pair electro by equatorial bond pair electrons.

Question 4.39.
Define hydrogen bond. Is it weaker stronger than the vander Waal's forces?
Answer:
Hydrogen bond can be defined as the attractiv force which binds hydrogen atom of one molecule wit the electronegative atom (F, O or N) of another molecul e.g.
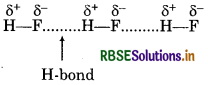
Hydrogen bond is stronger than van der Waal's forces.
Question 4.40.
What is meant by the term bond order Calculate the bond order of : N2, O2, O+2 and O2-.
Answer:
Bond order is defined as one half the differen between the number of electrons present in the bondin and the antibonding orbitals.
Bond order = 1/2 (Nb - Na )
Where N = Number of electrons in bonding orbital
Na = Number of electrons in antibonding orbits
N2
Total number of electrons = 7 + 7 = 14
Molecular orbital electronic configuration
(σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-4}{2}=3\)
O2
Total number of electrons = 8 + 8 = 16
Molecular orbital electronic configuration
(σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-6}{2}=2\)
O2-
Total number of electons = 16 - 1 = 15
Molecular orbital electronic configuration
(σ 1 s)2 (σ* 1 s)2 (σ 2 s)2 (σ* 2 s)2 (σ 2 pz)2 (π2 px2 = π2 py2) (π* 2 px1 = π*2 py1)(σ* 2 pz)0
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-5}{2}=2.5\)
Total number of electrons = 16 + 1 = 17

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter