RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 10 The s-Block Elements
RBSE Class 11 Chemistry The s-Block Elements InText Questions and Answers
Question 10.1.
What is the oxidation state of K in KO2?
Answer:
In KO2, super oxide (O-2) ion is present, whose oxidation state in -1. The compound is neutral.
Let oxidation state of K = x
x - 1 = 0
x = +1
Question 10.2.
The Eo for Cl2/Cl- is + 1.36, for I2/I- is + 0.53, for Ag+/Ag is + 0.79, Na+/Na is -2.71 and for Li+/Li is -3.04. Arrange the following ionic species in decreasing order of reducing strength:
I+, Ag, Cl-, Li, Na
Answer:
The species whose electrode potential is more negative has more reducing strength. Thus, the decreasing order of reducing strength is:
Li- > Na > I- > Ag > Cl-

Question 10.3.
Why is KO2 paramagnetic?
Answer:
Structure of O2- is 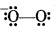
Since it has one unpaired electron in π°2p molecular orbital therefore it is paramagnetic.
Question 10.4.
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answer:
On moving down the group, cationic radius increases due to which lattice energy decrease much more than the hydration enthalpy, so the solubility of alkaline metal hydroxides increases as we go down the group.
Question 10.5.
Why does the solubility of alkaline earth metal carbonates and sulphates in water decrease down the group?
Answer:
Since the size of anions being much larger compared to cations, the lattice enthalpy will remain almost constant within a particular group. As the hydration enthalpies decrease down the group, solubility of alkaline earth metal carbonates and sulphates in water decreases down the group.
RBSE Class 11 Chemistry The s-Block Elements Textbook Questions and Answers
Question 10.1.
What are the common physical and chemical features of alkali metals?
Answer:
Physical Properties of Alkali Metals:
- Physical State: All elements are silvery white, soft and light metals.
- Oxidation State: Due to presence of 1 electron in valence shell, alkali metals show +1 oxidation state.
Example: 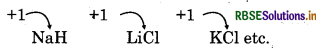
(iii) Boiling and Melting Points: Melting and boiling points of alkali metals are low because due to presence of one electron in valence electron, there is weak metallic bond between them. On moving down the group, melting point decreases with increasing atomic size.
Li > Na > K > Rb > Cs > Fr (M.P and B.P.)
(iv) Density: Alkali metals have low density because of their large atomic size. The density of first three elements Li, Na and K is lower than water. On moving down the group, atomic weight also increases with atomic size, hence density of metals increases.
Exception: The density of potassium is higher than sodium because of its larger size and vacant d-orbitals, which is an exception.
Li <Na < K < Rb < Cs < Fr
(v) Atomic Radius: Alkali metals have largest size in their period. Atomic size increases down the group from Li to Cs. It is due to following reason:
Reason: We know that the distance from valence shell of electrons and nucleus of atom is called atomic radius. As these are the first elements in the period, they have only one electron in outermost shell. Therefore, alkali metals have largest atomic radius in its period. Due to large atomic radius, value of nuclear charge is also very less. On moving down the group atomic size increases, because number of orbitals increases.
Li <Na < K < Rb < Cs < Fr
(vi) Ionic Radius: Alkali metals lose their valence electrons and form positive ions (cations). The M+ ion formed by lose of one electron is always smaller than their parent atom. Atomic radius increases down the group.
Explanation:
(i) Outermost shell of alkali metals has one electron. On losing this electron, outermost shell of atom is generally lost.
(ii) Cation is formed by losing of one electron from valence shell. Due to increase in number of protons as compared to electrons, effective nuclear charge increases and as result, size decreases. On moving down the group, number of shells increases, and as a result, ionic radius also increases.
Li < Na < K < Rb+ < Fr+
(vii) Electropositive Character: The tendency of an element to lose electron is called electropositive character. The electro-positivity of alkali metals is
maximum in periodic table. The tendency to lose electrons increases down the group because size increases and ionisation energy decreases.
Li< Na < K < Rb < Cs < Fr
(viii) Electronegativity: The tendency to attract electron towards it is called electronegativity. The electronegativity of alkali metals is very low. The value of electronegativity decreases down the group because atomic size increases.
Li > Na > K > Rb > Cs > Fr
Chemical Properties of Alkali Metals
(i) Reaction with Oxygen: The alkali metals tarnish in dry air due' to formation of their oxides which in turn react with moisture to form hydroxides.
4Li + O2 → 2Li2O ( Oxide)
2Na + O2 → Na2O2 (Peroxide)
M + O2 → MO2 (Superoxide)
(M = K, Rb, Cs)
(ii) Reaction with Water: The alkali metals react with water and other hydrogen containing compounds and releases dihydrogen.
2Na + 2H2O → 2NaOH + H2
(iii) Reaction towards Dihydrogen: The alkali metals react with dihydrogen at about 673K (Lithium at 1073K) to form hydrides.
2M + H2 → 2M + H-
2Na + H2 → 2NaH
(iv) Reaction with Halogens: The alkali metals readily react vigorously with halogens to form ionic halides, M+ X-
2M + X2 → 2M + X-
(v) Reaction with Liquid Ammonia: The alkali metals are soluble in liquid ammonia giving deep blue solutions which are conducting in nature.
M + (x + y)NH3 → [M(NH3)2]+ + [e(NH3),]

Question 10.2.
Discuss the general characteristics and gradation in properties of alkaline earth metals.
Answer:
General Characteristics and Gradation in Properties of Alkaline Earth Metals:
(i) Atomic Radius: Atomic and ionic radii of alkaline earth metals are smaller than corresponding members of alkali metals. This decrease in size is due to increase in effective nuclear charge. On moving down the group, atomic radius increases because of increase in number of shells and screening effect. The order is
Be < Mg < Ca < Sr < Ba < Ra
(II) Ionic Radlu. : All the elements in the group lose two electrons and form divalent cation (M2). On moving down the group, ionic radius increases but ionic radius is smaller than atomic size.
Be2+ < Mg2 < Ca2 < Sr2’ < Ba’ < Ra’
(iii) Ionisation Energy : The alkaline earth metals have low ionisation enthalpies due to fairly large size of the atoms. Since the atomic size increases down the group, their ionization enthalpy decreases. The first ionisation enthalpies of the alkaline earth metals are higher than those of the corresponding Group 1 metals. This is due to their small size as compared to the corresponding alkali metals and high nuclear charge. Electrons are removed from completely filled orbital (ns2). It is interesting to note that the second ionisation enthalpies of the alkaline earth metals are smaller than those of the corresponding alkali metals.
|
Element |
∆H1kJ mol-1 |
∆H2 kJ mol-1 |
|
Na (Group I) |
496 |
4562 |
|
Mg (Group II) |
737 |
1450 |
The second electron in sodium metal is removed from noble gas configuration where as magnesium can loose electron from a cation to attain noble gas configuration. On moving down the group, ionisation enthalpy of alkaline earth metals decreases due to increase in size and screening effect.
(iv) Oxidation Stat: Two electrons are present in valence shell of the group elements. Therefore, they show +2 oxidation state.
For example: +2 +2 +2
MgO BCl2 CaCl2 etc.
(v) Electro negativity: The value of clectronegativity of alkaline earth metals is very low. Electronegativity decreases down the group.
- Element Be Mg Ca Sr Ba
- Electronegativity 1.5 1.2 1.0 1.0 0.9
(vi) Metallic Nature: These are silvery white, lustrous and soft but harder than alkali metals.
(vii) Boiling and Melting PoInts: The melting and boiling points of these metals are higher than the corresponding alkali metals due to smaller sizes. In a group , there l.a no regular change in melting and boiling points.
(viii) Density : These are denser and harder than corresponding alkali metals. The density of these elements decreases from Be to Ca and then increases from Ca to Ba. The decrease in density upto Ca is weak ordered arrangement of atoms in lattice.
Chemical Properties of Alkaline Earth Metals:
(i) Reaction with Water: The electrode potential of Be is less negative and is least electronegative among the alkaline earth metals. Therefore, Be does not react even
with water or steam. On the other hand, Be react only with boiled water.
Mg + H2O → MgO + H2
or, Mg + 2H2O → Mg(OH)2 + H2
(ii) Reaction with Dioxygen: Due to less electropositive nature, alkaline earth metals are less reactive towards air or oxygen than alkali metals. beryllium and magnesium reacl with air and forms beryllium oxide and beryllium nitride.
2Be + O2 → 2BeO
3Be + N2 → Be3N2
Mg being more electropositive, burns in air with a brilliant glow and forms magnesium ôxide and magnesium nitride.
2Mg + O2 → 2MgO
3Mg + N2 → Mg3N2
Ca, Sr and Ba react with water rapidly.
(iii) Reaction with Dlhydrogen: All the elements except beryllium combine with hydrogen upon heating to form their hydrides,
M + H → MH2.
(iv) Reaction with Halogens: All the alkaline earth metals combine with halogens at high temperature and form corresponding halides.
M + X2 → MX2
(v) Reaction with Liquid Ammonia: Like alkali metals, the alkaline earth metals dissolve in liquid ammonia to give deep blue black solutions forming
ammoniated ions.
M + (x + y)NH3 → [M(NH3)x]2+ + [e(NH3)y]-
Question 10.3.
Why are alkali metals not found in nature?
Answer:
Alkali metals possess loosely held s-electron (ns1) in the outer most shell of their atoms, which makes them the most electropositive metals due to which they form M+ ions readily. Hence, alkali metals are not found in free state in nature.
Question 10.4.
Find out the oxidation state of sodium In Na2O2.
Answer:
In Na2O2 peroxide ion (O2-) is present, which has a oxidation state of -1.
Let oxidation state of Nax
2x + 2(-1) = 0
2x - 2 = 0
x = +1
Question 10.5.
Explain why is sodium less reactive than potassium?
Answer:
Since ionisation enthalpy of sodium is higher than that of potassium. so, it will lose electron less readily as compared to potassium. Thus, sodium is less reactive than potassium.
Question 10.6.
Compare the alkali metals and alkaline earth metals with respect to (i) ionisation enthalpy (ii) basicity of oxides and (iii) solubility of hydroxides.
Answer:
- Ionisation Enthalpy: The first ionisation enthalpies (I.E) of alkaline earth metals are higher than those of alkali metals due to increase in nuclear charge. However, second ionisation enthalpies (I.E2) of alkaline earth metals are lower as compared to corresponding alkali metals.
- Basicity of Oxides the basicity of oxides of alkaline earth metals are less than oxides of alkali metals due to increase in nuclear charge and high ionisation enthalpy.
- Solubility of Hydroxides: The hydroxides of alkaline earth metals are less soluble in water than the hydroxides of alkali metals.

Question 10.7.
In what ways lithium shows similarities to magnesium in its chemical behaviour?
Answer:
Lithium shows similarity with magnesium which is second element of group 2 and is placed diagonally to lithium. This relationship of lithium and magnesium is known as 'Diagonal Relationship'. This type of diagonal relationship is also shown by elements of second period.
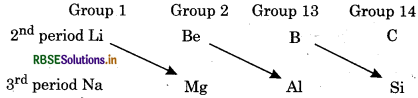
Lithium shows similarities with magnesium due to diagonal relationship: Some of them are:
1. Both have almost similar electronegativities.
2. Both Li and Mg are quite hard. They are harder and lighter than other members of their groups.
3. Both LiOH and Mg (OH)2 are weak bases.
4. Lithium and magnesium react with nitrogen to form nitride.
6Li + N2 → 2Li3N
3Mg + N2 → Mg3N2
5. The carbonates of both these metals decompose on heating to form oxide and evolve carbon dioxide.
Li2CO3 → Li2O + CO2
MgCO3 → MgO + CO2
6. Solid bicarbonates of both lithium and magnesium are not formed.
7. Both combine with oxygen to form monoxides. Other forms peroxides or superoxide.
4Li + O2 → 2Li2O
2Mg + O2 → 2MgO
8. Chlorides of both are deliquescent and crystallize from aqueous solution as hydrates.
Question 10.8.
Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
Answer:
Alkali and alkaline earth metals can not be obtained by chemical reduction methods due to following reasons:
- These act as strong reducing agents.
- These are highly electropositive metals.
Question 10.9.
Why are potassium and caesium, rather than lithium used in photoelectric cells?
Answer:
- Potassium and caesium posses low ionisation enthalpy due to which these metals eject electrons when exposed to light so, these are used in photoelectric cells.
- Lithium possesses highest ionisation enthalpy so it cannot be used in photoelectric cells.
Question 10.10.
When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change.
Answer:
The alkali metals are soluble in liquid ammonia giving deep blue solutions which are conducting in nature.

The blue colour of solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The electrical conductivity of solution is due to ammoniated cation and ammoniated electron.
Question 10.11.
Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Answer:
Due to small size of Be and Mg, their electrons are strongly bounded to their nucleus. So, they require large amount of energy for excitation of electrons to higher energy levels which is not available in the bunsen flame. So, these metals do not give colour to flame whereas other alkaline earth metals do so.
Question 10.12.
Discuss the various reactions that occur in the Solvay process.
Answer:
Solvay Process
(i) Sodium hydrogen carbonate (NaHCO3) gets precipitated in the reaction of sodium chloride with ammonium hydrogen carbonate. The later is prepared by passing CO2 to a concentrated solution of sodium chloride saturated with ammonia, where ammonium carbonate followed by ammonium hydrogen carbonate
are formed.
2NH3 + H2O + CO2 → (NH4)2CO3
(NH4)2CO3 + H2O + CO2 → 2NH4 HCO3
NH4 HCO3 + NaCl → NH4Cl + NaHCO3
Sodium hydrogen carbonate is insoluble due to common ion effect. It can be obtained by filtration.
(ii) Sodium hydrogen carbonate crystals get separate. These crystals are heated at 150°C to form sodium carbonate.
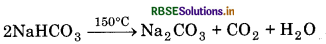
(iii) In this process, ammonia solution containing NH4Cl is treated with Ca(OH)2. Calcium chloride is obtained as a by-product.
CaCO3 → CaO + CO,
CaO + H2O → Ca(OH)2
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + 2H2O
Question 10.13.
Potassium carbonate can not be prepared by Solvay process. Why?
Answer:
Potassium carbonate can not be prepared by solvay process because potassium bicarbonate being more solube than sodium bicarbonate does not get precipitated when CO2 is passed through a concentrated solution of KlCl saturated with ammonia.
KCl + CO2 + NH3 + H2O → KHCO3 + NH4Cl

Question 10.14.
Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Answer:
Electropositive character of alkali metals increases down the group, which causes an increase in the stability of alkali carbonates. However, Li, CO is not so stable to heat, because it is covalent in nature. The ionic size of Li+ is very small so it polarise a large CO2-ion, to form more stable lithium oxide.
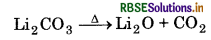
Hence, Li, CO, is decomposed at a lower temperature whereas Na2CO3 is decomposed at high temperature.
Question 10.15.
Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals:
(a) Nitrates,
(b) Carbonates,
(c) Sulphates.
Answer:
(a) Nitrates
Solubility: Nitrates of alkali and alkaline earth metals are highly soluble in water.
Thermal Stability: Alkali metal nitrates decompose to nitrites at high temperature except Lithium nitrate.
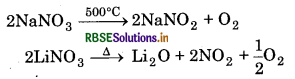
Alkaline earth metal nitrates decompose on heating into corresponding oxides with the evolution of mixture of NO2 and O2 except Be(NO3)2.

(b) Carbonates
Solubility: Carbonates of alkali metals are generally soluble in water while carbonates of alkaline earth metals are insoluble in water.
Thermal Stability: Carbonates of alkali metals are thermally stable upto 1273 K above which they are converted into oxides.
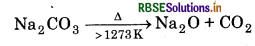
Lithium carbonate is considerably less stable and decomposes readily.
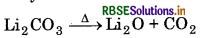
All the carbonates of alkaline earth metals decompose on heating to give carbon dioxide and the oxide. Beryllium carbonate is unstable and can be kept only in the atmosphere of CO2.
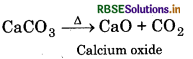
The thermal stability increases down the group with increase in size of cation.
(c) Sulphates:
Solubility: The sulphates of alkali metals are soluble in water except lithium sulphate. The sulphates of alkaline earth metals are also soluble in water. The solubility decreases form CaSO4 to BaSO4. The greater hydration enthalpies of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and so their Sulphates are soluble in water.
Thermal Stability: The sulphates of alkali metals are thermally stable except lithium sulphate whereas sulphates of alkaline earth metals are decomposed on heating.
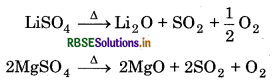
The thermal stability increases down the group with increase in size of cation.
Question 10.16.
Starting with sodium chloride how would you proceed to prepare:
(i) Sodium metal
(ii) Sodium hydroxide,
(iii) Sodium peroxide
(iv) Sodium Carbonate?
Answer:
(i) Sodium Metal: It is manufactured by electrolysis of a fused mixture of NaCl and CaCl2 in Down's cell at 873 K using iron cathode and graphite anode. Na is liberated at the cathode while Cl2 is evolved at anode.
At cathode: Na + e- → Na(l)
At anode: 2Cl- → Cl2 + 2e-
(ii) Sodium Hydroxide: It is manufactured by electrolysis of an aqueous solution of NaCl in Castner-Keller cell using mercury cathode and carbon anode. Sodium metal which is discharged at cathode combines with mercury to form amalgam. Cl2 gas is evolved at anode.
At cathode: Na + e- → Na;
Na + Hg → Na - Hg (Sodium amalgam)
At anode : 2Cl- → Cl2 + 2e-
Sodium amalgam thus obtained is treated with water to form sodium hydroxide and hydrogen gas.
2Na-Hg + 2H2O → 2NaOH + H2 + 2Hg
(iii) Sodium Peroxide: It is obtained by heating sodium in excess of air. The initially formed sodium oxide reacts with more oxygen to form sodium peroxide.
4Na + O2 → 2Na2O
2Na2O + O2 → 2Na2O2
(iv) Sodium Carbonate: It is obtained by Solvay-ammonia process. When carbon dioxide is passed through a concentrated solution of brine saturated with ammonia, NaHCO3 gets precipitated which on subsequent heating gives Na2CO3.
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
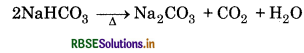
Question 10.17.
What happens when
(i) magnesium is burnt in air?
(ii) Quick lime is heated with silica?
(iii) Chlorine reacts with slaked lime?
(iv) Calcium nitrate is heated?
Answer:
(i) When Mg is burnt in air, it burns with dazzling brilliance to form magnesium oxide.
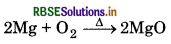
(ii) When quick lime is heated with silica, it forms calcium silicate.
CaO + SiO2 → CaSiO3
(iii) When chlorine reacts with slaked lime, it forms calcium hypochlorite, which is a constituent of bleaching powder.

(iv) When calcium nitrate is heated, it decomposes to give calcium oxide.
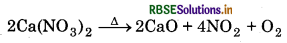

Question 10.18.
Describe two important uses of each of the following:
(i) Caustic soda,
(ii) Sodium carbonate
(iii) Quick lime
Answer:
|
Compound |
Use |
|
(i) Caustic soda |
a. It is used in refining of petroleum. |
|
|
b. It is used in paper, soap and textile industry. |
|
(ii) Sodium carbonate |
a. It is used in softening of hard water. |
|
|
b. It is used in the manufacture of glass, caustic soda etc. |
|
(iii) Quick lime |
a. It is used in the manufacture of dye stuffs. |
|
|
b. It is used in the purification of sugar. |
Question 10.19.
Draw the structure of
(i) BeCl2 (vapour),
(ii) BeCl2 (solid).
Answer:
(i) Structure of BeCl2 (Vapour):
In the vapour state, BeCl2 tends to form a chlorobridged dimer which dissociates into the linear monomer at high temperature of the order of 1200K.
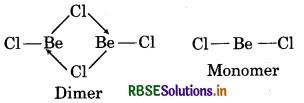
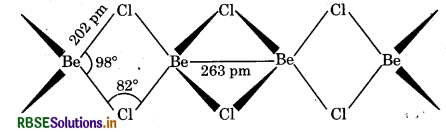
(b) Structure of BeCl2 (Solid): In the solid state, BeCl2 has a chain structure. In this structure, each Be atom is surrounded by chlorine atoms i.e., two Cl-atom by covalent bonds and other two Cl-atoms by co-ordinate covalent bonds. The chain structure is shown below:
Question 10.20.
The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts magnesium and calcium are sparingly soluble in water. Explain.
Answer:
Hydration energy of hydroxides and carbonates of sodium and potassium is more than their lattice energy. So, they are easily soluble in water. While in case of corresponding salts of magnesium and calcium, their hydration energy is less than their lattice energy. So, they are sparingly soluble in water.
Question 10.21.
Describe the importance of the following:
(i) Limestone,
(ii) Cement
(iii) Plaster of paris.
Answer:
(i) Limestone
- It is used as a building material in the form of marble and in manufacture of quick lime.
- It is used as an antacid, mild abrasive in toothpaste, a constituent of chewing gum and a filler in cosmetics.
- Specially precipitated CaCO3 is extensively used in manufacture of high quality paper.
(ii) Cement
- It is an important construction material.
- It is used in concrete and reinforced concrete, in plastering and in construction of buildings, bridges and dams.
(iii) Plaster of Paris
- It is used in dentistry.
- It is used in surgical bandages used for plastering broken or fractured bones of the body and for preparing blackboard chalks.
- It is used for making statues, models and other decorative materials.
Question 10.22.
Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Answer:
The ionic size of lithium is smallest among all alkali metals. So, lithium ion can polarize water molecules readily than other alkali methal ions. Due to this polarization, water molecules get attached to lithium salts as water of crystallization. Hence lithium salts are commonly hydrated. Since, ionic size increases down the group and polarizing power decreases. So, other alkali metal ions are usually anhydrous.

Question 10.23.
Why is LiF almost insoluble in water whereas LiCl is soluble not only in water but also in acetone?
Answer:
Lattice energy of LiF is high due to which LiF is almost insoluble in water. LiCl is soluble in water due to high hydration energy of Li+ ion. LiCl is also soluble in acetone due to its predominantly covalent nature. Covalent character increases with the increase in size of the anion.
Question 10.24.
Explain the significance of sodium, potassium, magnesium and calcium in biological fluids.
Answer:
Significance of Sodium:
(a) It is a major component of blood plasma.
(b) Important in trAnswer:mission of nerve signals and function of heart.
(c) Important in activation of some enzymes.
(d) Important in regulating the flow of water across cell membranes.
Significance of Potassium:
(a) In activating many enzymes.
(b) Participate in oxidation of glucose to produce energy rich ATP molecules.
(c) Protein synthesis.
(d) Participate with Na+ ions in sodium potassium pump for trAnswer:mission of nerve signals
Significance of Magnesium:
(a) M2+ ions catalyse many enzymatic reaction. All enzymes that utilize ATP in phosphate trAnswer:fer require magnesium as the cofactor.
(b) The main pigment for absorption of light in plant is chlorophyll which contains magnesium.
Significance of Calcium:
(a) About 99% to body calcium is present in bones and teeth.
(b) Calcium plays an important role neuromuscular function, inter neuronal trAnswer:mission, cell membrance integrity and blood coagulation.
Question 10.25.
What happens when:
(i) Sodium metal is dropped in water?
(ii) Sodium metal is heated in free supply of air?
(iii) Sodium peroxide dissolves in water?
Answer:
(i) When sodium metal is dropped in water, hydrogen gas is evolved with the release of extreme heat, which catches fire. The reaction is exothermic in nature. 2Na + 2H2O → 2NaOH + H2↑
(ii) When sodium metal is heated in free supply of air or oxygen, it burns with the formation of sodium peroxide and some quantity of sodium superoxide.
4Na + O2 → 2Na2O
2Na2O + O2 → 2Na2O2
(iii) When sodium peroxide is dissolved in water, it gives hydrogen peroxide.
Na2O2 + 2H2O → 2NaOH + H2O2
Question 10.26.
Comment on each of the following observations:
(a) The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ < K < Rb+ < Cs+
(b) Lithium is the only alkali metal to form a nitride directly.
(c) E+ for M2+(aq) + 2e- → M(s) (where M = Ca,
Sr or Ba) nearly constant.
Answer:
(a) The alkali metal ions are highly hydrated. The smaller the size of the ion, the greater is the degree of hydration. Thus, Li+ ion gets much more hydrated than Na+ ion which is more hydrated than K+ ion and so on. Therefore, the extent of hydration decreases from Li+ to Cs+. As a result of larger hydration of Li+ ion than Na+ ion the effective size of Li+ is much more than that of Na+ ion and ionic radii in water decreases as:
Li = > Na+ > K+ > Rb+ > Cs+
Therefore, the mobility of Li+ ion is slowest and that of Csion is maximum. Thus mobility of ions in water increases as:
Li+ < Na+ < K < Rb+ < Cs+
(b) Lithium is the only alkali metal to form a nitride directly due to its small size.
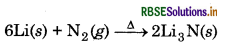
(c) E for M2+ (aq) + 2e → M(s) (where M = Ca, Sr or Ba) is nearly constant because of the fact that ER of M2+/M electrode depends upon following factors
(i) enthalpy of vaporisation.
(ii) ionisation enthalpy.
(iii) enthalpy of hydration.
Since the combined effect of above factors is nearly the same for Ca, Sr or Ba, therefore, their electrode potential, (E) are nearly constant:
Question 10.27.
State as to why:
(a) A solution of Na2CO3 is alkaline?
(b) Alkali metals are prepared by electrolysis of their fused chlorides?
(c) Sodium is found to be more useful than potassium?
Answer:
(a) Sodium carbonate (Na2CO3) gives weak acid (HCO3) and strong base (NaOH) in its aqueous solution. So, its solution is alkaline in nature.
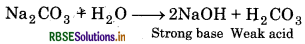
(b) The discharge potentials of alkali metals are much higher than that of hydrogen and therefore, when the aqueous solution of alkali metal chlorides are subjected to electrolysis, hydrogen is evolved preferably than alkali metal at the cathode. Therefore, to prepare alkali metals, electrolysis of their fused chlorides is carried out.
(c) Sodium is found to be more useful than potassium as it is highly reactive in nature. Sodium is used as a laboratory reagent for organic analysis.

Question 10.28.
Write balanced equations for reactions between.
(a) Na2O and water.
(b) KO2 and water.
(c) Nag and CO2.
Answer:
(a) Na2O2 + 2H2O → 2NaOH + H2O2
(b) 2KO2 + 2H2O → 2KOH + H2O2 + O2
(c) Na2O + CO2 → Na2CO3
Question 10.29.
How would you explain the following observations?
1. BeO is almost insoluble but BeSO, is soluble in water.
2. BaO is soluble but BaSO, is insoluble in water.
3. Lil is more soluble than KI in ethanol.
Answer:
- BeO is almost insoluble in water due to high lattice energy. BeSO4 is soluble in water due to high hydration energy of Be2+ ion. The high hydration energy of Be in BeSO4 overcomes the lattice energy factor and therefore BeSO4 is soluble in water.
- BaO is soluble in water because lattice energy of BaO is much smaller than that of its hydration energy. Whereas BaSO4 is insoluble in water because lattice energy predominates over the hydration energy.
- Lil is more soluble than KI in ethanol due to more covalent nature of Lil than KI.
Question 10.30.
Which of the alkali metal is having least melting point:
(a) Na
(b) K
(c) Rb
(d) Cs
Answer:
(d) Cs
Reason: Due to large size of Cs.
Question 10.31.
Which one of the following alkali metals gives hydrated salts.
(a) Li,
(b) Na,
(c) K,
(d) Cs?
Answer:
(a) Li
Reason: Due to small size of Li+ ion.
Question 10.32.
Which one of the alkaline earth metal carbonates is thermally the most stable :
(a) MgCO3,
(b) CaCO3,
(c) SrCO3,
(d) BaCO3?
Answer:
(d) BaCO3
Reason: Due to high electropositive nature of Ba than other corresponding metals.

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter
- RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure