RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 8 Redox Reactions
RBSE Class 11 Chemistry Redox Reactions InText Questions and Answers
Question 8.1.
In the reactions given below, identify the species undergoing reduction:
(i) H2S (g) + Cl2(g)
(ii) 3Fe3O4 (s) + 8AI(g)
(iii) 2Na(s) + H2(g)
Answer:
(i) H2S (g) + Cl2(g) → 2HCl(g) + S(g)
Since, a more electronegative element i.e., chlorine is added to hydrogen, so, H2S is oxidised and chlorine is reduced due to addition of hydrogen to it.
(ii) 3Fe3O4(s) + 8Al (s) → 9Fe (s) + 4Al2O3(s)
Since, oxygen is added to aluminium so aluminium is oxidised. Oxygen is removed from Fe3O4 (ferrous/ferric oxide), so, Fe3O4 is reduced.
(iii) 2Na (s) + H2(g) → 2NaH (s)
It can be explained on the basis of electron transfer
rections:
2Na (s) → 2Nag + 2e- (Oxidation)
H2(g) + 2e- → 2H (g) (Reduction)
So, sodium is oxidised and hydrogen is reduced.

Question 8.2.
Justify that the reaction :
2Na(s) + H2(g) → 2NaH (s) is a redox reaction.
Answer:
For the reaction,
2Na(s) + H2(g) → 2NaH(s)
The two half-reactions are:
2Na (s) → 2Na+(g) + 2e-
(Oxidation half reaction)
H2(g) + 2e- → 2H-(g)
(Reduction half reaction)
Since, in above reaction, sodium is oxidised and hydrogen is reduced. Hence, it is clear that it is a redox reaction.
Question 8.3.
Using stock notation, represent represent the following compounds :
HAuCl4, Tl2O, FeO, Fe2Ó3 CuI, CuO, MnO and MnO2.
Answer:
First of all, calculate the oxidation number of metal:
|
Compound |
Oxidation Number of Metal |
Stock Notation |
|
HAuCl4 |
1 + x + 4(-1) = 0 1 + x - 4 = 0 x = 3 |
HAu(III)Cl4 |
|
TI2O |
2x + (-2) = 0 2x - 2 = 0 x = 1 |
TI 2(I)O |
|
FeO |
x - 2 = 0 x = 2 |
Fe(II)O |
|
Fe2O3 |
2x + 3(-2) = 0 2x - 6 = 0 x = 3 |
Fe2 (III)O3 |
|
CuI |
x - 1 = 0 x = 1 |
Cu(I)I |
|
CuO |
x - 2 = 0 x = 2 |
Cu(II)O |
|
MnO |
x - 2 = 0 x = 2 |
Mn(II)O |
|
MnO2 |
x + 2 (-2) = 0 x - 4 = 0 |
Mn(IV)O2 |
Question 8.4.
Justify that the reaction:
2Cu2O(g) + Cu2S (s) 6Cu(s) + SO2(g)
is a redox reaction. Identify the species I oxidised/reduced, which acts as an oxident and which acts as a reductant.
Answer:
First of all, assign the oxidation number of each of the species in the reaction:
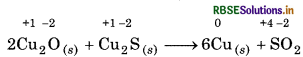
In above reaction, the oxidation state of Cu decreases from +1 to 0, so, copper is reduced and oxidation state of Sincreases from 2 to + 4, so, sulphur is oxidised. Hence, this reaction is a redox reaction.
Question 8.5.
Which of the following species do not show disproportionation reaction and why?
ClO ̄, ClO-2, ClO-3 and ClO-4
Also write reaction for each of the species that disproportionates.
Answer:
First of all, assign the oxidation number to chlorine in each oxoanion of chlorine,
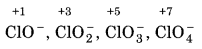
Since, in ClO4, chlorine is present in its highest oxidation state i.e., +7, so, it does not disproportionate. The disporportionation reactions for the other three oxoanions of chlorine are given as below:
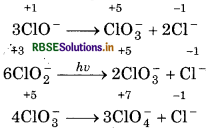
Question 8.6.
Suggest a scheme of classification of the following redox reactions:
(a) N2(g) + O2(g) → 2NO(g)
(b) 2 Pb(NO3)2(s) → 2PbO(g) + 4NO2(g)
(c) NaH(s) + H2O(l) → NaOH(aq) + H2(g)
(d) 2NO2(g) + 2OH-(aq) → NO-2(aq) + NO-3(aq) + H2O(l)
Answer:
(a) N2(g) + O2(g) → 2NO (g)
Since, this reaction shows the combination of N2 and O2 to form NO, so, it is a combination redox reaction.
(b) 2Pb(NO3)2(s) → 2PbO(g) + 4NO2(g) + O2(g)
This reaction shows the breaking down of lead nitrate nto three components, SO it is regarded decompositon redox reaction.
c) NaH (s) + H2O(l) →NaOH(aq) + H2(g)
In this reaction, hydrogen of water has been displaced by Hion into dihydrogen gas. So, it is referred to as displacement redox reaction.
(d) 2NO2(g) + 2OH-(aq) → NO-2 (aq) + NO3- (aq) + H2O(l)
This reaction involves disproportionation of NO2 (+4 oxidation state) into NO2 (+3 oxidation state) and NO3 (+5 oxidation state), so, it is referred to as disproportio- nation redox reaction.

Question 8.7.
Why do the following reactions proceed differently?
Pb3O4 + 8HCl → 3PbCl2 + Cl2 + 4H2O
and
Pb3O4 + 4HNO3 → 2Pb(NO3)2 + PbO2 + 2H2O
Answer:
Pb3O4 is a stoichiometric mixture of 2 mol of PbO and 1 mol of PbO2.
In PbO, the oxidation state of Pb = + 2
In PbO2, the oxidation state of Pb = + 4
So, PbO2 can act as an oxidising agent and so, it can oxidise Cl- ion of HCl into chlorine. PbO is a basic oxide. Hence, the reaction,
Pb3O4 + 8HCl → 3PbCl2 + Cl2 + 4H2O
can be splitted into two reactions:
2PbO + 4HCl → 2PbCl2 + 2H2O (Acid-base reaction)
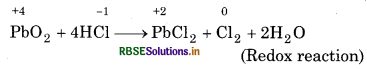
Now, since, HNO3 itself is an oxidising agent, so, it is unlikely that the reaction may occur between PbO2 and HNO3. However, the acid base reaction occurs between PbO and HNO3 as:
2PbO + 4HNO3 → 2Pb(NO3)2 + 2H2O
It is the passive nature of PbO2 against HNO3 that makes the reaction different from the one that follows with HCl.
Question 8.8.
Write the net ionic equation for the reaction of potassium dichromate (VI), K2Cr2O7 with sodium sulphite (Na2SO3) in an acid solution to give chromium (III) ion and the sulphate ion.
Answer:
The net ionic equation can be written in following steps:
Step 1: The skeletal ionic equation is:
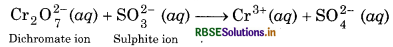
Step 2: Assign oxidation number to each atom of ionic equation:
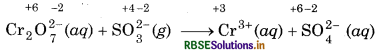
In this equation, the oxidation state of Cr decreases from +6 to +3, so Cr2O2 acts as oxidant and the oxidation state of S increases from +4 to +6, so, SO2 acts as
reductant.
Step 3: Oxidation state of Cr decreases from +6 to +3, so, there is decrease of +3 in oxidation state of chromium on R.H.S. of the equation. Oxidation state of sulphur increases from +4 to +6, so, there is an increase of +2 in the oxidation state of sulphur on R.H.S. of the equation. To make the increase and decrease of oxidation state equal, put 2 before chromium ion on R.H.S. and 3 before SO2 on R.H.S. and balance the Cr and S atoms on both the sides of equation. So, we get,
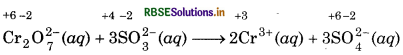
Step 4: The reaction takes place in the acidic medium and ionic charges are not equal on both the sides. Add 8H+ ions on the left to make to make ionic charges equal.
Cr2O72-(aq) + 3SO2-3(aq) + 8H+ → 2Cr3+ (aq) + 3SO42- (aq)
Step 5: Finally, balance the hydrogen atoms and add appropriate number of water molecules i.e., 4H2O on the R.H.S. to obtain balance redox equation:
Cr2O72-(aq) + 3SO32-(aq) + 8H+(aq) → 2Cr3+ (aq) + 3SO42- (aq) + 4H2O (l)
Question 8.9.
Permanganate ion reacts with bromide ion in basic medium to give manganese dioxide and bromate ion. Write the balanced ionic equation for the reaction.
Answer:
The balanced ionic equation for the reaction can be written in following steps:
Step 1: The skeletal ionic equation is :
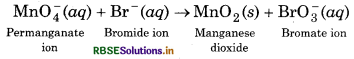
Step 2: Assign oxidation numbers to each atom of ions in the equation,
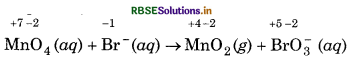
It shows that MnO4- is the oxidising agent and Br- is the reducing agent.
Step 3: Oxidation state of Mn decreases from +7 to +4, so, there is decrease of +3 oxidation state of manganese on R.H.S. of equation.
Oxidation state of Br increases from -1 to +5, so, there is an increase of +6 oxidation state of Br on R.H.S. of equation.
To make increase and decrease of oxidation state equal, put 2 before MnO, on R.H.S. and balance the Mn and Br atoms on both the sides of the equation, so, we get,
2MnO4(aq) + Br-(aq) → 2MnO2 (s) + BrO-3(aq) + 2OH-(aq)
Step 4: Since, the reaction takes place in basic medium and the ionic charges are not equal on both sides. so, add 2OH- ions on the R.H.S. to make ionic charges equal.
2MnO-4 (aq) + Br-(aq) + H2O(l) → 2MnO2 (s) + BrO-3(aq) + 2OH-(aq)
Step 5: Finally, balance the number of hydrogen atoms and add appropriate number of water molecules i. e., one H2O on the L.H.S. to obtain balanced redox equation.
2MnO4(aq) + Br ̄ (aq) + H2O(l) → 2MnO2 (s) + BrO3(aq) + 2OH- (aq)

Question 8.10.
Permanganate (VII) ion, MnO in basic solution oxidises iodide ion, I to produce molecular iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent this redox reaction.
Answer:
Step 1: Write the skeletal ionic equation,
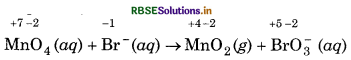
Step 2: Write the two half reactions :
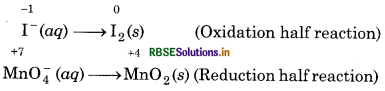
Step 3: Balance of iodine atoms in the oxidation half reaction.
2I-(aq) → I2(s)
Step 4: Balance the oxygen atoms in the reduction half reaction, add two water molecules on the R.H.S.
MnO-4 (aq) → MnO2 (s) + 2H2O(l)
Now, add four H+ ions on the L.H.S. to balance the hydrogen atoms.
MnO4-(aq) + 4H+(aq) + 4OH-(aq) → MnO2 (s) + 2H2O(l) + 4OH-(aq)
Since, the reaction occurs in basic medium, so, for 4H+ ions, add 4OH- ions to both sides of the equation,
MnO-4(aq) + 4H+(aq) + 4OH- (aq) → MnO2 (s) + 2H2O(l) + 4OH- (aq)
MnO-4 (aq) + 2H2O (l) → MnO2 (s) + 4OH-(aq)
Step 5: Balance the charges of the two half reactions.
2I(aq) → I2 (s) + 2e ̄
MnO-4(aq) + 2H2O(s) + 3e → MnO2 (s) + 4OH- (aq) To equalise the number of electrons, multiply the oxidation half reaction by 3 and reduction half reaction by 2.
6I-(aq) → 3I2 (s) + 6e ̄
2MnO-4 (aq) + 4H2O(l) + 6e- → 2MnO2 (s) + 8OH-(aq)
Step 6: Add two half reactions to obtain the net reactions after cancelling electrons on both sides
6I-(aq) + 2MnO4(aq) + 4H2O(l) → 3I2 (s) + 2MnO2(s) + 8OH(aq)
Step 7: A final verification shows that the equation is balanced in respect of the number of atoms and charges on both sides.
RBSE Class 11 Chemistry Redox Reactions Textbook Questions and Answers
Question 8.1.
Assign oxidation number to the underlined elements in each of the following species:
(a) NaH2PO4
(b) NaHSO4
(c) H4P2O7
(d) K2MnO4
(e) CaO2
(f) NaBH4
(g) H2S2O7
(h) KAI(SO4)2.12H2O.
Answer:
(a) O.N. of P in NaH2PO4
Let the oxidation number of P = x
+1 + 2 (+1) + x + 4 (-2) = 0
3 + x - 8 = 0
∴ x = + 5
(b) O.N. of S in NaHSO4
Let the oxidation number of H = x
+1 +1 + x + 4 (-2) = 0
2 + x - 8 = 0
∴ x = + 6
(c) O.N. of P in H4P2O7
Let the oxidation number of P = x
4(+1) + 2x + 7 (-2) = 0
4+ 2x - 14 = 0
2x - 10 = 0
∴ \(x=\frac{10}{2}=+5\)
(d) O.N. of Mn in K2MnO4
Let the oxidation number of Mn = x
2 (+1) + x + 4 (-2) = 0
2 + x - 8 = 0
∴ x = + 6
(e) O.N. of O in CaO2
Let the oxidation number of O = x
2 + 2x = 0
2x = -2
∴ x = -1
(f) O.N. of B in NaBH4
Let the oxidation number of B = x
1 + x + 4 (-1) = 0
1 + x 4 = 0
∴ x = + 3
(g) O.N. of S in H2S2O7
2(1) + 2x + 7 (-2) = 0
2 + 2x - 14 = 0
2x - 12 = 0
∴ x = +6
(h) O.N. of S in KAI(SO4)2.12H2O
Let the oxidation number of S = X
+ 1 + 3 + 2x + 8(−2) + 12 × (0)
= 0 + 4 + 2x - 16 = 0
[Oxidation number of K = 1, Oxidation number of Al = 3]
2x = 12
X = +6
Question 8.2.
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
(a) KI3
(b) H2S4O6
(c) Fe3O4
(d) CH3CH6CH
(e) CH3COOH
Answer:
(a) O.N. of I in KI3
KI is actually made up of K+I- and I2
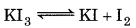
Oxidation no. of I in K+I is -1, whereas it is 0 in I2.
∴ Oxidation no. of I in KI is -1
(b) O.N. of S in H2S4O6
The structure of H2S4O6 is represented as,
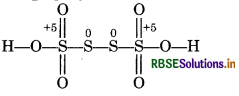
The oxidation number of each of S-atom linked to each other in the middle is zero while the oxidation number of the two S atoms is +5 each.
∴ Oxidation number (average) of S in H2S4O6 = +5/2
(c) O.N. of Fe in Fe3O4
(i) Fe3O4 is a mixture of FeO and Fe2O3 having that composition FeO. Fe2O3. The oxidation of Fe in FeO is +2, whereas it is +3 in Fe2O3. [By O stoichiometry].
(ii) By conventional method:
3x + 4 (-2) = 0
or x = + 8/3
The value of 8/3 for oxidation number of Fe as obtained by conventional method is infact the average of the actual oxidation number of all the atoms in the formula, e. g.,(1 × 2 + 2 × 3)/3 = + 8/3.
(d) O.N. of C in CH3 CH2OH
idation number of underlined carbon atoms can be
culated as follows:
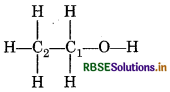
C2 is attached to three H atoms (less electronegative than and one -CH2OH (more electronegative than C) idation number of C2 is
3(+1) + x + 1 (−1) = 0
+ 3 + x - 1 = 0
x = - 2
or each covalent bond between two disimilar atoms, sign an oxidation number of +1 to the less ctronegative atom and -1 to the more electronegative elment]
C1 is attached to one OH [Oxidation No. - 1] one -CH3 group [O. No. +1], two H atoms [O.No. = + 1]
∴ O Number of C1 is
+ 1 + 2 (+1) + x - 1 = 0
+ 1 + 2 + x -1 = 0
x = -2
(e) O.N. of C in CH3COOH
By conventional method
CH3COOH = C2H4O2
2(x) + 4(1) + 2(-2) = 0
2(x) + 4 - 4 = 0
2x = 0
x = 0
chemical bonding method,
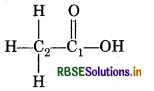
C2 is attached to three H-atoms (less electronegative n carbon) and one-COOH group (more electronegative than carbon).
∴ Oxidation number of C2 = 3 (+1) + x + 1 (−1) = 0
3 + x - 1 = 0
x + 2 = 0
x = 2
However C1 is attached to one oxygen atom by a dou bond, one OH group (Oxidation number CH3 group oxidation number = + 1)
∴ Oxidation number of C1 = 1 + x + 1 (-2) + 1(-1) = 0
1 + x - 2 - 1 = 0
1 + x - 3 = 0
x - 2 = 0
x = + 2

Question 8.3.
Justify that the following reactions a redox reactions :
(a) CuO(s) + H2(g) → Cu(s) + H2O(g)
(b) Fe2O3 (s) + 3CO(g) → 2Fe(s) + 3CO2(g)
(c) 4BCl3g (g) + 3LiAlH4(s) → 2B2H6(g) + 3LiCl(s) + 3AICl3
(d) 2K(s) + F2(g) → 2K+F ̄ (s)
(e) 4NH3 (g) + 5O2 (g) → 4NO(g) + 6H2O
Answer:
(a) Assign oxidation numbers of each atom in t given reaction
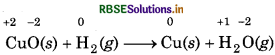
Since, the oxidation number of Cu decreases from +2 to so, Cu undergoes reduction and the oxidation number hydrogen increases from 0 to +1, so, hydrogen underg oxidation. Hence, this reaction is a redox reaction.
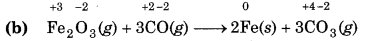
Since, the oxidation number of Fe decreases from +3 to so Fe undergoes reduction and the oxidation number carbon increases from +2 to +4, so, carbon underg oxidation. Hence, this reaction is a redox reaction.
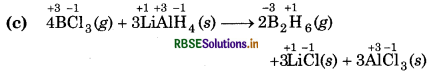
Since, the oxidation number of B decreases from +3 to so, B undergoes reduction i. e., BCl3 is reduced to B2H The oxidation number of H increases from -1 to +1, hydrogen undergoes oxidation i.e., BCl, is reduced B2H6. The oxidation number of H increases from -1 +1, so, hydrogen undergoes oxidation i.e., LiAlH4 oxidised. Hence, this reaction is a redox reaction.
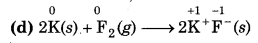
Since, the oxidation number of K increases from 0 to +1 so, K undergoes oxidation and the oxidation number O decreases from 0 to -1, so, Fundergoes reduction. Hence, this reaction is a redox reaction.
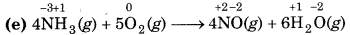
Since, the oxidation number of N increases from -3 to +2, so N undergoes oxidation and the oxidation number of O decreases from 0 to -2, so, O undergoes reduction. Hence, this reaction is a redox reaction.
Question 8.4.
Fluorine reacts with ice and results in the change :
H2O(s) + F2(g) → HF(g) + HOF(g)
Justify that this reaction is a redox reaction.
Answer:
First of all, assign oxidation number to each atom g) in the given reaction.
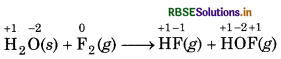
In this reaction, the oxidation number of F decreases from 0 to -1, which shows that F, is reduced to HF and the oxidation number of F increases from 0 to +1, which shows that F2 is oxidised to HOF. So, it is a redox che reaction.
However, HOF is highly unstable molecule and it decomposes into HF and O, as follows:
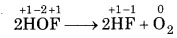
In above reaction, F of HOF is reduced while O of HOF is bes oxidised. So, it is a redox reaction and more specificially,
it is a disproportionation reaction.
Question 8.5.
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O2-7 and NO3- Suggest structure of these compounds. Count for the fallacy.
Answer:
H2SO5:
Let oxidation number of S = X.
∴ 2(1) + x + 5x (-2) = 0
2 + x - 10 = 0
x = +8
But oxidation number of 'S' can not be more than 6 so because S has only 6 valence electrons. This fallacy is to removed by calculating oxidation number of S by to chemical bonding method.
Structure of H2SO is represented as :
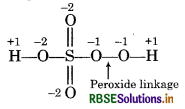
Chemical bonding method:
2(+1) + 3(-2) + x + 2 (−1) = 0
(for H) (for 3 'O) (S) (for 0 - 0)
2 - 6 + x = 0
2 + x - 8 = 0
Cr2O72-:
Let, oxidation number of Cr = x
2(x) + 7(-2) = -2
2x - 14 = 2
x = +6
Structure of Cr2O2 ion is represented as :
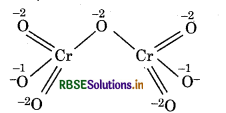
Chemical bonding method
4(-2) + 2(-1) + 1(-2) + 2x = 0
(for 4'0') (for 2'0') (for 1 '0') (Cr)
-8 - 2 - 2 + 2x = 0
-12 + 2x = 0
x = +6
∴ Oxidation number of Cr in Cr2O2 is found to be same
i.e.,+6 calculated by conventional method or by chemical bonding method. So, there is no fallacy.
NO-3:
Let, oxidation number of N = x
x + 3(-2) = -1
x - 6 = -1
x = +5
Structure of NO3- ion is represented as :
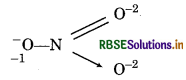
Chemical bonding method:
1(-1) + x + 1(-2) + 1(-2) = 0
(for O) (for N) (for O) (for → 0)
- 1 + x - 2 - 2 = 0
x = +5
Since, the oxidation of N in NO3 is found to be same i.e., +5 by conventional method or by chemical bonding method. So, there is not fallcy.
Question 8.6.
Write fourmulas for the following compounds:
(a) Mercury (II) chloride
(b) Nickel (II) sulphate
(c) Tin (IV) oxide
(d) Thallium (I) sulphate
(e) Iron (III) sulphate
(f) Chromium (III) oxide
Answer:
|
Compound |
Formula |
|
(a) Mercury (II) chloride |
HgCl2 |
|
(b) Nickel (II) sulphate |
NiSO4 |
|
(c) Tin (IV) oxide |
SnO2 |
|
(d) Thallium (I) sulphate |
TI2SO4 |
|
(e) Iron (III) sulphate |
Fe2(SO4); |
|
(f) Chromium (III) oxide |
Cr2O3 |
Question 8.7.
Suggest a list of the substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Answer:
Substance of Carbon:
|
Compound |
Oxidation State of C |
|
CH4 |
-4 |
|
C2H6 |
-3 |
|
CH2CH4 |
-2 |
|
C2H2 |
0 |
|
CH2CI2 |
+1 |
|
C6Cl6 |
-1 |
|
CHCl3 |
-2 |
|
(COOH)2 |
+3 |
|
CCl4 |
+4 |
Substance of nitrogen:
|
Compound |
Oxidation State of N |
|
NH3 |
-3 |
|
N2H4 |
-2 |
|
N2H2 |
-1 |
|
N2 |
0 |
|
N2O |
+1 |
|
NO |
+2 |
|
N2O3 |
+3 |
|
N2O4 |
±4 |
|
N2O5 |
+5 |
Question 8.8.
While sulphur dioxide and hydroge peroxide can act as oxidizing as well reducing agents in their reactions, ozor and nitric acid act only as oxidants. Why
Answer:
It can be explained by oxidation number concept SO2:
Let oxidation number of S
= X
x + 2(-2) = 0
x - 4 = 0
x = + 4
Sulphur can exhibit minimum oxidation number -2 a maximum oxidation number +6. So, in SO2, S can eith decrease or increase its oxidation number. Hence S can act both as oxidizing as well as reducing agent. H2O2:
Let, oxidation number of O = x
2(1) + 2(x) = 0
2 + 2x = 0
2x = - 2
x = - 2
x = -1
Since, oxygen can exhibit minimum oxidation number and maximum oxidation number 0. So, in H2O2, '0' either decrease or increase its oxidation number. Hen H2O2 can act both as oxidizing as well as reduc agent.
O3: Oxidation number of O in Og is zero. It can increase is oxidation number. However, it can decre its oxidation number from 0 to -1 or -2. Hence, oz (O3) can act only as an oxidizing agent.
HNO3: Let, oxidation number of N = x
1 + x + 3(-2) = 0
1 + x - 6 = 0
x = +5
It is maximum value of oxidation number of nitrog So, it can only decrease its oxidation number. Her HNO3 can act only as an oxidizing agent.

Question 8.9.
Consider the reactions:
(a) 6CO2(g) + 6H2O(l) → C6H12O6 (aq) + 6O2
(b) O2 (g) + H2O2(l) → H2O(l) + 2O2(g)
Why it is more appropriate to write th reactions as :
(a) 6CO2(g) + 12H2O(l) → C6H12O6 (aq) + 6H2O(l) + 6O2
(b) O3(g) + H2O2(I) → H2O(l) + O2(g) + O2
Also suggest a techniqe to investigate the path of the above (a) and (b) redox reactions.
Answer:
(a) This reaction can be explained by using mechanism of photosynthesis. It involves following stes:
Step - I: Decomposition of water
12H2O(l) → 12H2(g) + 6O2(g)
Step-II: Reduction of CO2
6CO2(g) + 12H2(g) → C6H12O6(s) + 6H2O(l)
Overall reaction:
nd 6CO2(g) + 12H2O(l) → C6H12O6 (s) + 6H2O(l) + 6O2(g)
Above steps show that 12H2O molecules are use:
step-I and 6H2O molecules are obtained per molecule of glucose.
step-II. So, this overall reaction is more appropriate reaction.
(b) This reaction can be explained by using mechanism of reaction of ozone with H2O2.
It involves following steps:
Step-1: O2(g) → O2 (g) + O(g)
Step-II: H2O2(l) + O(g) → H2O(l) + O2(g)
Overall reaction:
H2O2(l) + O3(g) → H2O(l) + O2(g) + O2(g) Above steps show that O2 molecule is released in each ce, step. So, this overall reaction is more appropriate ing reaction.
Question 8.10.
The compound AgF2 is unstable compound. However, if formed, the compound acts as a very strong oxidising agent. Why?
Answer:
AgF 2:
Let the oxidation number of Ag = x
x + 2(-1) = 0
x = + 2
It is highly unstable therefore it readily accepts an electron to get +1 oxidation state which is more stable.
Ag2+ + e- → Ag+
Hence, AgF2 acts as a strong oxidising agent.
Question 8.11.
Whenever a reaction between an oxidizing agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. Justify this statement giving three illustrations.
Answer:
This statement can be justified by following examples:
(i) When sodium is reducing agent and O2 is oxidising agent, then following reactions take place.
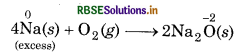
Excess of reducing agent (Na) forms a compound of lower Th oxidation state.
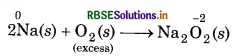
Excess of oxidising agent (O2) forms a compound of higher oxidation state.
(ii) When carbon is reducing agent and O2 is oxidising in agent, then following reactions take place:
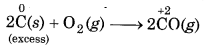
When carbon (reducing agent) is taken in excess, a compound i. e., CO of lower oxidation state is obtained.
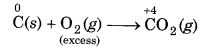
If O2 (oxidising agent) is taken in excess, a compound (CO2) of higher oxidation state is formed.
(iii) When P1 is reducing agent and Cl2 is oxidising agent, then following reactions take place :
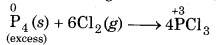
Excess of reducing agent (P4) forms a compound having lower oxidation state.
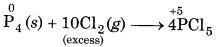
Excess of oxidising agent (Cl2) forms a compound having higher oxidation state.
Question 8.12.
How do you count for the following observations?
(a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene
we use alcoholic potassium permanganate Ans as an oxidant. Why? Write a balanced redox equation for the reaction.
(b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
Answer:
(a) Alcoholic KMnO4 is preferred to acidic or alkaline KMnO4 in the manufacturing of benzoic acid because both the reactants are mixed well in alcoholic solution and form a homogeneous solution. The rate of reaction in homogeneous medium is higher than hterogeneous medium. In neutral medium, OH ions e produced in the reaction itself.
The balanced redox equation for the reaction is given as
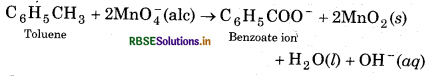
(b) When concentrated sulphuric acid is added to an rganic mixture containing chloride, we get colourless ngent smelling gas HCl because hydrogen chloride is eak reducing agent. It can not reduce H2SO4 to SO2.
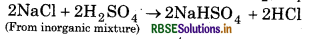
if the mixture contains bromide than we get redours of bromine because HBr is a strong reducing nt. It reduces H2SO4 to SO2 and itself oxidsed to Br1⁄2.
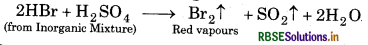
Question 8.13.
Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions :
(a) 2AgBr(s) + C6H6O2(aq) → 2Ag(s) + 2HBr(aq) + C6H4O2(aq)
(b) HCHO(l) + 2[Ag(NH3)2]+(aq) + 3OH-(aq) → 2Ag(s) + HCOO-(aq) + 4NH3 (aq) + 2H2O(l)
(c) HCHO(l) + 2Cu2+ (aq) + 5OH- (aq) → Cu2O(s) + HCOO-(aq) + 3H2O(l)
(d) N2H4 (l) + 2H2O2(l) → N2(g) + 4H2O(l)
(e) Pb(s) + PbO2(s) + 2H2SO4 (aq) → 2PbSO4 (s) + 2H2O(l)
Answer:
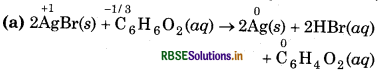
Substance oxidised = C6H6O2(aq)
Reducing agent = C6H6O2 (aq)
Substance reduced = AgBr (s)
Oxidising agent = AgBr (s)
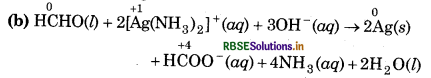
Substance oxidised = HCHO(l)
Reducing agent = HCHO(l)
Substance reduced = [Ag(NH3)2]+(aq)
Oxidising agent = [Ag(NH3)2]+(aq)
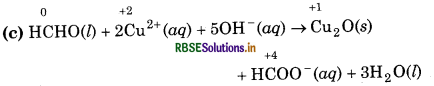
Substance oxidised = HCHO(l)
Reducing agent = HCHO(l)
Substance reduced = Cu2+(aq)
Oxidising agent = Cu2+(aq)
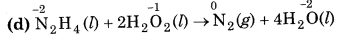
Substance oxidised = N2H4 (l)
Reducing agent = N2H4 (l)
Substance reduced = H2O2 (l)
Oxidising agent = H2O2(l)
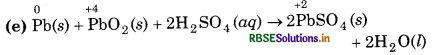
Substance oxidised = Pb(s)
Reducing agent = Pb(s)
Substance reduced = PbO2(s)
Oxidising agent = PbO2 (s)
Question 8.14
Consider the reactions :
2S2O2-3 + I2(s) → S4O62-(aq) + 2I-(aq)
S2O32-(aq) + 2Br2(l) + 5H2O(l) → 2SO24-(aq) + 4Br(aq) + 10H+(aq)
Why does the same reductant, thiosu hate react differently with iodine a bromine ?
Answer:
Assign oxidation number to each atom reactions:
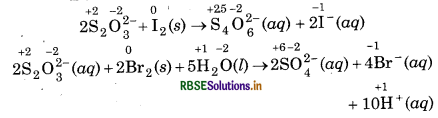
In above reactions, thiosulphate acts as reductant bromine (Br2) and iodine (I2) act as oxidising ag Bromine is strong oxidising agent as compared to iod Br, oxidises S of S2O2 to higher oxidation state + 6 in SO42-. While, I2 oxidises S of S2O32- to a lower oxidastate 2.5 in S4O2. That's why the same reduct thisoulphate reacts differently with iodine and bromide.

Question 8.15.
Justify giving reactions that among halogens, fluorine is the best oxidant among hydrohalic compounds, hydroid acid is the best reductant.
Answer:
The relative order of oxidising power of halogen
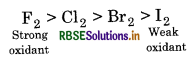
It is due to the fact that F, oxidises all other halide ions i.e., Cl ̄, Br ̄ and I ̄ into corresponding halogens, while iodine (I2) can not oxidise any halide ion.
F2(g) + 2Cl (aq) → 2F-(aq) + Cl2(g)
F2(g) + 2Br-(aq) → 2F-(aq) + Br2 (g)
F2(g) + 2I-(aq) → 2F-(aq) + I2 (g)
The relative order of reducing power of hydrohalic acids is :

It is due to the fact that HI reduces strong oxidising agent i.e., H2SO4 and is itself oxidised to I2, while HF HCl does not reduce H2SO4
2HI + H2SO4 → SO2 + 2H2O + I2
2HF + H2SO4 → No reaction
HCl also does not react with H2SO4-
Question 8.16.
Why does the following reaction occur?
XeO64- (aq) + 2F-(aq) + 6H+(aq) → XeO3(g) + F2(g) + 3H2O(l)
What conclusion about the compound Na4 XeO6 (of which XeO64- is a part) can be drawn from the reaction?
Answer:
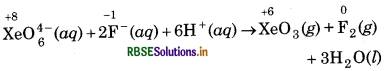
Since, in above ionic equation, the oxidation number of Xe decreases from +8 to +6, so, it is reduced and oxidation number of F increases from -1 to 0, so, it is oxidised. Hence, it is a redox reaction. Sodium xenate (Na XeO6) is a powerful oxidising agent as it oxidises F to F2 and itself reduced to XeO3.
Question 8.17.
Consider the reactions :
(a) H3PO2(aq) + 4AgNO3 (aq) + 2H2O(l) → H3PO4 (aq) + 4Ag(s) + 4HNO3(aq)
(b) H3PO2(aq) + 2CuSO4 (aq) + 2H2O(l) → H3PO4 (aq) + 2Cu(s) + H2SO4 (aq)
(c) C6H5CHO(l) + 2[Ag(NH3)2] (aq) + 3OH (aq) → C6H5COO-(aq) + 2Ag(s) + 4NH3 (aq) + 2H2O(l)
(d) C6H5CHO(l) + 2Cu2+(aq) + 5OH (aq) → No change observed.
What inference do you draw about the S behaviour of Ag+ and Cu2+ from these reactions?
Answer:
Assign the oxidation number to Ag and Cu in each reaction:
(a) H3PO2(aq) + 4AgNO3 (aq) + 2H2O(l) → H3PO4 (aq) + 4Ag(s) + 4HNO3(aq)
In this reaction, Ag+ ions have been reduced to Ag(s) so, S it acts as an oxidising agent and oxidises H3PO2 to H3PO4.
(b) H3PO2(aq) + 2CuSO4 (aq) + 2H2O(l) → H3PO4 (aq) + 2Cu(s) + H2SO4(aq)
In this reaction, Cu2+ ions are reduced to Cu(s). Thus it acts as an oxidising agent and oxidises H3PO2 H3PO4.
(c) C6H5CHO(l) + 2[Ag(NH3)2] (aq) + 3OH (aq) → C6H5COO-(aq) + 2Ag(s) + 4NH3 (aq) + 2H2O(l)
In this reaction, Ag+ of [Ag(NH3)2]+ is reduced to Ag(s). It acts as oxidising agent and oxidises C6H5CHO to C6H5COO-.
(d) C6H5CHO(l) + 2Cu2+(aq) + 5OH (aq) → No change observed.
Cu2+ ions can not oxidise C6H5CHO to C6H5COOH. This indicates that Ag+ is a stronger oxidising agent than Cu2+.
Question 8.18
Balance the following redox reactions by ion-electron method:
(a) MnO4 (aq) + I-(aq) → MnO2 (s) + I2 (s) (in basic medium)
(b) MnO4 (aq) + SO2(g) → Mn2+(aq) + HSO4 (aq) (in acidic solution)
(c) H2O2(aq) + Fe2+ (aq) → Fe3+ (aq) + H2O(l) (in acidic solution)
(d) Cr2O72-(aq) + SO2 (g) → Cr3+ (aq) +SO42-(aq) (in acidic solution)
Answer:
(a) MnO4 (aq) + I-(aq) → MnO2 (s) + I2 (s) (in basic medium)
Step 1: Write the skeletal ionic equation and assign oxidation number to each atom
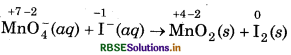
step 2: Write two half-reactions.
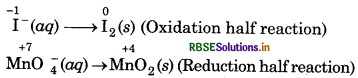
step 3: Balance the iodine atoms in oxidation half eaction :
2I-(aq) → I2(s)
step 4: Balance O-atoms in the reduction half reaction, ad two water molecules to R.H.S.
MnO4(aq) → MnO2 (s) + 2H2O(l)
Now, to balance H-atoms, add 4H+ ions to L.H.S.
MnO4(aq) + 4H+(aq) → MnO2 (s) + 2H2O(l)
since, the reaction occurs in basic medium, so for 4H+ ns, we add 4OH- ions to both sides of the equation.
MnO4-(aq) + 4H+(aq) + 4OH-(aq) → MnO2(s) + 2H2O(l) + 4OH-(aq)
or
MnO4 (aq) + 4H2O(l) → MnO2 (s) + 2H2O(l) + 4OH-(aq)
MnO4(aq) + 2H2O(l) → MnO2 (s) + 4OH-(aq)
step 5: Now, balance the charges of the both half actions,
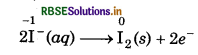
To equalize the number of electrons, multiply the dation half reaction by 3 and the reduction half action by 2, then we get
6I-(aq) → 3I2 (s) + 6e ̄
2MnO4-(aq) + 4H2O(l) + 6e- → 2MnO2 (s) + 8OH-(aq)
step 6: Add above two half reactions after cancelling - electrons on both sides, we get
6I-(aq) + 2MnO4(aq) + 4H2O(l) → 3I2 (s) + 2MnO2 (s) + 8OH-(aq)
step 7: A final verfication shows that the equation is anced in respect of number of all atoms and charges both sides.
MnO4- (aq) + SO2 (g) → Mn2+(aq) + HSO-4 (aq) (in acidic solution)
step 1 : Write the skeletal ionic equation and assign the dation number to each atom
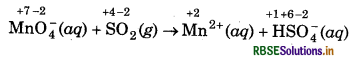
step 2 : Write two half reactions :
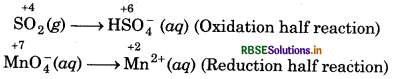
Step 3: Balance O-atoms in each half reaction.
In oxidation half reaction, add 2H2O molecules to the L.H.S.
SO2 (g) + 2H2O(l) → HSO4(aq)
In reduction half reaction, add 4H2O molecules to the R.H.S.
MnO4(aq) → Mn2+ (aq) + 4H2O(l)
Now, balance H-atoms in each half reaction, in oxidation half reaction, add 3H+ ions to the R.H.S.
SO2(g) + 2H2O(l) → HSO4(aq) + 3H+(aq)
In reduction half reaction, add 8H+ ions to the L.H.S.
MnO4(aq) + 8H+(aq) → Mn2+(aq) + 4H2O(l)
Step 4: Balance the charges of two half reactions:
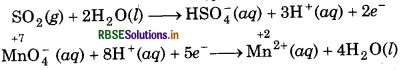
To equalize the number of electrons, multiply th oxidation half reaction by 5 and reduction half reactio by 2, we get
5SO2(g) + 10H2O(l) → 5HSO4(aq) + 15H+(aq) + 10e-
2MnO4(aq) + 16H(aq) + 10e- → 2Mn2+(aq) + 8H2O
Step 5: Add above half reactions after cancelling th electrons and other species on both sides, we get
5SO2 + 2MnO4-(aq) + H+(aq) + 2H2O(l) → 5HSO4(aq + 2Mn2+(aq)
Step 6: A final verification shows that the equation balanced in respect of the number of atoms and charg on both sides.
(c) H2O2(aq) + Fe2+ (aq) → Fe3+(aq) + H2O(l) (in acidic solution)
Step 1: Write skeletal ionic equation and assig oxidation number to each atom
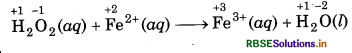
Step 2: Write two half reactions,
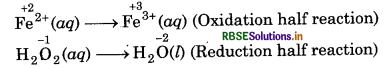
Step 3: Balance O atoms in reduction half reaction.
H2O2(aq) → 2H2O(l)
Now, to balance H-atoms, add 2H+ ions to L.H.S.
H2O2(aq) + 2H+(aq) + 2H2O(l)
Step 4: Balance the charges on both sides
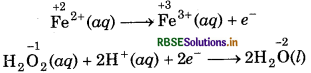
To equalize the number of electrons, multiply the e oxidation half reaction by 2 we get
2Fe2+ (aq) → 2Fe3+(aq) + 2e-
Step 5: Now, add above half-reactions after cancelling the electrons, we get,
2Fe2+(aq) + H2O2(aq) + 2H+(aq) → 2Fe3+ (aq) + 2H2O(l)
Step 6: A final verification shows that the equation is balanced in respect of the number of atoms and charges
on both sides.
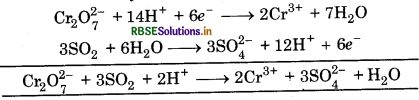
(d) Cr2O27- + SO2 → Cr3+ + SO42-
First half reaction : 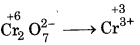 (Reduction)
(Reduction)
Balancing Cr, Cr2O72- → 2Cr3+
(i) To balance oxygen atoms, add H2O to right side
Cr2O72 → 2Cr3+ + 7H2O
(ii) To balance hydrogen atoms, add H+ ions to left side
Cr2O72- + 14H+ → 2Cr3+ + 7H2O
(iii) To balance charge, electrons are added to left side
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O ...... (i)
Second half reaction: 
(iv) To balance oxygen atom, add H2O to left side
SO2 + 2H2O → SO42-
(v) To balance hydrogen atom, add H+ ions to right side
SO2 + 2H2O- → SO2 + 4H+
(vi) To balance charge, add electrons to right side
SO2 + 2H2O → SO42- + 4H+ + 2e- ...(2)
Multiply eq. (1) by 1 and eq. (2) by 3, and add, we get

Question 8.19.
Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
(a) P4 (s) + OH-(aq) → PH3(g) + HPO2 (aq)
(b) N2H4 (4) + ClO3 (aq) → NO(g) + Cl-(g)
(c) Cl2O7 (g) + H2O2(aq) → ClO2-(aq) + O2(g) + H+
Answer:
(a) P4 (s) + OH-(aq) → PH3 (g) + HPO2- (aq)
lon-electron Method:
First half reaction, P4 → P3H3
Balancing P, P4 → 4PH3
(i) To balance H- atoms, add H+ ions to left side.
P4 + 12 H+ → 4PH3
∴ Reaction is in basic medium, add OH- ions to right side.
P4 + 12 H2O → 4PH3 + 12OH ̄
(ii) To balance charge, electrons add to left side.
P4 + 12H2O + 12e → 4PH3 + 12OH-
second half reaction,
Balancing P, P4 → 4HPO2-
(i) To balance hydrogen atoms, add H2O to left side.
P4 + 8H2O → 4HPO2
(ii) To balance hydrogen atom, add H+ions to right side.
P4 + 8H2O → 4HPO2- + 12H+
∴ Reaction is in basic medium, add OH to left side
P4 + 8H2O + 12OH- → 4HPO2 + 12H2O
(iii) To balance charge, electrons are added to right side.
P4 + 8H2O + 12OH- → 4HPO2- + 12H2O + 8e- ...(2)
Multiply eq. (1) by 2 and eq (2) by 3, and add we get.
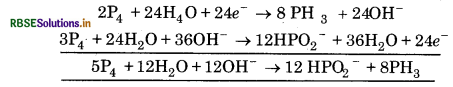
This is balanced equation.
Oxidation Number Method:
Step 1: Write the skeletal ionic equation,
P4 (s) + OH(aq) → PH3(g) + HPO2 (aq)
Step 2: Assign the oxidation number for P.
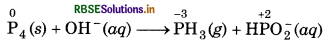
This indicates that the P acts as both oxidant and reductant.
Step 3. Calculate the increase and decrease of oxidation number and make them equal, multiply PH3(g) by 8HPO2- (aq) by 12 and P4 (s) by 5.
5P4 (s) + OH(aq) → 8PH3 (g) + 12HPO2 (aq)
Step 4: Balance the O and H-atoms on left side of the equation, add 12 H2O molecules to left side.
5P4 (s) + 12H2O(l) + 12OH-(aq) → 8PH(g) + 12HPO2 (aq)
(b) N2H4(l) + ClO3-(aq) → NO(g) + Cl-(g)
Ion-electron Method
First half reaction: N2H4 → NO (Oxidation)
Balancing N, N2H4 → 2NO
(i) To balance oxygen atoms, add H2O to left side.
N2H4 + 2H2O → 2NO
(ii) To balance hydrogen atoms. add H+ ions to right side.
N2H4 + 2H2O → 2NO + 8H
∴ Reaction is in basic medium, add OH to left side:
N2H4 + 2H2O + 8OH- → 2NO + 8H2O + 8e- ...... (i)
Second half reaction,
(i) To balance O-atoms, add H2O to right side.
ClO-3 → Cl + 3H2O
(ii) To balance hydrogen atoms, add H+ ions to left side.
ClO2 + 6H+ → Cl- + 3H2O
∴ Reaction is in basic medium, add OH to right side
ClO3 + 6H2O → Cl- + 3H2O + 6OH-
(iii) To balance charge, add electrons to left side.
ClO3- + 6H2O + 6e- → Cl ̄ + 3H2O + 6OH- ...(2)
Multiply equation (1) by 3 equation (2) by 4 and add, we get.
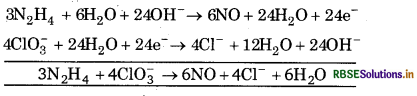
This is balanced equation.
Oxidation Number Method:
Write the skeletal ionic equation and asign oxidation number
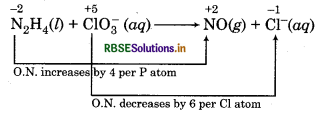
Multiply NO by 2 because in N2H4 there are 2N atoms.
N2H4 (l) + ClO3(aq) → 2NO(g) + Cl-(aq)
Total increase in oxidation number of
N = 2 x 4 = 8 (8e- lost)
Total decrease in oxidation number of
Cl = 1 × 6 = 6 (6e- gained)
So, to balance increase or decrease in oxidation number.
multiply N2H4 by 3, 2NO by 3 and ClO-3, Cl- by 4.
3N2H4 (l) + 4ClO2 (aq) → 6NO(g) + 4Cl-(aq)
Question 8.20.
What sorts of information can you draw from the following reaction?
(CN)2(g) + 2OH-(aq) → CN-(aq) + CNO-(aq) + H2O(l)
Answer:
Assign oxidation number to carbon atoms in the given reaction :
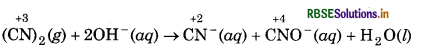
In this reaction, oxidation number of carbon decreases from +3 to +2 in the formation of CN-(aq) from (CN)2(g) while, the oxidation number of carbon increases from +3 to +4 in the formation of CNO-(aq) from (CN)2 (g).
The following informations can be drawn from the above reaction:
(a) Decompostion of cyanogen in the cyanide ion (CN) and cyanate ion (CNO) occurs in basic medium.
(b) Cyanogen (CN)2 acts as both reducing agent as well as oxidising agent.
(c) The reaction is an example of disproportionation reaction (a special type of redox reaction).
(d) Cyanogen (CN)2 is called pseudohalogen while CN ̄,
CNO ions are called pseudohalide ions.
Question 8.21.
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+ MnO2 and H+ ion. Write a balanced ionic
equation for the reaction.
Answer:
A balance ionic equation for the reaction can be written by using following steps.
Step 1: Write skeletal ionic equation and assign oxidation number.
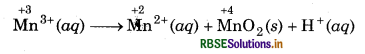
Step 2: Write two half reactions:
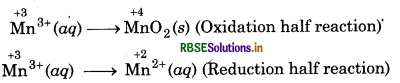
Step 3: To balance O-atoms, add two H2O molecules to the left side of oxidation half reaction.
Mn3+ (aq) + 2H2O(l) → MnO2 (s)
To balance H-atoms, add 4H+ ions to the right side.
Mn3+ (aq) + 2H2O(l) → MnO2 (s) + 4H+(aq)
Step 4: Balance the charges on both sides
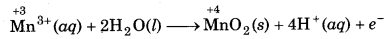
Step 5: Add above half reactions after cancelling the
electrons, we get,
2Mn3+ (aq) + 2H2O(l) → Mn2+ (aq) + MnO2 (s) + 4H+(aq) ́
This representes the balanced redox reaction (dispport- ionation reaction).

Question 8.22.
Consider the elements:
Cs, Ne, I and F
(a) Identify the element that exhibits only negative oxidation state.
(b) Identify the element that exhibits only positive oxidation state.
(c) Identify the element that exhibits only positive and negative oxidation states. (d) Identify the element which exhibits neither the negative nor the positive oxidation state.
Answer:
(a) F exhibits only negative oxidation state as it is most electronegative element i. e., -1.
(b) Cs exhibits only positive oxidation state as it is most electropositive element i. e., +1.
(c) I exhibits both positive and negative oxidation states due to presence of vacant d-orbital i. e.,-1, +1, +3, +5 and +7.
(d) Ne exhibits neither the negative nor the positive oxidation state as it is an inert gas. The oxidation state of Ne is zero.
Question 8.23.
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
Answer:
A balanced equation for this redox change can be written in following steps:
Step 1: Write skeletal ionic equation and assign the oxidation number to each species.
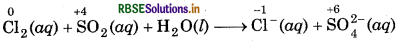
Step 2: Write two half reactions,
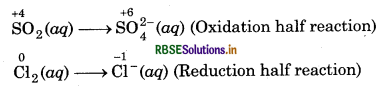
Step 3: To balance O-atoms in oxidation half reaction, add two H2O molecules to the left side.
SO2 (aq) + 2H2O(l) → SO2-(aq)
To balance H-atoms, add 4H+ ions to the right side SO2 (aq) + 2H2O(l) SO2-(aq) + 4H+(aq),
To balance Cl-atoms in reduction half reaction, multiply Cl-(aq) by 2
Cl2 (aq) → 2Cl-(aq)
Step 4: Balance the charges on both sides,
+4
SO2 (aq) + 2H2O(l) → SO2-(aq) + 4H+(aq) + 2e-
Cl2 (aq) + 2e- → 2Cl-(aq)
Step 5: Add above half-reactions after cancelling the electrons, we get
Cl2 (aq) + SO2 (aq) + 2H2O(l) → 2Cl-(aq) + SO24-(aq) + 4H+(aq)
This represents the balanced redox reaction.
Question 8.24.
Refer to the periodic table given in you book and now answer the following questions:
(a) Select the possible non metals that can show disproportionation reaction.
(b) Select three metals that can show disproportionation reaction.
Answer:
(a) Phosphorus, sulphur and chlorine can show disproportionation reactions in the alkaline medium.
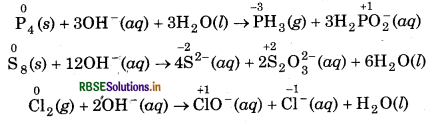
(b) Copper, manganese and gallium can show disproportionation reactions.
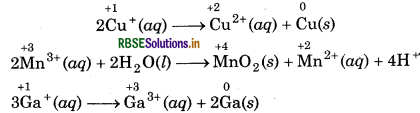
Question 8.25.
In Ostwald's process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g of ammonia and 20.00 g of oxygen?
Answer:
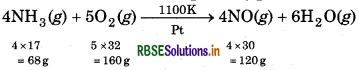
68g NH3 reacts with = 160 g O2
1g NH3 reacts with = \(\frac{160 \times 1}{68} \mathrm{~g} \mathrm{O}_2\)
∴10g NH3 reacts with = \(\frac{160 \times 10}{68}=23.5 \mathrm{~g} \mathrm{O}_2\)
But available amount of O2 is 20.0 g which is less than the amount which is required to react with 10 g NH3. So, O2 is the limiting reagent and it limits the amount of NO produced. From the above balanced equation,
∵ 160 g of O2 produces 120 g NO
1g of O2 produces \(\frac{120 \times 1}{160} \mathrm{~g} \text { NO }\)
∵20 g of O2 will produce \(\frac{120 \times 1 \times 20}{160}=15 \mathrm{~g} \mathrm{NO}\)
= 15 g NO
Question 8.26.
Using the standard electrode potentials given below predict if the reaction between the following is feasible:
(a) Fe3+ (aq) and I-(aq)
(b) Ag+(aq) and Cu(s)
(c) Fe3+(aq) and Cu(s)
(d) Ag(s) and Fe3+(aq)
(e) Br2(aq) and Fe2+ (aq).
Given, E° I2/I = + 0.54 V
E°Fe3+/Fe2+ = + 0.77 V
E° Cu2+/Cu = + 0.34 V
E° Ag+/Ag = + 0.80 V
E° Br/Br3+ = + 1.09 V
Answer:
(a) Fe3+(aq) and I ̄(aq)
Hence the reaction is given as
Fe3+ (aq) + 2I-(aq) → Fe2+ (aq) + I2 (s)
E° Fe3+/Fe2+ = + 0.77 V
E°I2/I- = + 0.54 V
E° cell = E°cathode - E°anode
= E°Fe3+/Fe2+
= 0.77 - 0.54
= + 0.23 V
∵ E° has +ve value, so, this reaction is feasible.
(b) Ag+(aq) and Cu(s)
The reaction is given as :
Cu(s) + Ag+(aq) → Cu2+ (aq) + Ag(s)
E° Ag+/Ag = + 0.80 V
E° Cu2+/Cu = + 0.34 V
E° cell = E°cathode - E°anode
= E°Ag+/Ag - E°Cu2+/Cu
= 0.80 - 0.34
= 0.46 V
∵E° cell has +ve value, so this reaction is feasible.
(c) Fe3+(aq) and Cu(s)
The reaction is given as :
Cu(s) + Fe 3+ (aq) → Cu 2+ (aq) + Fe2+(aq)
E° Fe3+/Fe2+ = 0.77 V
E° Cu2+/Cu = + 0.34 V
E°cell = E° cathode - E° anode
= E° Fe3+/Fe2+ - Cu2+/Cu
= 0.77 - 0.34 = + 0.43 V
The +ve value, so, this reaction is feasible:
[d) Ag(s) and Fe3+ (aq)
The reaction between Ag(s) and Fe3+ (aq) is given as :
Ag(s) + Fe3+ (aq) → Ag+(aq) + Fe2+(aq)
E°Ag+/Ag = + 0.80 V
E° Fe3+/Fe2+ = + 0.77 V
E°cell = E°cathode - E°anode
= E°Fe3+/Fe2+ - E°Ag/Ag
= 077 - 0.80
= - 0.03 V
The -ve value of E° cell indicates that this reaction is not
(e) Br2(aq) and Fe2+ (aq).
The reaction is given as :
Br2(aq) + Fe2+(aq) → 2Br-(aq) + Fe3+ (aq)
E°Br2/Br = + 1.09 V
E° Fe3+/Fe2+ = + 0.77 V
E° cell = E° cathode - anode
E°Br2/Br- - Fe3+/Fe2+
= 1.09 - 0.77
= + 0.32 V
The +ve value of Eo feasible.

Question 8.27.
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
(iii) A dilute solution of H2SO4 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Answer:
(i) Electrolysis of an aqueous solution of AgNO3 with silver electrodes is given as below:
At cathode : Ag+ + e- → Ag
At anode : Ag → Ag+ + e-
(ii) Electrolysis of an aqueous solution of AgNO3 with platinum electrodes is given as below:
At cathode : Ag+ + e- → Ag
At anode : OH → OH + e-
4OH → 2H2O + O2
So, oxygen gas will be liberated at anode.
(iii) Electrolysis of a dilute solution of H2SO4 with platinum electrodes is given below:
H2SO4 → 2H+(aq) + SO2-(aq)
H2 → OH + OH-
At cathode : 2H + + 2e
∴ Hydrogen gas is liberated at cathode.
At anode : OH- → OH + e-
4OH → 2H2O + O2
∴ Oxygen gas is liberated at anode.
(iv) Electrolysis of an aqueous solution of CuCl2 with platinum electrodes is given as below:
CuCl2 → Cu2+ + 2Cl-
H2O → H + OH ̄
At cathode: Cu2+ + 2e- → Cu
∴ Capper is deposited at cathode.
At anode : 2Cl- → Cl2 + 2e-
∴ Chlorine will be discharged at anode due to over voltage.
Question 8.28.
Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Mn
Answer:
It can be explained by the relative position of these metals in the electrochmical series. The metal placed lower in the series can displace the metals having ahigher position in the series present as its salt. So, the order of metals in which they displace each other from the solution of their salts is:
Question 8.29.
Mg > Al > Zn > Fe > Cu > Ag
Given the standard electrode potentials:
K+/K = 2.93 V
Ag/Ag = 0.80 V
Hg2+/Hg = 0.79 V
Mg2+/Mg = -2.37 V
Cr3+/Cr = 0.74 V
Arrange these metals in their increasing order of reducing power.
Answer:
Since we know that, lower the standard reduction potential of metal, more easily the metal is oxidised and so greater is the reducing power of metal.
Hence, increasing order of reducing power of metals is given below:
AgHg < Cr < Mg < K
Question 8.30.
Depict the galvanic cell in which the reaction.
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place, further show :
(i) which of the electrode is negatively charged?
(ii) the carriers of the current in the cell, and
(iii) individual reaction of each electrode.
Answer:
The reaction is given as below:
Zn(s) + 2 Ag+(aq) → Zn2+ (aq) + 2Ag(s)
Cell representation is:
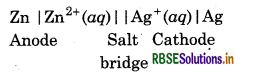
(i) In this reaction Zn acts as anode so it is negatively charged.
(ii) Loss of electrons takes place at anode i.e.,
Zn → Zn2+ + 2e-, electrons flow from Zn electrode (anode) to

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter
- RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure