RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
RBSE Class 11 Chemistry Hydrocarbons InText Questions and Answers
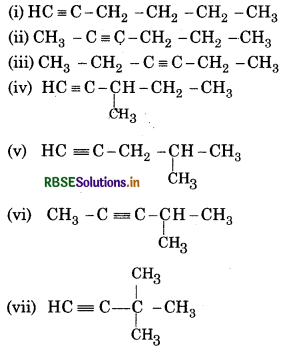
Question 13.1.
Write structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also write their IUPAC names.
Answer:
Molecular formula = C6H14
Chain isomers
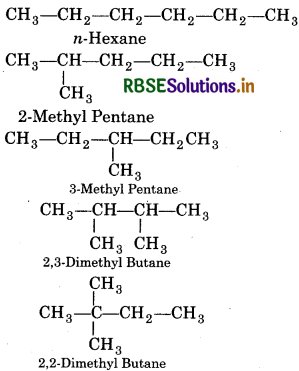
Question 13.2.
Write structures of different isomeric alk C5H11. Write IUPAC names of alcohols ol carbons of the chain.
Answer:
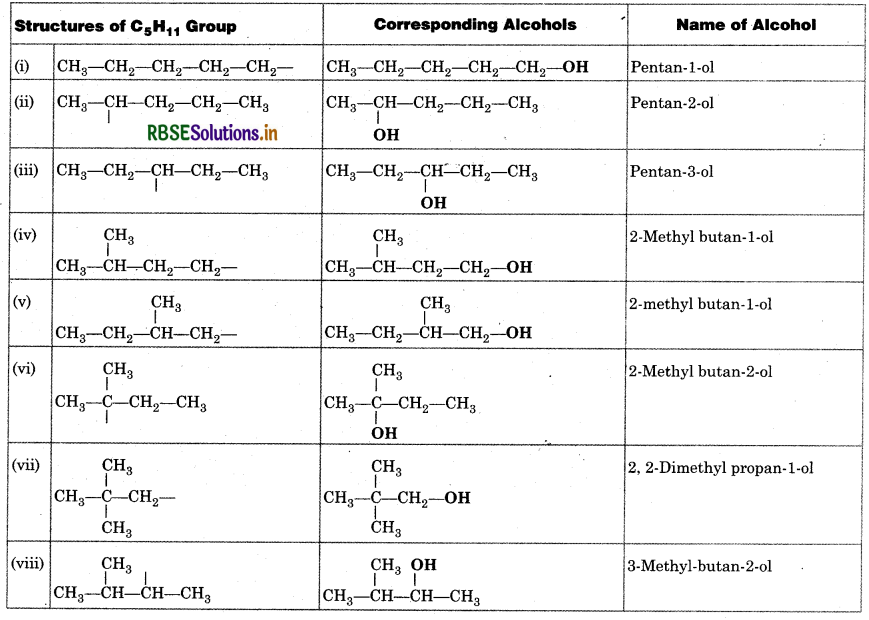

Question 13.3.
Write IUPAC names of the following compounds:
(i) (CH3)3 CCH2C(CH3)3
(ii) (CH3)2C(C2H5)2
(iii) Tetra-tert-butyl methane
Answer:
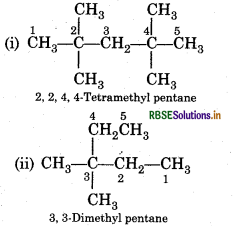
Question 13.4.
Write structural formulae of the following compounds :
(i) 3, 4, 4, 5-Tetramethyl heptane
(ii) 2, 5-Dimethyl hexane
Answer:
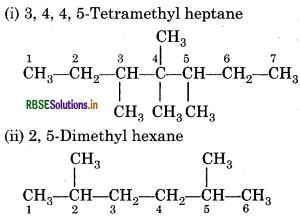
Question 13.5.
Write structures for each of the following compounds. Why are the given names incorrect? Write correct IUPAC names.
(i) 2-Ethyl pentane
(ii) 5-Ethyl-3-methyl heptane
Answer:
(i) 2-Ethyl pentane
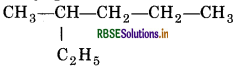
The IUPAC name of the compound given is incorrect, because the longest chain contains six carbon atoms and not five. So, the correct name is 3-Methyl hexane.
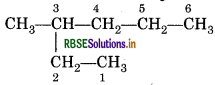
(ii) 5-Ethyl-3-methyl heptane
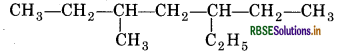
The IUPAC name of the compound given is incorrect, because, numbering is to be started from the end which gives lower number to ethyl group. So, the correct name is 3-Ethyl-5-methyl heptane.
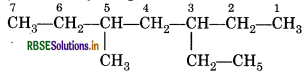
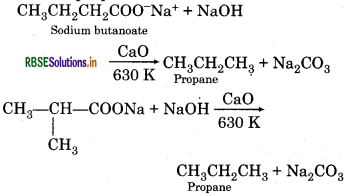
Question 13.6.
Sodium salt of which acid will be needed for the preparation of propane ? Write chemical equation for the reaction.
Answer:
Sodium salt of butanoic acid is required for the preparation of propane.

Question 13.7.
Write IUPAC names of the following compounds:
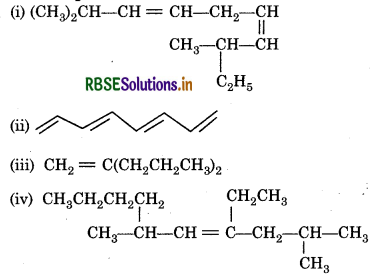
Answer:
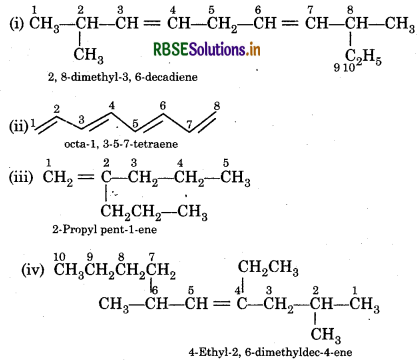
Question 13.8.
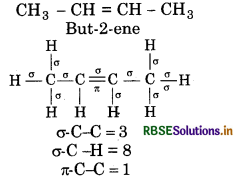
Calculate number of sigma (σ) and pi (i) bonds in the above structures (i-iv).
Answer:
(i) σ-bonds = 33
(ii) o-bonds = 17
(iii) σ-bonds = 23
(iv) o-bonds = 41
π-bonds = 2
π-bonds = 4
π-bond = 1
π-bond = 1
Question 13.9.
Write structures and IUPAC names of different structural isomers of alkenes corresponding to C5H10.
Answer:
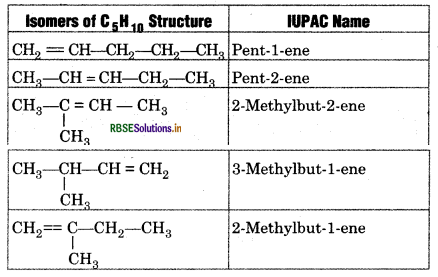
Question 13.10.
Draw cis and trans isomers of the following compounds. Also write their IUPAC names.
(i) CHCI = CHCI
(ii) C2H5 CCH2 = CCH3 C2H5
Answer:
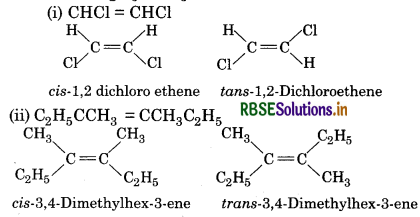
Question 13.11.
Which of the following compounds will show cis-trans isomerism?
(i) (CH3)2 C = CH-C2H5
(ii) CH2 = CBr2
(iii) C6H5 CH = CH CH3
(iv) CH3-CH=CCICH2
Answer:
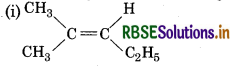
It will not not show cis-trans isomerism due to presence of two identical groups attached to one of the doubly bonded carbon atom.
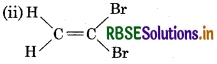
It will not show cis-trans isomerism due to presence of two identical groups attached to one of the doubly bonded carbon atom.
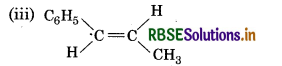
It will show cis-trans isomerism.
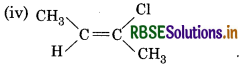
It will show cis-trans isomerism.

Question 13.12.
Write IUPAC names of the products obtained by addition reactins of HBr to hex-1-ene :
(i) in the absence of peroxide and
(ii) in the presence of peroxide
Answer:
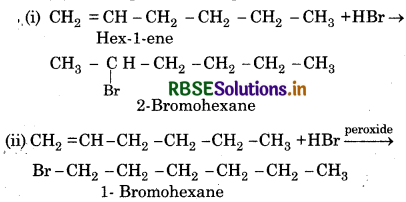
Question 13.13.
Write structures of differnt isomers corresponding to the 5th member of alkyne series. Also write IUPAC names of all the isomers. What type of isomersim is exhibited by different pairs of isomers?
Answer:
|
Structure |
IUPAC Name |
|
|
Hex-1-yne |
Position isomers : (i) and (ii), (i) and (iii), (ii) and (iii), (v) and (vi).
Chain isomers : (i) and (iv), (i) and (v), (i) and (vi), (i) and (vii), (ii) and (iv), (ii) and (v), (ii) and (vi), (ii) and (vii), (iii) and (iv), (iii) and (v), (iii) and (vi), (iii) and (vii), (iv) and (v) and (vii).
Question 13.14.
How will you convert ethanoic acid into benzene?
Answer:
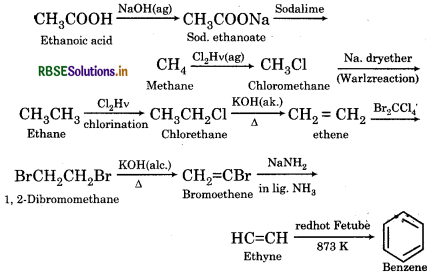
RBSE Class 11 Chemistry Hydrocarbons Textbook Questions and Answers
Question 13.1.
How do you account for the formation of ethane during chlorination of methane ?
Answer:
Chlorination of methane takes place through free radical mechanism. It involves following three steps: Initiation Step:
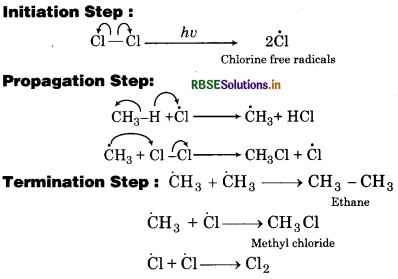
So, in third step, ethane is formed during chlorination of methane.
Question 13.2.
Write IUPAC names of the following compounds :
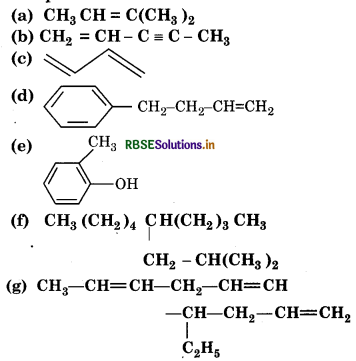
Answer:
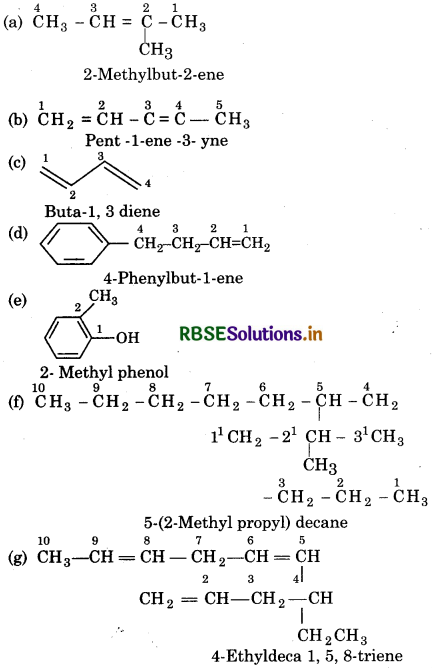

Question 13.3.
For following compound, write structure formulae and IUPAC names for all possible isomers having the number of double or triple bonds as indicated:
(a) C4Hg (one double bond)
(b) C5Hg (one triple bond)
Answer:
(a) Isomers of C4 Hg having one double bond
IUPAC Name
Structure
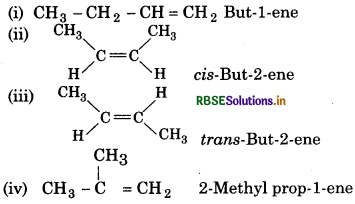
(b) Isomers of C5H8 having one triple bond
Structure
(i) CH3CH2CH2C = CH
(ii) CH3-CH2-C=C-CH3
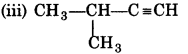
IUPAC Name:
Pent-1-yne
Pent-2-yne
3-Methylbut-1- yne
Question 13.4.
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) Pent-2-ene
(ii) 3,4-Dimethylhept-3-ene
(iii) 2-Ethylbut-1-ene
(iv) 1- Phenylbut-1-ene
Answer:
(i) Ozonolysis of Pent-2-ene
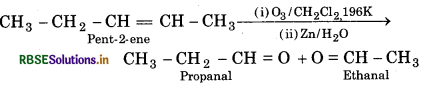
(ii) Ozonolysis of 3, 4- Dimetnyl hept-3-ene
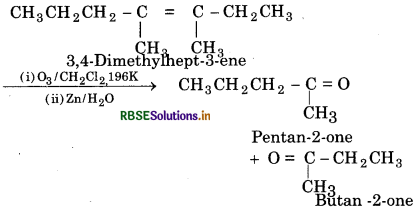
(iii) Ozonolysis of 2- Ethyl but-1-ene
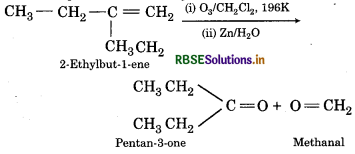
(iv) Ozonolysis of 1-Phenylbut-1-ene
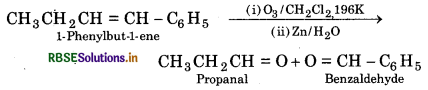
Question 13.5.
An alkene ‘A' on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of 'A'.
Answer:
Structure of Products :
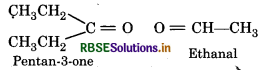
Oxygen atoms in above products is removed and join the two ends by a double bond, So, the structure of alkene 'A' is.
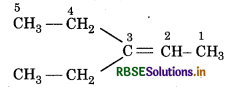
IUPAC name : 3-Ethylpent-2-ene
Question 13.6.
An alkene ‘A' contains three C-C, eight C -H, o-bonds and one C-C T-bond. 'A' on ozonolysis give two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Answer:
Aldehyde having molar mass 44 u = Ethanal (CH3CHO)
Two moles of aldehydes i.e.,
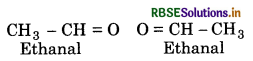
Remove the O-atoms in above aldehydes and join them by a double bond, the stucture of alkene ‘A' is

Question 13.7.
Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Answer:
Products of ozonolysis of alkene
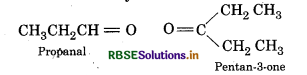
Remove the O-atoms in above products and join them by a double bond, the stucture of alkene is
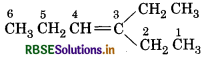
IUPAC name: 3-Ethyl hex-3-ene
Question 13.8.
Write chemical equations for combustion reaction of the following hydrocarbons:
(i) Butane
(iii) Hexyne
(ii) Pentene
(iv) Toluene
Answer:
(i) Combustion of Butane

(ii) Combustion of Pentene

(iii) Combustion of Hexyne
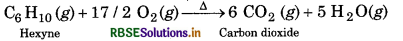
(iv) Combustion of Toluene
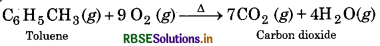
Question 13.9.
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Answer:
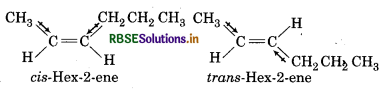
Cis-isomer posseses higher dipole moment than trans isomer so, cis-isomer will have higher boiling point than trans-isomer.
Question 13.10.
Why is benzene extraordinarily stable though it contains three double bonds?
Answer:
It contains three double bonds. Its structure can also be shown as below:
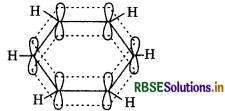
It has six electrons of the p-orbitals which cover all the six carbon atoms and are said to be delocalized and show resonating structures. As a result of delocalization, it is more stable molecule.

Question 13.11.
What are the necessary condition for any system to be aromatic?
Answer:
The necessary condition for any system to be aromatic are:
- Planaritym,
- Complete delocalization of the π-electron in the ring,
- Presence of (4n+2) π electrons in the ring where n is an integer (n = 0,1,2,...,) i.e. it follows Huckel's rule.
Question 13.12.
Explain why the following system are not aromatic?
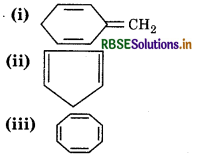
Answer:
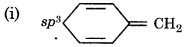
It has sp3 hybridized carbon atom, so it is not planar.
It does not contain 6 π-еlectrons in a cycle, so, it does not follow (4n+2) л electron rule.
Hence, it is not an aromatic compound.
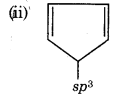
It has sp3 hybridized carbon atom, so it is not planar,
It contains 4л electrons, so it does not follow (4n+2) л electrons rule.
Hence, it is not an aromatic compound.
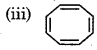
It has 8πelectrons, so it does not follow (4n + 2) леlectron ule.
Hence, it is not an aromatic compound.
Question 3.13.
How will you convert benzene into:
(i) p-nitrobromobenzene
(ii) m - nitrochlorobenzene
(iii) p-nitrotoluene
(iv) acetophenone?
Answer:
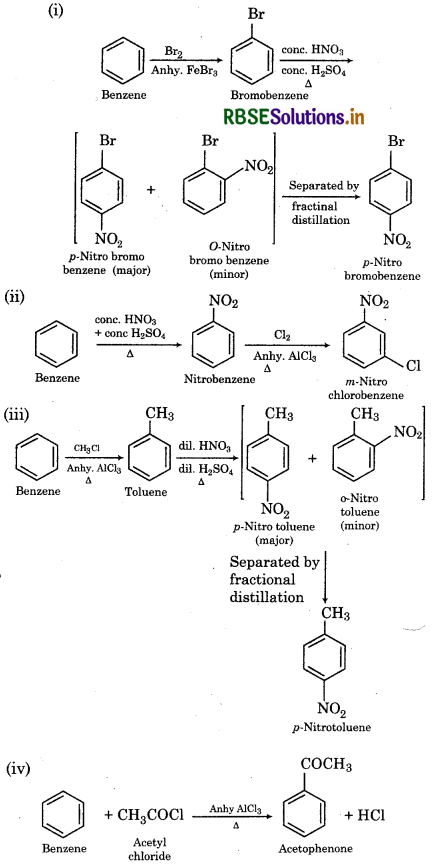
Question 13.14.
In the alkane HC-CH2 – C(CH3)2 -CH2-CH-(CH3)2, identify 1o, 2o, 3o carbo atoms and give the number of H aton bonded to each one of these.
Answer:
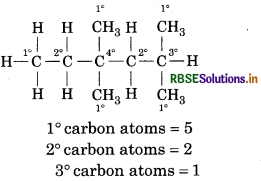

Question 13.15.
What effect does branching of an alka chain has on its boiling point?
Answer:
As branching of an alkane chain increases, boili point of the alkane decreases.
Question 13.16.
Addition of HBr to propene yield 2-bromopropane, while in the presence benzoyl peroxide the same reaction yiel 1-bromopropane. Explain and gi mechanism.
Answer:
Addition of HBr to propene is an ionic electrophil addition reaction in which the electrophile (H+) first a to give a move stable 2° carbocation. In the second st the carbocation is rapidly attacked by the nucleophi (Br) to give 2-bromopropane.
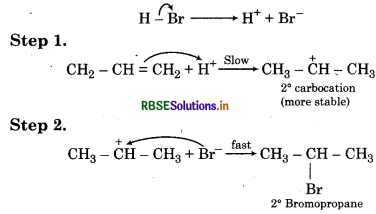
In presence of benzoyl peroyl peroxide, the reaction still electrophilic but the electrophile here is a Br° fr radical which is obtained by the action of HBr on benzo peroxide.
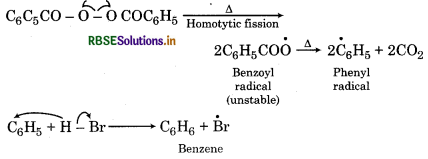
In the first step BrTM radical adds to propene in such a way so as to generate a more stable secondary (2°) free radical. In the second step the free radical thus obtained rapidly abstracts H-atom from HBr to form on 1-bromopropane.
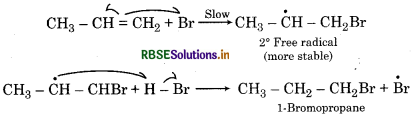
Question 13.17.
Write down the products of ozonolysis of 1,2 dimethyl benzene (o-xylene). How does the result support Kekule strucutre for benzene?
Answer:
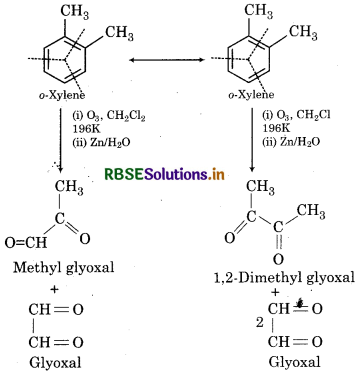
Hence, all the three products can not be obtained from any one of the two Kekule structures, this shows that o-xylene is a resonance hybrid of the Kekule structures (I and II).

Question 13.18.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Answer:
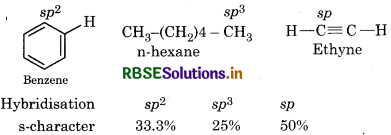
As the s-character of the orbital increases the acidic character increases.
In ehtyne carbon is sp-hybridised. it has highest percentage of s-character hence it is highly electronegative in nature. So it can attracts shared pair of electron towards istself easily so it can release H+, ion easily. Hence most acidic in nature. In Benzene, carbon atom is sp2 hybridised i.e. less electronegative in nature hence it will release H*ion. less easily while in n-hexane carbon atom is sp3 hybridised so it is least electronegative and cannot release H+ ion easily. Hence the nature of n-hexane is least acidic in nature. Hence, decreasing order of acidic behaviore is Ethyne > Benzene > n-Hexane
Question 13.19.
Why does benzene undergo electrophilic A substitution reaction easily and r nucleophilic substitution with difficulty?
Answer:
Benzene possesses electron cloud of 6л electrons A above and below the plane of the ring. It is a rich source g of electrons. So, it attracts the electrophiles towards it c and repels nucleophiles. As a result benzene undergoes electrophic substitution reaction easily and nucleophilie substitution with difficulty.
Question 13.20.
How would you convert the following compounds into benzene?
(i) Ethyne (ii) Ethene (iii) Hexane
Answer:
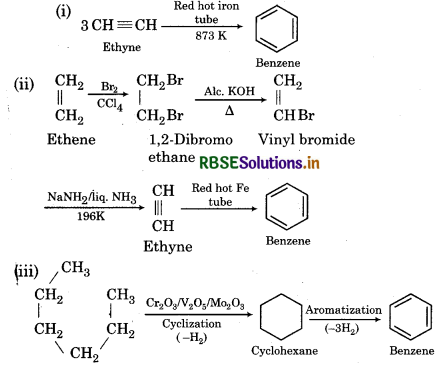
Question 13.21.
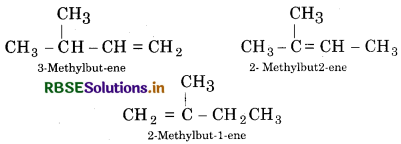
Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer:
The skeleton structure of of 2- methyl butane is
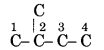
Show double bonds at different positions and satisfying al the tetracovalency of each carbon atom.

Question 13.22.
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile E+.
(a) Chlorobenzene, 2, 4-dinitro chloroben- zene, p-nitrochlorobenzene
(b) Toluene, p-H3 C - С6 H1 – NO2,
p-O2N - C6H4 NO2
Answer:
(a) Benzene shows electrophilic substitution eaction. Higher the electron density in the benzene ring more reactive is the compound towards these reactions. s we know that - NO2 shows more I effect then Cl, so reater the number of nitro groups less reactive is the ompound.
Hence, decreasing order of reactivity of compounds with n electrophile is:
Chlorobenzene >p-nitrochlorobenzene > 2,4 dinitrochlorobenzene
As we know that -CH3 group is electron releasing nd -NO2 group is electron withdrawing. So the aximum electron density is shown by toluene, followed y p-nitrotoluene then p-dinitro benzene, Hence ecreasing order of rectivity of compounds with an lecrophile is :
oluene > p-H3C-C6 H4 −NO2 > p-O2N −CH4 – NO2
Question 13.23.
Out of benzene, m-dintro benzene and toluene which will undergo nitration most easily and why?
Answer:
In toluene-CH3 group is present which is electron eleasing group while in m-dinitrobenzene, -NO2 roup is present which is electron withdrawing group. o, maximum electron density is present in toluene, llowed by benzene and least in m-dinitrobenzene. ence toluene will undergo nitration most easily as ompared to benzene and m -dinitrorbenzene.
Question 13.24.
Suggest the name of a lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer:
Anhydrous FeCl3
Question 13.25.
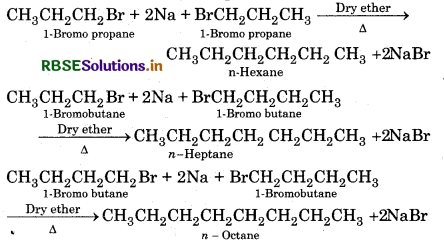
Why is Wurtz reaction not preferred for the preparation of alkanes contaning odd number of carbon atoms? Illustrate your answer by taking one example.
Answer:
A mixture of two alkyl halides is used to prepare Ikanes having odd number of carbon atoms. Since two kyl halides can react in three different ways, so a mixture of three alkanes instead of the desired alkar would be formed e.g. Wurtz reaction between bromopropane and 1-bromobutane gives a mixture three alkanes i.e. hexane, heptane and octane.

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter
- RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure