RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 11 The p-Block Elements
RBSE Class 11 Chemistry The p-Block Elements InText Questions and Answers
Question 11.1.
Standard electrode potential values, E° for Al3+/Al is - 1.66V and that of Tl3+/Tl is + 1.26V. Predict about the formation of M3 ion in solution and compare the electropositive character of the two metals.
Answer:
Given,
E°Al3+/Al = 1.66V
E°Tl3+/Tl = +L26V
Since the standard reduction potential of aluminium is lesser than thallium, so, it is clear that aluminium has high tendency to make Al3+(aq) ions, whereas TI3+ is not only unstable in solution but is a powerful oxidising agent. So, TI is more stable in solution than TI3+. Aluminium being able to form +3 ions easily, is more electropositive as compared to thallium.
Question 11.2.
White fumes appear around the bottle of anhydrous aluminium chloride. Give reason.
Answer:
White fumes appear around the bottle of anhydsous aluminium chloride because it is partially hydrolyred with atmospheric mositure to liberate hydrogen chloride gas. Moist HCl appears white in colour.

Question 11.3.
Boron ¡s unable to form BF+ ion. Example.
Answer:
Boron is unable to form BF+ ion due to non-availability of d-orbitais, so it can not expand its octet. The maximum covalence of boron can not exceed 4.
Question 11.4.
Why is boric acid considered as a weak acid?
Answer:
Boric acid is considered as a week acid because it can not release H+ ions on its own, It accepts OH- ions from water molecule to complete its octet and in turn releases H+ ions.
Question 11.5.
Select the member’s of group 14 that
1. forms the most acidic dioxide,
2. is commonly found in +2 oxidation state,
3. used as semiconductor.
Answer:
- Carbon forms the most acidic dioxide.
- Lead is commonly found in + 2 oxidation state.
- Silicon and Germanium are used as semiconductors,
Question 11.6.
[SiF6]2- Is known whereas [SiCl6]2- not. Give possible reasons.
Answer:
[SiF6]2- is known whereas [SiCl6]2- not because of the following reasons:
- Six large Cl’ ions can not be acœmmodated around Si4 due to limitation of its ize.
- Interaction between ions pair of Cl ion and Si4 is not very strong.
Question 11.7.
Diamond is covalent, yet It has hig melting point. Why?
Answer:
Diamond has a three dimensional network involving strong C-C bond, which are very difficult to break bond, in turn has high melting point.

Question 11.8.
What are silicones?
Answer:
Simple silicones consist of imm chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. These groups are hydrophobic in nature.
RBSE Class 11 Chemistry The p-Block Elements Textbook Questions and Answers
Question 11.1.
Discussa the pattern of variation in the oxidation states of:
(i) B to TI and
(ii) C to Pb.
Answer:
(i) General electronic configuration of Group 13 elements = ns2 np1. oxidation state = + 3
Except B and AI, other elements show + 1 and + 3 oxidation states. B and Al exhibit + 3 oxidation state. It is due to meet pair effect. The two s-electrons in outermost orbital do not participate in bonding as they are strongly attracted by the nucleus. The inert pair effect becomes more prominent on moving down this group. So, + 1 oxidation state of Ga is unstable and + I oxidations state of TI ta very stable. The stability of the + 3 oxidetion state gets decreased on moving down the group.
(ii) General electronic configuration of Groups 14
elements = ns2 np2
Most common oxidation state = 4 On moving down the group + 2 oxidation state becomes more stable and the hihgper oxidation state becomes less stable due to inert pair effect Si and C mostly exhibit + 4 oxidetion state. Although. Ge, Pb and Sn show both + 2and + 4 oxidation state.
Question 11.2.
How can you explain higher stability of BCl3 as compared to TICl3?
Answer:
BCl3 is more stable as compared to TICl3 because + 3 oxidations stflte of thallium is highly oxidising and it reverts back to more stable + I oxidation state.
Question 11.3.
Why does boron trifluoride behave as a lewis acid?
Answer:
5B = 2, 3 = 1s2, 2s2 2p1
Boron contains three valence electrons. So. it can form only 3 covalent bonds which meAnswer: that there are only 6 electrons around boron and its octet remains incomplete. Hence, BF8 remains electrons behaves and as a Lewies acid.

Question 11.4.
Consider the compounds, BCl and CCl4. How will they behave with water? Justify.
Answer:
Since, BCl3 is electron-deficient, so, it readily hydrolyses to form boric acid.
BCI3 + 3H2O → H3BO3 + 3HCl
CCl4 resista hydrolysis because it does not have any vacant orbital to accept electrons.
CCl4 + H2O → No reaction
Question 11.5.
Is boric acid a protic acid 7 Explain.
Answer:
Boric acid is not a protic acid bcaui it does not ionize in water to give a proton. But it acts Lewis acid because it accepts ectrons from hydroxide ion.
Question 11.6.
Explain what happens when boric acid heated?
Answer:
When boric acid is heated at 370K or above, it heating to yield boric oxide. Changes to metaboric acid which on further,
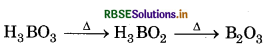
Question 11.7.
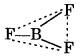
Describe the shapes of DF3 and BH4. Assign the hybrldisation of boron in these species.
Answer:
DF3: It is formed by the sp2 hybridation of boron orbitais. Its geometry is triangular planar.
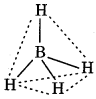
BH4: It is formed by the sp3 hybridisation of born orbitais. Its geometry is tetrahedral.
Question 11.8.
Write reactions to justii amphoteric nature of aluminium.
Answer:
Aluminium dissolves in mineral acids and aqueous alkalies and therefore its shows amphoteric nature.
(i) Ahiminium dissolves in dilute acid and liberates dihydrogen. which shows its basic nature.
2Al + 6HCl(aq) → 2Al3+ + 6Cl2-(aq) + 3H2(g)
(ii) Aluminium reacts with aqueous alkali and liberates dihydrogen, which shows its acidic nature.
2Al(s) + 2NaOl(aq) + 6H2O(l) → 2Na[Al(OH)4]-(aq) + 3H2
Question 11.9.
What are electron deficient compounds? Are BCl3 and SICl4 electron deficient species? Explain.
Answer:
Electron deficient compounds are those compounds in which there are only six electrons in the valency shell of central atom after the formation of the molecule. Electron deficient bonds are ‘often described as 3-center-2-electron boflds, BCl3 is electron deficient compound because boron has only 6 valence electrons and is short of 2 electrons, Therefore, it can accept a pair of electrons from nucleophiles. SiCI4 is not electron deficient compound because silicon has 8 valence electrons.

Question 11.10.
Write the resonance structure of CO32- and HCO3-.
Answer:
Resonance Structur.s of CO32-
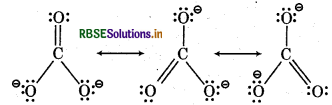
Resonanc Structures of HCO3-
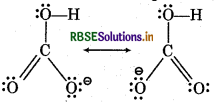
Question 11.11.
What is the state of hybridisation of carbon (a) CO (b) diamond (e) graphite?
Answer:
(a) CO: sp2 hybridisation
(b) Diamond: sp3 hybridisation
(C) Graphite: sp2 hybridisation
Question 11.12.
Explain the difference in properties of diamond and graphite on the basis of their structure.
Answer:
Differences between Diamond and Graphite
|
Property |
Diamond |
Graphite |
|
1. It has a layered structure |
It has a layered structure |
It has a layered structure |
|
2. Each carbon atom is sp2 hybridised and is bonded three other carbon atoms through a bond. |
Each carbon atom is sp2 hybridised and is bonded three other carbon atoms through a bond. |
Each carbon atom is sp2 hybridised and is bonded three other carbon atoms through a bond. |
|
3. The 4th electron forms a it -bond. |
The 4th electron forms a it -bond. |
The 4th electron forms a it -bond. |
|
4. It has a planar geometry |
It has a planar geometry |
It has a planar geometry |
|
5. 141.5 pm |
141.5 pm |
141.5 pm |
|
6. It is quite soft and its layers can be separated earily. |
It is quite soft and its layers can be separated earily. |
It is quite soft and its layers can be separated earily. |
Question 11.13.
Rationalise the given statements and given chemical reactions:
(i) Lead (II) chloride reacts with Cl2 to give PbCl4.
(ii) Lead (IV) chloride is highly unstable towards heat.
(iii) Lead is known not to form an iodide, Pbl4.
Answer:
(i) Lead (II) chloride reacts with Cl2 to give PbCl4 because lead exists in +2 and +4 oxidation states.
PbCl2 + Cl2 → PbCl4
(ii) Lead tetrachioride (PbCl4) is highly unstable towards heat because the stability of +4 oxidation state decreaseei on moving down the group in the periodic table due to inert pair effect. So, +2 oxidation state of Pb is moro stable. It decomposes on heating to give lead dichionde and chlorine gas.
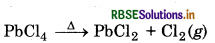
(iii) In PbI4, Pb (+4 oxidations state) is oxidising in nature and Jis reducing in nature. A combination of Pb
(iv) and iodide ion is unstable. Pb (TV) oxidises I- to I2 and itself gets reduced to Pb (II)
PbI4 → PbI2 + I2
So. Lead is known not to form an iodide, PbI4.

Question 11.14.
Suggest reasons why the B-F bond lengths in BF3 (130 pm)and BF4- (143pm)differ.
Answer:
BF3 is an electron-deficient species. Due to presence of vacant p-orbital on boron, the fluorine and boron atoms undergo pπ - pπ back bonding to remove thus deficiency. This provides a double bond character to the B.- F bond. This double bond character shortens the bond length in BF3 i.e. 130 pm However, in BF-4, BF3 is coordinated to F- ion, which changes its hybridisation from sp2 (in BF3) to sp3 (in BF-4). In this species, B forms 4 σ bonds end the double bond character is bai This accounts for a B - F bond length of 143 pm in BF4- ions.
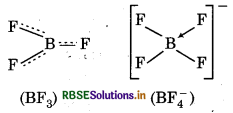
Question 11.15.
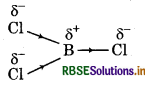
If B-Cl bond has a dipole moment, explain why BCl3 molecule has a zero dipole moment.
Answer:
B - Cl bond is polar in nature due to difference in the electxqnativities of B and Cl. However, BCl3 molecule is qon-polar. This is due to the fact that it has trigonal planar geometry and it is a symmetrical molecule. S, the respective dipole moments of B - Cl bonds cancel each other, So. BCl3 molecule has a zero dipole flioment.
Question 11.16.
AlumInium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF, Aluminum trifluoride precipitates Out olthe resulting solution when gaseous BF3 is bubbled through. Give reasons.
Answer:
HF pooiesaee a very strong inter-molecular hydrogen bonding. So, it does not give ions and AIF3 does not dissolve in it. Whole NaF is an ionic compound
and when it is added to the mixture, AIF3 dissolves. It is due to presence of free F- ions.
AIF3 + 3NaF → Na[AIF6]
When BF3 is added to the solution, AIF3 gets precipitated out of the solution. It is due to the fact that the tendency of boron to from complexes is much more than that of aluminium. So, when HF3 is added to the solution, B replaces Al from the complex according to the following reaction.
Na3[AIF6] + 3BF3 → 3NaBF4 + AIF3

Question 11.17.
Suggest a reason as to why COis poisonous.
Answer:
Carbon monoxide (CO) is highly poisonous in nature. Air containing even less than 1% of carbon monoxide can be fatal, if breath it for about 10 to 15 minutes. It combines with Haemoglobin in the blood and form carboxy haemoglobin, which reduces the oxygen carrying capacity of blood. The oxy-haemoglobin carries oxygen to various body parts but when carbon monoxide is combined, it stops the normal circulation of oxygen and may cause death due to suffocation.
Question 11.18.
How is excessive content of CO responsible for global warming?
Answer;
Carbon dioxide has the property to absorb I.R. radiations, (heat) reflected from the earth surface, when sun radiations come to the earth surface. Higher the concentration of CO2 in the atmosphere, higher is the amount of heat trapped. This results in the increase in the average temperature of earth’s surface. It is called green house effect which causes global warming all around us.
Question 11.19.
Explain structures of dilithane and boric acid .
Answer:
Structure of Diborane:
Diborane (B2H6) has a three centre electron pair bond. Two types of hydrogen i.e., terminal hÿrogen and bridging hydrogen are present in. this structure.
Boron - Terminal hydrogen Two centre electron pair bond
Boron - Bridging hydrogen Three centre electron pair bond.
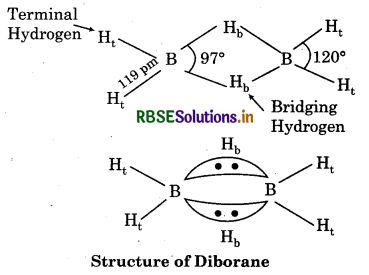
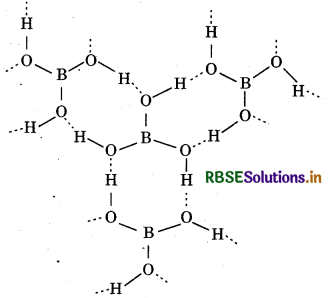
(ii) Structure of Boric acid:
Boric acid possesses a layered structure. Each planar BO3 unit is linked to one another through hydrogen atoms. The hydrogen atoms form a covalent bond with BO unit, whereas a hydrogen bond is formed with another BO3 unit.
Question 11.20.
What happens when:
(i) Borax is heated strongly.
(ii) Boric acid is added to water.
(iii) Aluminium is treated with dilute NaOH.
(iv) BF3 is reacted with ammonia?
Answer:
(i) When borax is heated strongly. It first loses water molecules and swells then it is converted into transparent liquid, solidifying to form a glass-like material called borax bead.
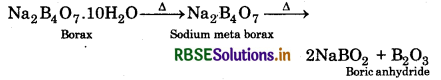
(ii) When boric and is added to water, it accepts electrons from OH- to from [B(OH)4]- ion and H3O+ ion.
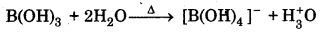
(iii) When aluminium is treated with dilute NaOH it forms sodium tetrahydrxo aluminate (Ill) with the liberation of hydrogen gas.
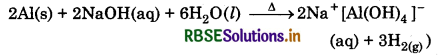
(iii) When BF3 is reacted with ammonis, then it accepts electron pair from ammonia to form an adduct.
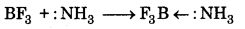
Question 11.21.
Explain the following reactions:
(i) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(ii) Silicon dioxide is treated with hydrogen fluoride.
(iii) CO s heated with ZnO.
(iv) Hydrated alumina is treated with aqueous NaOH solution.
Answer:
(i) When silicon is heated with methyl chloride at high temperature (537 K) in the presence of copper, it forms methyl substituted chiorosilene followed by hydrolysis and polymerisation.
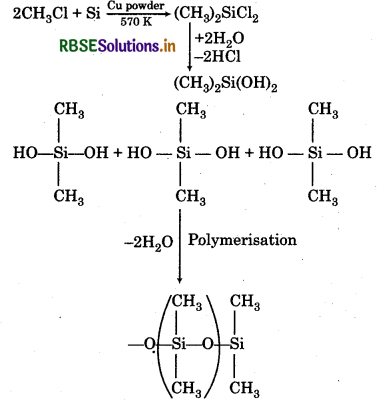
(ii) When silicon dioxide is treated with hydrogen fluoride (HF), it form silicon tetrafluoride.
SiO2 + 4HF → SiF4 + 2H2O
SiF4 formed in this reaction further reacts with HF to form hydro-fluorosilicic acid.
SiF4 + 2HF → H2SiF8
(iii) When CO is heated with ZnO, it reduces ZnO to Zn. In this reaction, CO arts as a reducing agent.
ZnO(s) + CO(g) → Zn(s) + CO2(g)
(iv) When hydrated alumina is treated with aqueous NaOH solution, the hydrated alumina dissolves in aqueous NaOH solution to form sodium metsaluminate.


Question 11.22.
Given reasons:
(i) Cone, HNO3 can be transported In aluminium container.
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain.
(iii) Graphite is used as lubricant.
(iv) Diamond is used as an abrasive.
(v) Aluminium alloys are used to make aircraft body.
(vi) Aluminium utensils should not be kept in water overnight.
(vii) Aluminium wire Is used to make transmission cables.
Answer:
(i) Conc. HNO3 can be transported in aluminium container because it reacts with aluminium to form a thin protective oxide layer on the aluminium surface. This layer renders aluminium passive.
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain because in this reaction, hydrogen gas is produced and the pressure of the gas is used to
2Al + 2NaOH + 6H2O → 2Na+[Al(OH)4]- + 3H2
(iii) Graphite possesses layered structure. The layers are held together by weak vander Waal’s forces. These layers can slide over each other due to which graphite is soft and slippery. Hence, graphite is used as lubricant.
(iv) Diamond possesses covalent bonds, which are present through out the surface, giving it a rigid three dimensional structure. It is very difficult to break extended covalent bonding so, diamond is the hardest substance. Hence, it is used as an abrasive.
(v) Aluminium alloys are used to make aircraft body because of high tensile strength and light weight of aluminium.
(vi) The oxygen present in water reacta with aluminium to form a thin layer of aluminium oxide. This Layer prevents aluminium from further reaction. However, when water is kept in an aluminium vessel for long period of time, some amount of aluminium oxide may. dissolve in water. As aluminium ions are harmful, water should not be stored in aluminium vessels overnight.
(vii) Silver, copper and aluminium are the best conductors f electricity. Silver is an expensive metal and silver wires are very expensive. Copper is quite expensive and is also very heavy. Aluminium is a very ductile metal. Thus, aluminium is used in making wires for electrical conduction.
Question 11.23.
Explain why there Is phenomenon decrease in ionization enthalpy from carbon to silicon.
Answer:
From carbon to silicon there is increase in atomic size and screening effect. These two effects overweigh the effect of increasing nuclear charge. Therefore, the outermost electron becomes less and 1cm tightly held by nucleus and hence ionization csergy decreases.
Question 11.24.
How would you explain the lower atomic radius of Ga as compared to AI?
Answer:
The atomic radius of Ga is less than atomic radius of Al, because in between Ga and Al. there are ten L elements of first transition series which have electrons L in inner d-orbital. The inner d-orbital do not shield the nucleus effectively due to their shape and poor penetration power. As a result, effective nuclear charge in Ga becomes more than in Al and atomic radius of Ga is L less than Al.
Question 11.25.
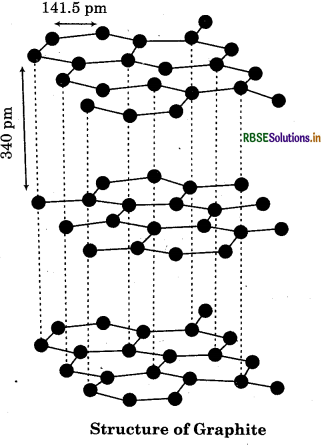
What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Answer:
Allotrop When an element is found in more than one form, having the same chemical properties but different physical properties, then such forms are called allotropes.
e. q. Allotropes of carbon - Diamond and Graphite
Structure of Diamond:
It has three dimensional structure. It has sp3 hybridised carbon. It is one of the hardest naturally-occurring substances. It is used as an abrasive and for cutting tools.
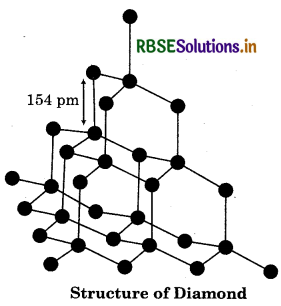
Structure of Graphite:
It has layered structure. The carbon atom is sp2 hybridised. The layers are held together by weak Vander Waal’s forces. These layers can slide over each other, making graphite soft and slippery. It is used as lubricant.
Question 11.26.
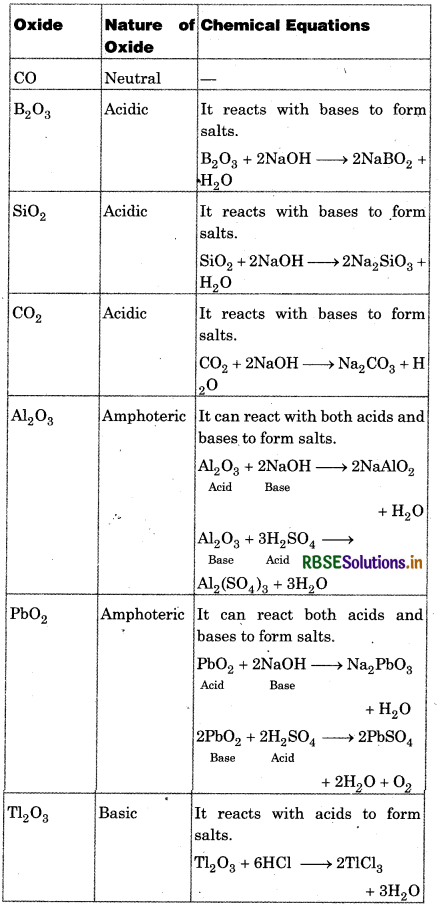
(i) Classify following oxides as neutral, acidic, basic or amphoteric:
CO, B2O3, SiO2, CO2, Al2O3, PbO2, TI2O3
(ii) Write suitable chemical equations to show their nature.
Answer:
Question 11.27.
In some of the reactions, thallium resembles aluminium, whereas in others it resembles with group I metals. Sipport this statement by giving some evidences.
Answer:
Thallium exists in Group 13. It shows +1 and +3 oxidation states. On moving down the group, the +1 state becomes more stable as compared to +3 state due to inert pair effect. The two electrons present in the s-sub shell (ns2 np1) are stronglý attracted by the nucleus and do not participate in bonding. Since, thallium shows both + 1 and +3 oxidation states. So, it resembles with Group I alkali metals (+ 1 oxidation state) and aluminium (+3oxidation state). It forms compounds like TICl3 etc. (like aluminium) and TICl etc. (like alkali metals).

Question 11.28.
When metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HCl to form compound (C). The compound (A) when heated strongly gives (D), which is used to extract metal. Identify (X), (A), (B), (C) and (D). Write suitable equations to support their identities.
Answer:
Since, metal (X) gives a white precipitate with NaOH, which is soluble in excess of NaOH. So, X must be aluminium (Al). The white precipitate (A) obtained is aluminium hydroxide. In excess of NaOH, it gives soluble complex (B) i.e. , sodium tetrahydroxy aluminate III.
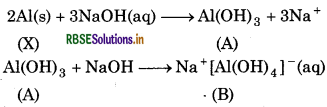
Aluminium hydroxide (A) is soluble in dilute HCl to form aluminium chloride (C).
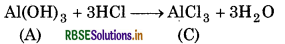
When aluminium hydroxide (A) is heated strongly, it gives alumina (D) which is used to extract aluminium
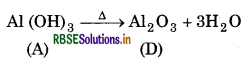
Question 11.29.
What do you understand by:
(i) inert pair effect?
(ii) allotropy?
(iii) catenation?
Answer:
(i) Inert Pair Effect: The reluctance of s- electrons in participating in chemical bonding. It is due to the poor shielding of d and f block electrons of inner shell which increases the effective nuclear charge. The term ‘inert pair’ was first proposed by N. Sidgwick in 1927. The name suggests that the s-electrons are more tightly bo.md to the nucleus and therefore more difficult to ionize. For example, the p.block elemento of the 4th, 5th and 6th period come after d.block elements but the electrons present in the intervening d. (and f) orbitais do not effectively shield the s-electrons of the valence shell. Due to this, the inert pair of ns electrons remains more tightly held by. the nucleus and therefore participates less in bonding.
(ii) Allotropy: Allotropy is the property of some chemical elemento to exist in two or more different forms, in the same physical state, known as allotropes of these elements. Allotropes are different structural modifications of an element, the atoms of the elements are bonded together in a different manner. For example, allotropes of carbon include diamond, graphite and fullerenes.
(iii) Catenation: It is the linkage of atoms of the same element into longer chains. Catenation occurs most readily in cerbon, which forms covalent bonds with other carbon atoms to form longer chains and structures. This property is due to strong C - C bonds.
Question 11.30.
A certain salt X, gives following results:
(I) Its aqueous solution is alkaline to litmus.
(II) It swells up to a glassy material Y on strong heating.
(III)When conc. H2SO4 la added to a hot solution of X, white crystals of an acid is formed.
Identify X. Y, Z and write the equations for the above reactions. V
Answer:
(i) Since the aqueous solution of sait X is alkaline to litmus, It must be the salt of strong base and a weak acid.
(ii) Since the salt X swells up to a glassy material Y on heating, therefore X must be borax and Y must be a mixture of sodium meta.borat.e and boric anhydride.
(iii) When conc. sulphuric acid is added to a hot solution of X (borax), white crystals of an acid Z separates out. Therefore, Z must be ortho-boric acid. The reactions Involved are:
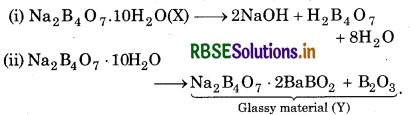
(iii) Na2B4O7 .10H2O + H2SO4 → 4H3BO3 + Na2SO4 + 5H2O
Question 11.31.
Write balanced equations for:
(i) BF3 + LiH →
(ii) B2H6 + H2O →
(iii) NaH + B2H6 →
(iv) H3BO3 →
(v) Al + NaOH →
(vi) B2H6 + NH3 →
Answer:
(i) 2BF3 + 6LiH → B2H6 + 6LiF
(ii) B2H6 + 6H2O → 2B(OH)3(aq) + 6H2(g)
(iii) 2NaH + B2H6 → 2NaBH4
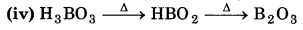
(v) 2Al + 2NaOH + 6H2O → 2Na+[Al(OH)4]-(aq) + 3H2(g)
(vi) 2B2H6 + 6NH3 → 3[BH2(NH3)2]+[BH]- → 2B3N3H6 + 12H2
Question 11.32.
Give one method for industrial preparation and one for laboratory preparation of CO and CO2 each.
Answer:
Industrial Preparation of CO CO2 is prepared commercially by passing steam over red hot coke.

Laboratory Preparation of CO: It is prepared by the dehydration of formic acid in the presence of conc. H2SO4 at 373 K.
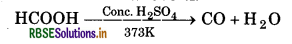
Industrial Preparation of CO2:
CO2 is prepared commercially by heating limestone.
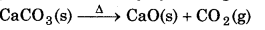
Laboratory Preparation of CO2:
It is prepared by treating dii. HCl with calcuium carbonate.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Question 11.33.
An aqueous solution of borax is:
(i) neutral
(ii) amphoteric
(iii) basic
(iv) acidic
Answer:
(iii) basic
Reason: In aqueous solution, borax gives strong base (NaOH) and weak acid (H3B03), so, it is basic in nature.

Question 11.34.
Boric acid is polymeric due to:
(a) its acidic nature.
(b) the presence of hydrogen bonds.
(c) its monobsic nature.
(d) its geometry.
Answer:
(b) the presence of hydrogen bonds
Question 11.35.
The type of hybridisation of boron in diborane is;
(i) sp
(ii) sp2
(iii) sp3
(iv) dsp2
Answer:
(iii) sp3
Question 11.36.
Thermodynamically the most stable form of carbon is:
(i) diamond
(ii) graphite
(iii) fullerene
(iv) coal
Answer:
(ii) graphite
Question 11.37.
Elements of group 14.
(i) exhibit oxidation state of +4 only.
(ii) exhibit oxidation state of +2 and +4
(iii) form M2 and M” ions.
(iv) form M2 and M4 ions.
Answer:
(ii) exhibit oxÍdation state of +2 and +4
Reason: It is due to the inert pair effect.
Question 11.38.
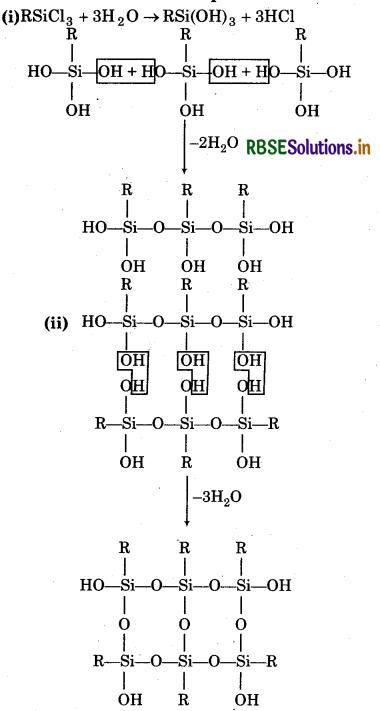
If the starting material for the manufacture of silicones is RSiCl3, write the structure of the product formed.
Answer:

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter
- RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure