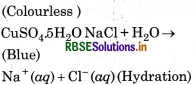RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 9 Hydrogen
RBSE Class 11 Chemistry Hydrogen InText Questions and Answers
Question 9.1
Comment on the reactions of dihydrogen with:
(i) Chlorine
(ii) Sodium and
(iii) Copper (II) oxide.
Answer:
(i) Dihydrogen reacts with chlorine to give hydrogen chloride.
H2(g) + Cl2 (g) → 2HCl(g)
In this reaction, dihydrogen reduces chlorine into Clion d and itself gets oxidised to H+ ion by chlorine.
(ii) Dihydrogen reacts with sodium at high temperature 1. to form sodium hydride.
H2(g) + 2 Na(g) → 2 NaH(s)
In this reaction, an electron is transfered from Na to H leading to the formation of an ionic compound Na+ H-.
(iii) Dihydrogen reacts with copper (II) oxide (CuO) to ee give copper and water..
H2 (g) + CuO(s) → Cu(s) + H2O(l)
In this reaction, dihydrogen reduces CuO to copper in zero oxidation state and itself gets oxidised to H2O.

Question 9.2.
Would you expect the hydrides of N, O and F to have lower boiling points than hydrides of their subsequent group members? Given reasons.
Answer:
On the basis of molecular masses of NH3, H2O and HF, their boiling points are expected to be lower than those of the subsequent group member hydrides. However, the magnitude of H-bonding in their hydrides will be quite appreciable to high electronegativities of N, O and F. Hence, the boiling points of NH3, H2O and HF will be higher than the hydrides of their subsequent group members.
Question 9.3.
Can phosphorus with outer electronic configuration 3s2, 3p3 form PH?
Answer:
Phosphorus with outer electronic configuration on not form PH5, although it exhibits +3 and +5 oxidation states. High A, H value of dihydrogen and AgH value of hydrogen do not favour to exhibit the highest oxidation state of P with hydrogen.
Question 9.4.
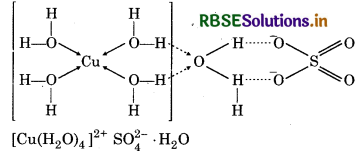
How many hydrogen bonded water molecule (s) are assoicated in CuSO4.5H2O?
Answer:
In CuSO4.5H2O. only one water molecule is Ma hydrogen bonded, which is outside the brackets i.e. coordination sphere. The other four molecules of water are coordinated.
Question 9.5.
Calculate the strength of 10 volume solution of hydrogen peroxide.
Answer:
Hydrogen peroxide decomposes on heating according to the equation:
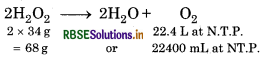
By definition, 10 volume soution of H2O2 means that 1 L of this will give 10 L of O2 at NTP.
From the equation, 22.4 L of O2 at NTP are obtained from 2 × 34 or 68 g of H2O2.
∴ 10 L of O2 at NTP will be obtained from
\(\mathrm{H}_2 \mathrm{O}_2=\frac{68}{22.4} \times 10\)
Therefore, strength of H2O2 in 10 volume
H2O2 = 30.36 g L2 = 3% HO2 solution
RBSE Class 11 Chemistry Hydrogen Textbook Questions and Answers
Question 9.1.
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
1H = 1s1
It hows resemblance with alkali metal of Group 1 as it sesses ns configuration. It can form H+ ion as alkali cals form cation. hows resemblance with halogen of group 17 as it has dency to gain one electron and acquire inert gas figuration. Its position is anomalous in Periodic Table.

Question 9.2.
Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes ?
Answer:
Isotopes of Hydrogen
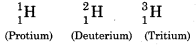
Mass Ratio
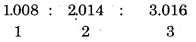
Question 9.3.
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer:
1H = 1s1
attain stablility, it acquires the electronic figuration of nearest noble gas (He = 1s2), hydrogen ms share their single electron with each other So, rogen exists in a diatomic form.
Question 9.4.
How can the production of dihydrogen, obtained from coal gasification, be increased?
Answer:
The production of dihydrogen takes place as follows:
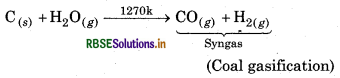
production of dihydrogen obtained from coal ification can be increased by reacting CO of syngas steam in the presence of iron chromate as catalyst.

Question 9.5.
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in this process?
Answer:
Electrolytic method for preparation of ydrogen: A small quantity (15% - 20%) of acid or alkali is added to water to make it good conductor electricity. Dihydrogen gas is released at the catho and oxygen is released at anode.
H2SO4 → 2 H+ + SO2 -
H2O → H+ + OH-
At cathode: 2 H+(aq) + 2e- → 2H
H + H → H2(g)
At anode : 4 OH ̄ (aq) → 4 OH + 4e ̄
4 OH → 2 H2O(l) + O2(g)
In this process, the role of an electrolyte is to conduct t solution.
Question 9.6.
Complete the following reactions.
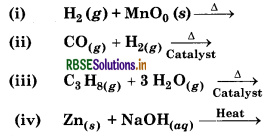
Answer:
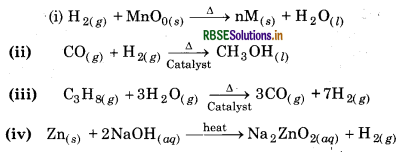

Question 9.7.
Discuss the consequences of high enthalp of H-H bond in terms of chemical reactivi of dihydrogen.
Answer:
Dihydrogen is quite stable due to high bo dissociation enthalpy of H-H bond (i.e. 435.88 kJ molTM So, the dissociation of dihydrogen into its atoms is on 0.081% at 2000K which increases to 95.5% at 500K. Dihydrogen is found to be chemically inert or stable room temperature due to high H-H pond enthalpy.
Question 9.8.
What do you understand by:
(i) electron-deficient
(ii) electron-preci and
(iii) electron-rich compounds hydrogen ? Provide justification wi suitable example.
Answer:
The term 'hydride' is given to compounds form by hydrogen with elements. These are categorised as
(i) Electron-deficient Hydrides: They have too fe electrons for writing their conventional Lew structure. e.g. Diborane (B2H6).
(ii) Electron-Precise Hydrides: They have t required number of electrons to write the of de conventional Lewis structure. e.g. Hydrides of group 14 elements such as CH4.
(iii) Electron-rich Hydrides: These hydrides have excess electrons which are present as lone pairs. e.g. hydrides of group 15-17 elements such as NH3 (1 lone pair), H2O (2 lone pairs). HF (3 lone pairs)
Question 9.9.
What characteristics do you expect from an electron deficient hydride with respect to its structure and chemical reaction?
Answer:
Electron-deficient hydride has too few electron to he form normal covalent bonds. To make up its deficiency, such hydrides generally exists as polymeric species. e.g. BH3 exists as B2H6 (Diborane). These hydrides act as electron acceptors i.e. Lewis acids and therefore they form complex with Lewis bases.
B2H6 + (CH3)3 N → 2BH3 + N(CH3)3

Question 9.10.
Do you expect the carbon hydrides of the type (CnH2n+2) to act as Lewis acid or base ? Justify your answer .
Answer:
Carbon hydrides of the type (CnH2n+2)
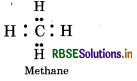
In such hydrides, carbon has neither extra electrons nor less electron. So these are electron precise hydrides. by They will act neither as Lewis acids or bases.
Question 9.11.
What do you understand by the term "non-stoichiometric hydrides" ? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer.
Answer:
Non-Stoichiometric Hydrides: These hydrides are formed when dihydrogen reacts d-block and f-block elements. However, the metals of group 7, 8 and 9 do not form hydrides. Due to small size of dihydrogen in these se hydrides, hydrogen occupies interstices in the metal of lattice producing distortion without change in its type. th Such hydrides are also called interstitial hydrides.
e.g. La H 2.87, Yb H 2.55; Ti H15-18 etc.
This types of the hydrides can not be formed by alkali = metals beacuse such hydrides (like NaH) ew stoichiometric.
Question 9.12.
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer:
The metallic hydrides are useful for hydrogen storage due to following reasons:
- The property of adsorption of hydrogen on transition (i metals is widely used in catalytic reduction or th hydrogenation reactions for the preparation of large number of compounds.
- Some metals like Pt, Pd can accomodate a very large volume of hydrogen.
Question 9.13.
How does atomic hydrogen or oxy hydrogen torches function for cutting and welding purposes? Explain.
Answer:
Atomic hydrogen is produced when molecular hydrogen is passed through an electric arc struck between tungsten electrodes at 3773 - 4273 K.
H2 → 2H; ∆H = 435.9 kJ/mol
The life time of atomic hydrogen is very small and hence it immediately gets converted into molecular form by b liberating a large amount of energy which may be used for cutting or welding purposes in the form of atomic hydrogen torch.
Question 9.14.
Among NH3, H2O and HF, which would you expect to have highest magnitude of A hydrogen bonding and why?
Answer:
Among NH3, H2O and HF, HF will have highest magnitude of hydrogen bonding beacuse F is most electronegative elements than N and O.
Question 9.15.
Saline hydrides are known to react with water violently producing fire. Can CO2, a well known fire extinguisher be used in this case? Explain.
Answer:
Saline hydride i.e. NaH reacts with water violently and liberates hydrogen gas. It is an exothermic reaction and the evolved H 2 gas catches fire.
NaH(s) + H2O(l) → NaOH(aq) + H2(g)
The fire so produced can not be distinguished by CO2 because it gets reduced by hot metal hydride
NaH + CO2 → HCOONa
Question 9.16.
Arrange the following:
(i) CaH2, BeH2 and TiH, in order of increasing electrical conductance.
(ii) LiH, NaH and CsH in order of increasing ionic character.
(iii) H-H, D-D and F-F in order of increasing bond dissociation enthalpy.
(iv) NaH, MgH2 and H2O in order of increasing reducing property.
Answer:
(i) Greater the ionic charactr of hydride, greater will be its electrical conductance.'
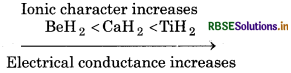
(ii) Greater the size of cation of hydride, greater will be ionic character of that hydride.
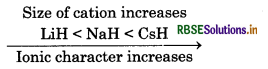
(iii) Lesser the bond length, greater will be the bond ssociation enthalpy of that bond.
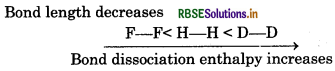
(iv) Metal hydrides are strong reducing agents than non- etal hydrides. Moreover, alkali metal hydrides have ore reducing property than alkaline metal hydrides ecause of small size of alkali metal.
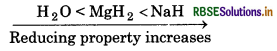

Question 17.
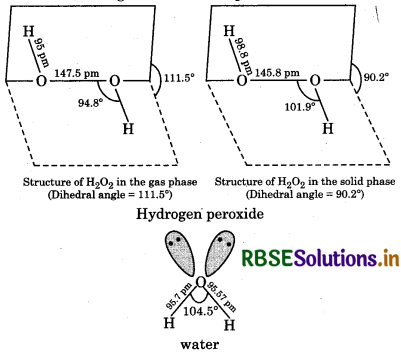
Compare the structure of H2O and H2O2.
Answer:
In water, oxygen is bonded to the two hydrogen coms by two O-H covalent bonds and there are two ne-pairs of electrons on the oxygen atom. Due to the resence of two lone-pairs of electrons on the O atom, the -O-H bond angle is 104.5°, which is slightly less an the tetrahedral angle of 109°28′. Therefore, the ructure of water molecule is an angular or bent structure.
hydrogen peroxide is non-linear, non-planar molecule. has an open book structure. In this, two 0-0 atoms re bonded to each other by a single covalent bond and ach O is further bonded to a hydrogen atom by a single valent bond. The O-H bonds are in different planes. he dihedral angle between two planes is 111.5°C
Question 9.18.
What do you understand by the tern 'auto-protolysis' of water? What is it significance ?
Answer:
The term auto-protolysis of water means it self-ionization, which takes place as follows:
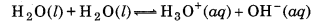
Its significance is that water is amphoteric in nature. behaves both as acid as well as base. For example :
Question 9.19.
Consider the reaction of water with F2 and suggest in terms of oxidation an reduction, which species are oxidised reduced.
Answer:
Water reacts with F2 to form hydrogen fluorid and oxygen
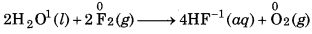
In this reaction, water acts as reducing agent and hence itself gets oxidised to O2. While, F2 acts as oxidisin agent and hence itself gets reduced to F.
Question 9.20.
Complete the following chemical reactions:
(i) PbS(s) + H2O2 (aq) →
(ii) MnO4- (aq) + H2O2 (aq) →
(iii) CaO (s) + H2O(g) →
(iv) AlCl3 (g) + H2O (l) →
(v) Cag N2 (s) + H2O(l) →
Classify the above into (a) hydrolysis (b) redox and (c) hydration reaction.
Answer
Chemical Reactions :
(i) PbS (s) + 4H2O(aq) → PbSO4 (s) + 4H2O(l) (Redox reaction)
(ii) 2MnO4 (aq) + 5H2O2 (aq) + 6H+(aq) → 2Mn2+ (aq) + 8H2O(l) + 5O2(g) (Redox reaction)
(iii) CaO(s) + H2O(aq) → Ca(OH)2(aq) (Hydrolysis reaction)
(iv) AlCl3 (aq) + 6H2O(7) → [Al(OH2)6] + 3 Cl-(aq)] (Hydration reaction)
(v) Ca3N2 (s) + 6H2O(l) → 3(Ca(OH)2(aq) + 2NH3(aq) (Hydrolysis reaction)

Question 9.21.
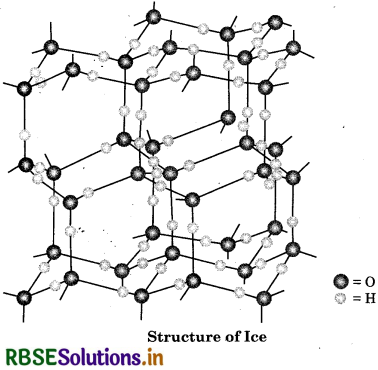
Describe the structure of the common form of ice.
Answer:
Ice is crystalline form of water. Ice has a highly s ordered three dimensional structure containing hydrogen bond. X-ray studies show that each oxygen atom is surrounded tetrahedrally by four other oxygen t atoms at a distance of 276 pm. One hydrogen atom lies in between each pair of oxygen atoms. So, each and every hydrogen atom is covalently bonded to an oxygen atom and linked to another oxygen atom by a hydrogen bond. This arrangement leads to a packing with large open spaces and results in lower density of ice than that of water. Hence, ice floats on water.
Question 9.22.
What causes the temporary permanent hardness of water?
Answer:
Temporary hardness is caused by the presence of bicarbonates of calcium and magnesium in water. Permanent hardness is caused by the presence of chlorides and sulphtes of calcium and magnesium in water.
Question 9.23.
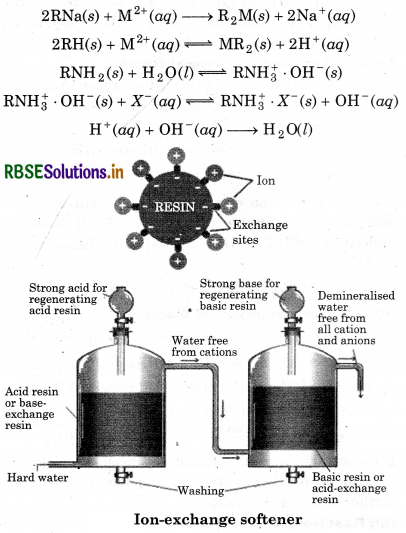
Discuss the principle and method of softening of hard water by synthetic ion-exchange resins.
Answer:
Synthetic lon-exchange Resin Method: Hard water is softened by using synthetic cation exchangers. O This method is more efficient than zeolite process. Cation exchange resins contain large organic molecule with -SO, H group and are water insoluble. Ion exchange resin (RSO3H) is changed to RNa by treating it with NaCl. The resin exchanges Na+ ions with Ca2+ and Mg2+ ions present in hard water to make the water soft.
Riesin anion. The resin can be regenerated by adding aqueous NaCl solution. Pure de-mineralised (de-ionized) water free from all soluble mineral salts is obtained by passing water successively through a cation exchange (in the H form) and an anion- exchange (in the OH form) resins. In this cation exchange process, H exchanges for Na+, Ca2+, Mg2+ and other cations present in water. This process results in proton release and thus makes the water acidic. In the anion exchange process, OH exchanges for anions like Cl, HCO, SO4 etc. present in water. OH-ions, thus, liberated neutralise the H+ ions set free in the cation exchange.
H+(aq) + OH(aq) → H2O(l)
The exhausted cation and anion exchange resin beds are regenerated by treatment with dilute acid and alkali solutions respectively.
Question 9.24.
Write chemical reactions to show the amphoteric nature of water.
Answer:
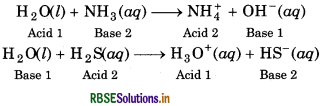
Water has the ability to act both as an acid as well as a base, so, it has amphoteric nature. Following chemical reactions show the amphoteric nature of water.
Hydrogen 81
H2O(l) + NH3(aq) → NH(aq) + OH(aq)
Acid Base
H2O(l) + H2S(aq)- → H3O+(aq) + HS-(aq)
Base Acid

Question 9.25.
Write chemical reaction to justify that hydrogen peroxide can function as an oxidising as well as reducing agent.
Answer:
Hydrogen peroxide (H2O2) acts both as oxidising agent and reducing agent in acidic medium.
Oxidising Nature
In acidic medium
PbS(s) + 4H2O2(aq) → PbSO4 (s) + 4 H2O(l)
In basic medium
MnSO4 + H2O2 + 2 NaOH → MnO2 + Na2SO4 + 2H2O
Reducing Nature
In acidic medium
K2Cr2O7 (g) + 4 H2SO4 (aq) + 3 H2O2(aq) → K2SO4 + Cr2(SO4)3 + 7H2O + 3O2
In basic medium
I2 + H2O + 2OH- → 2I- + 2H2O + O2
Question 9.26.
What is meant by 'demineralized' water? How is it obtained?
Answer:
Water free of all cations and anions is called de-mineralized water. It is obtained with the help of ion exchange resins. Hard water is first passed through cation exchange resin and then through anion exchange resin.
In cation exchange resin :
2 Resin H+ + 2M+(aq) → M (Resin)2 + 2H+
In anion exchange resin :
Resin- NH3 + OH- + Cl- → Resin NH3 + Cl- + OH-
Finally following reaction occurs :
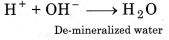
Question 9.27.
Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer:
No, demineralised or distilled water is not useful for drinking purposes. It can be made useful by adding essential minerals in appropriate quantities in demineralised or distilled water.
Question 9.28.
Describe the usefulness of water in biosphere and biological system.
Answer:
Water in useful for photosyntesis in plants. Plants produce food (in the form of glucose) by using CO2 and water in the presence of sunlight and chlorophyll.

Metabolism of glucose in our body forms CO2 and H2O vapours with the release of energy
C6H12O6 + 6O2(g) → 6CO2 (g) + 6H2O(g), ∆H = -Q
This reaction is exothermic in nature. Energy released is utilized by human body to do work.
Question 9.29.
What properties of water make it useful as a solvent? What types of compound can it
(i) dissolve and
(ii) hydrolyse?
Answer:
Water is useful as a solvent because of its high dipole moment and high dielectric constant.
(i) Ionic compounds and covalent compounds (such as ethanol which can form hydrogen bonds) can dissolve in water.
(ii) Many metallic and non- metallic oxides, hydrides etc can be hydrolysed by water.
e.g. CaH2 (s) + 2 H2O(l) → Ca(OH)2(aq) + 2H2(g)
Question 9.30.
Knowing the properties of H2O and D2O do you think that D2O can be used for drinking purposes?
Answer:
D2O can not be used for drinking purposes because i it is harmful to human beings as the rate of biochemical reactions decreases in heavy water (D2O).

Quetion 9.31.
What is the difference between the term 'hydrolysis' and 'hydration'?
Answer:
|
Hydrolysis |
Hydration |
|
It means the interaction of water with salt to give acidic or basic solution. |
It means addition of water to ions or molecules to form hydrated ions or hydrated salts. |
Question 9.32.
How can saline hydrides remove traces of water from organic compounds?
Answer:
In saline hydrides (MH), H ion is a strong Bronsted base. It reacts with traces of water present in organic compounds to liberate hydrogen gas.
H2O(l) + H-(s) → H2(g) + OH(aq)
Question 9.33.
What do you expect the nature of hydrides is, if formed by elements of atomic number 15, 19, 23 and 44 with di-hydrogen? Compare their behaviour towards water.
Answer:
(i) Element with Z = 15 is a non-metal phosphorous) and hence forms covalent hydride (PH3).
(ii) Element with Z = 19 is an alkali metal (potassium K) and hence forms ionic hydride (KH).
(iii) Element with Z = 23 is a transition metal of group 5 vanadium V) and hence forms metallic or interstitial nydride (VH1.6).
iv) Element with Z = 44 is a transition metal of group 8 ruthenium) and therefore it does not form any hydride. Behavior towards water: Only ionic hydrides react with water evolving H2 gas as,
2KH(s) + 2H2O(aq) → 2KOH(aq) + 2H2 (1)
Question 9.34.
Do you expect different products in solution when aluminium (III) cholride and potassium chloride treated seperately with:
(i) normal water.
(ii) acidified water and
(iii) alkaline water? Write equation where-ever necessary.
Answer:
(i) Reactions in Normal Water
Aluminium (III) chloride (AlCl3) undergoes hydrolysis n normal water to form strong acid (HCl) and weak base Al(OH)3.
AlCl3 (s) + 3H2O(l) → Al(OH)3 (s) + 3H+ (aq) + 3Cl(aq)
Whereas, potassium chloride (KCl) is a salt of strong acid (HCl) and strong base (KOH), So, it does not undergo hydrolysis in normal water. It dissociates to give K+(aq) and Cl- (aq) ions
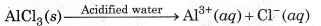
(ii) Reactions in Acidified Water:
In acidified water, H+ions formed by hydrolysis of AlCl3 n normal water react with Al(OH) to form Al3+ (aq) ons and H2O. Therefore, in acidified water, AlCl3 exists as Al3+ (aq) and Cl-(aq) ions.
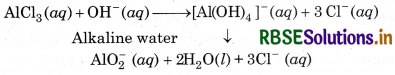
However, the aqueous solution of potassium chloride is neutral in acidified water. So, the ions do not react urther and remain as such.
(iii) Reactions in Alkaline Water:
AlCl3 reacts with alkaline water to form soluble cetrahydroxy aluminate complex or meta aluminate-ion AlCl3 (aq) + OH-(aq)-

However, the aqueous solution of KCl is neutral in alkaline water. So, the ions do not react further and remain as such.
Question 9.35.
How does H2O2 behave as a bleaching agent?
Answer:
H2O2 behaves as a bleaching agent due to formation of nascent oxygen.
H2O2 → H2O + [O]
Colured matter + [O] → Colurless matter H2O2 bleaches material such as silk, hair etc.
Question 9.36.
What do you understand by the term :
(i) Hydrogen economy
(ii) Hydrogenation
(iii) Syngas
(iv) Water-gas shift reaction
(v) Fuel cell
Answer:
(i) Hydrogen economy: Dihydrogen releases large quantities of heat on combustion. Hydrogen economy is an alternative. Its basic prinicple is the transportation and storage of energy in the form of liquid or gaseous dihydrogen. Advantage of hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power.
(ii) Hydrogenation: It means addition of hydrogen to the compounds having multiple bonds in the presence of catalyst to form saturated compounds :
iom
(iii) Syngas: It is a mixture of CO and H, produced from coal gasification.

Now-a-days syngas is produced from sewage, saw-dust scrap wood, news papers etc.
(iv) Water-gas- shift reaction:
It involves reaction of carbon monoxide (CO) with steam in the presence of iron chromate as catalyst. This reaction increases the production of dihydrogen.

Carborn dioxide is removed by scrubbing with sodium arsenite solution.
(v) Fuel Cell: It is a device which converts chemical energy into electrical energy.
e.g. H2 → O2 fuel cell
Hydrogen 83
The reaction taking place are as following:
At anode: 2H2(g) + 4OH-(aq) → 4H2O(l) + 4 e-
At cathode : O2(g) + 2H2O(l) + 4e → 4OH(aq)
Overall reaction : 2H2(g) + O2(g) → 2H2O(l)

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter
- RBSE Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure