RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques Textbook Exercise Questions and Answers.
RBSE Class 11 Chemistry Solutions Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
RBSE Class 11 Chemistry Organic Chemistry - Some Basic Principles and Techniques InText Questions and Answers
Question 12.1
How many σ- and -bonds are present in each of the following molecules?
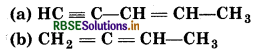
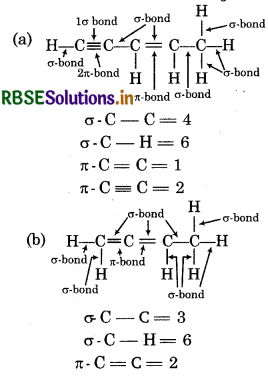
Answer:
Question 12.2.
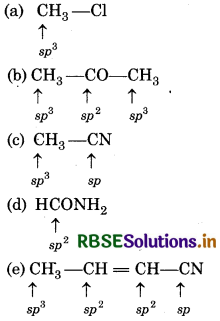
What is the type of hybridisation of each carbon in the following compounds?
(a) CH3Cl
(b) (CH3)2 CO
(c) CH3CN
(d) HCONH2
(e) CH3 CH = CH - CN
Answer:
Question 12.3.
Write the state of hybridisation of carbon in the following compounds and shapes of each of the molecules:
(a) H2C = 0
(b) CHF
(c) HC = N
Answer:
(a) H2C = 0
Hybridisation = sp2
Shape = Trigonal planar
(b) CH2F
Hybridisation = sp3
Shape Tetrahedral
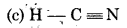
Shape = Linear
Hybridisation = Sp

Question 12.4.
Expand each of the following condensed formulae into their complete structural formulae :
(a) CH3 CH2COCH2CH3
(b) CH2CH = CH(CH2)3 CH3
Answer:
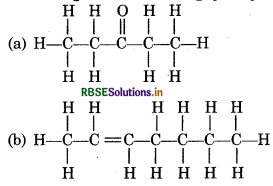
Question 12.5.
For each of the following compounds, write a condensed formula and also their bond-line formula.
(a) HOCH2 CH2 CH2 CH(CH3)2 CH HO(CH2)3 CH(CH3)CH(CH3)2
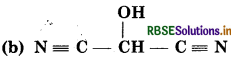
Answer:
(a) Condensed formula :
HO(CH2)3 CH(CH3)CH(CH3)2
Bond-line formula

(b) Condensed formula
HOCH(CN)2
Bond-line formula
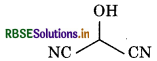
Question 12.6.
Expande each of the following bond-line formulae to show all the atoms including carbon and hydrogen.
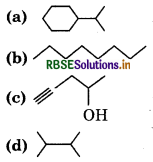
Answer:
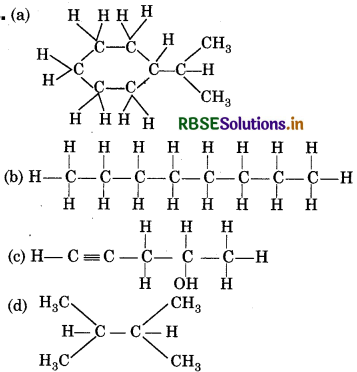
Question 12.7.
Structures and IUPAC names of som hydrocarbons are given below. Explai why the names given in the parenthese are incorrect.
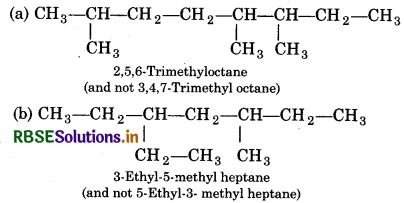
Answer:
(a) Lowest locant number, 2,5,6 is lower than 3,5,7
(b) Since the substituents are present in equivaler position, so lower number is given to the one tha comes first in the name according to alphabetica order.

Question 12.8.
Write the IUPAC name of compounds i-i from the given structures.
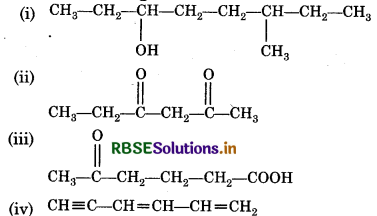
Answer:
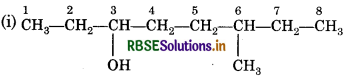
The functional group present is an alcohol (OH). Hence the suffix is ‘-ol'. The longest chain containing -OH has eight carbon atoms. Hence the corresponding saturated hydrocarbon is octane. The -OH is on carbon atom 3. In addition, a methyl group is attached at 6th carbon. Hence, the systematic name of this compound is 6-Methyloctan-3-ol.
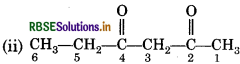
The functional group present is ketone (>C = O), hence suffix '-one'. Presence of two keto groups is indicated by 'di', hence suffix becomes ‘dione'. The two keto groups are at carbons 2 and 4. The longest chain contains 6 carbon atoms, hence, parent hydrocarbon is hexane. Thus, the systematic name is hexane- 2,4-dione.
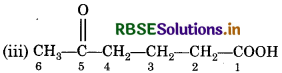
Here, two functional groups namely ketone and carboxylic acid are present. The principal functional group is the carboxylic acid group; hence the parent chain will be suffixed with 'oic' acid. Numbering of the chain starts from carbon of – COOH functional group. The keto group in the chain at carbon 5 is indicated by 'oxo'. The longest chain including the principal functional group has 6 carbon atoms; hence the parent at hydrocarbon is hexane. The compound is, therefore,
named as 5-oxohexan-1-oic acid.
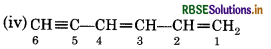
The two C=C functional groups are present at carbon atoms 1 and 3, while the C=C functional group is present at carbon 5. These groups are indicated by suffixed 'diene' and 'yne' respectively. The longest chain containing the functional groups has 6 carbon atoms; hence the parent hydrocabron is hexane. Therefore, the name of the compound is Hexa-1, 3-dien-5-yne.
Question 12.9.
Derive the sturcture of:
(i) 2-chlorohexane
(ii) Pent-4-en-2-ol
(iii) 3-Nitrocyclohexene
(iv) Cyclohex-2-en-1-ol
(v) 6-hydroxy-heptanal
Answer:
(i) 2-Chloro hexane:
Hexane: Indicates the presence of 6 C-actoms in the chain with single bonds.
2-Chloro: indicates the presence of chloro group at position-2.
∴Structure is represented as:
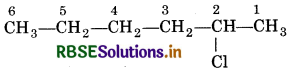
(ii) Pent-4-en-2-ol
Pent: indicates the presence of 5 C-atoms in the chain 4-en-corresponds to C C at postion-4. 2-ol-corresponds to alcholic group ie-OH, at Position 2.
∴ Structure is represented as:
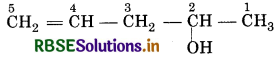
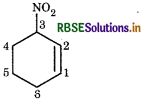
(iii) 3-Nitro cyclohexene
Cyclohexene: Indicates six membered ring with one carbon double bond.
3-Nitro: indicates the presence of nitro group in position-3.
∴ Structure is represented as:
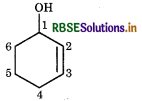
(iv) Cyclohex-2-en-1-ol
Cyclo-hex: indicates six membered ring.
2-en-indictes the presene of one double bond at position-2
1-ol-idicates the presence of alcoholic group (-OH) at position-1.
∴ Structure is represented as:
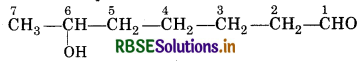
(v) 6-Hydroxy heptanal
Heptanal-indicates the presence of 7 C-atoms in the chain with aldehyde group (CHO).
6-Hydroxy-Indicates the presence of alcholic group (-OH) at position-6.
∴ Sturtures is represinted as :

Question 12.10.
Write the strucutal formula of:
(a) o-Ethyl anisole
(b) p-Nitro aniline.
(c) 2.3 Dibromo-1-phenyl pentane
(d) 4-Ethyl-1-fluoro-2 nitro benzene
Answer:
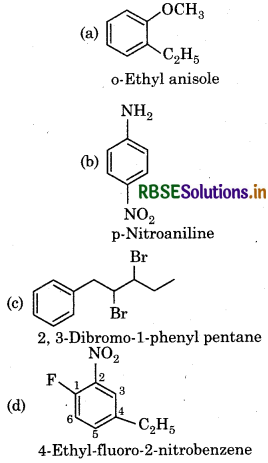
Question 12.11.
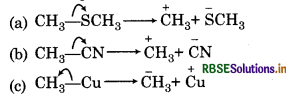
Using covered-arrow notation, show the formation of reactive intermediates when the following covalent bonds undergo heterolytic cleavage.
(a) CH3 - SCH
(b) CH3-CN
(c) CH3-Cu
Answer:
Question 12.12.
Giving justificiatin categories the following molecules ions as nucleophile or electrophile: HS-, BF3, C2H5O-
(CH3)3 N:, Cl+, 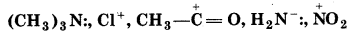
Answer:
Nulcleophilcs
HS-, C2H5O-, (CH3)3 N:, H2N-
These species posses unshared pair of electrons which an be donated and shared with an electrophile.
Electrophilcs:
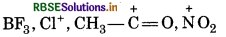
These species are electron deficient which can accept elctron pair form a nucleophile.
Question 12.13.
Identify electrophilic centre in th following: CH3-CHO, CH2 CN, CH2
Answer:
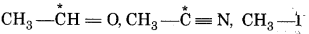
In above species, the starred carbon atom are electopil centres as they will have partial positive charge due polarity of the bond.
Question 12.14.
Which bond is more polar in the followin pairs of molecules :
(a) H3C - H, H3C - Br
(b) H3C - NH2, H3C - OH
(c) H3C - OH, H2C - SH
Answer:
(a) Among C-H and C-Br bond, C-Br is more pola due to high electronegativity of Br than H.
(b) Among C-N and C-O bond, C-O bond is mo polar due to high electronegativity of O than N.
(c) Among C-O and C-S bond, C-O bond is mo polar due to high electronegativity of O than S.
Question 12.15.
In which C-C bond of CH2 CH2 CH2 Br th inductive effect is expected to be the least?
Answer:
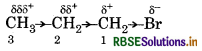
The magnitude of inductive effect decreases as th number of intervening bond increases. so, the inductiv effect is least in the bond between carbon-3 an hydrogen.

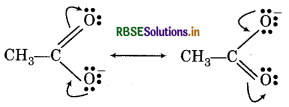
Question 12.16.
Write resonance structures of CH3COO and show to movement of electrons curves arrows
Answer:
Question 12.17.
Write resonance structures of CH2 = CH - CHO. Indicate relative stability the contributing structures.
Answer:
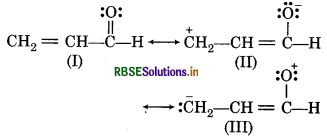
Relative order stability
I > II > III
Structure It has more number of covalent bonds. Each carbon and oxgyen atom possesses an octet and no separation of opposite charge, so it must stable.
In structure II, negative charge is present on more electronegative atom and positive charge is present on more electropositive atom. Structure III does not contribute as oxygem has positive charge and carbon has negative charge, so, it is least stable.
Question 12.18.
Explain why the following two structures, I and II can not be the major contributers to the real structures of CH3COOCH3.
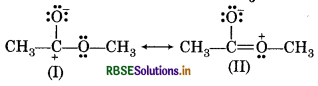
Answer:
These two strucrtures, I and II can not be the major contributers to the real structure of CH3COOCH, as they involve charge separation. Moreover, struture I contains a carbon atom with an incomplete octet.
Question 12.19.
Explain why (CH3)3 C+ is more stable than CH3 CH2 and CHg is the least stable catain.
Answer:
Relative order of stability among Carbocations:
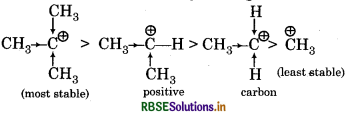
(CH3)3 C+ carbonium ion is most stable because its shows more +I effect as it contains three alkyl group which release their bonding electrons towards carbon atom having positive charge due to which the existence of positive charge decreases on that carbon atom and it can not attack easily with nucleophile so, its reactivity is lesser and stability is greater than CH3 CH2+ and CH3.
Question 12.20.
On complete combustion, 0.246g of an organic compound gave 0.198g of carbondioxide and 0.1014 of water. Determine the percentage composition of carbon and hydrogen in the compound.
Answer:
Given,
Weight of organic compound = 0.246 g
Weight of CO2 =0.198 g
Weight of water = 0.1014 g
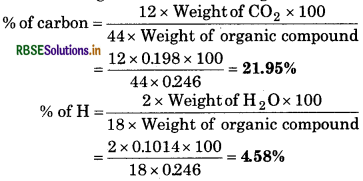
Question 12.21.
In Duma's method for estimation of nitrogen, 0.3 g of an organic compound gave 50 mL of nitrogen collected at 300 K temperature and 715 mm pressure, calculete the percentage composition of nitrogen in the compound. (Aqueous tension at 300 K = 15 mm)
Answer:
Given,
Weight of organic compound = 0.3 g
Volume of nitrogen collected = 50 mL
Temperature = 300 K
Aqueous tension at 300 K = 15 mm
∴ Actual pressure (P) = 715 - 15 = 700 mm
Volume of nitrogen at STP = \(\frac{\mathrm{P} \times \mathrm{V} \times 273}{760 \times T}\)
\(=\frac{700 \times 50 \times 273}{760 \times 300}=41.9 \mathrm{~mL}\)
∴ 22, 400 mL of N, at STP weights = 28 g
∴ 41.9 mL nitrogen weights = \(\frac{28 \times 419}{22400} \mathrm{~g}\)
∴ \(\% \mathrm{~g} \mathrm{~N}=\frac{28 \times 41.9 \times 100}{22400 \times 0.3}=\mathbf{1 7 . 4 6} \%\)

Question 12.22.
During estimation of nitrogen present in an organic compound by Kjeldahl's method, the ammonia evolved from 0.5 g of the compound in Kjeldahl'c estimation of nitrogen neutralized 10 ml of 1M. H2SO4. Find out the percentage of nitrogen in the compound.
Answer:
Given,
Weight of compound = 0. 5 g
1 M of 10 mL H2SO4 = 1 M of 20 mL NH3
∴ 1000 mL of 1 M ammonia contains 14g of nitrogen
∴ 20 mL of 1M ammonia contains:
\(\% \text { of } \mathrm{N}=\frac{14 \times 20 \times 100}{1000 \times 0.5}=\mathbf{5 6} \%\)
Question 12.23.
In carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 of AgBr. Find out the percentage of bromine in the compound.
Answer:
Given,
Weight of organic compound = 0.15 g
Weight of AgBr = 0.12 g
Molor mass of AgBr = 108 + 80 = 188 g mol-1
∵ 188 g AgBr contains 80g bromine.
∴ 0.12 g AgBr contains = \(\frac{80 \times 0.12}{188} \mathrm{~g}\)
∴ \(\frac{80 \times 0.12 \times 100}{188 \times 0.15}\)
= 34.04%
Question 12.24.
In sulphur estimation, 0.157 g of an organic compound gave 0.4813 of barium sulphate. What is the percentage of sulphur in the compound?
Answer:
Given, Mass of organic compound = 0.157 g
Mass of BaSO4 = 0.4813 g
Molor mass of BaSO4 = 137 + 32 + 64 = 233 g
∴ 233 g of BaSO4 contains 32 g sulphur
∵ 0.4813 of BaSO4 contain = \(\frac{32 \times 0.4813}{233}\) g sulphur
∵ \(\% \text { of } \mathrm{S}=\frac{32 \times 0.4183 \times 100}{233 \times 0.157}=\mathbf{4 2 . 1 0} \%\)
RBSE Class 11 Chemistry Organic Chemistry - Some Basic Principles and Techniques Textbook Questions and Answers
Question 12.1.
What are hybridisation states of each carbon atom in the following compounds?
CH2 = C = O, CH3 CH = CH2, (CH3)2CO
CH2 = CHCN, C6H6
Answer:
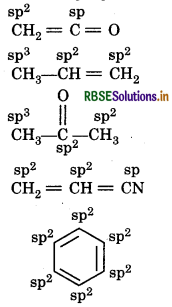
Question 12.2.
Indicate the σ and π-bond in the following molecules:
C6H6, C6H12, CH2Cl2, CH2 = C = CH2, CH3 NO2, HCONHCH3
Answer:
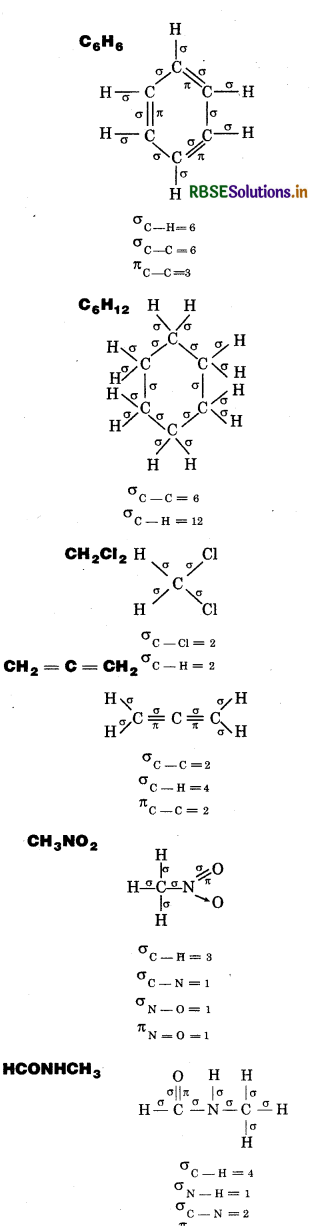
Question 12.3.
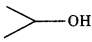
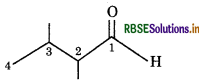
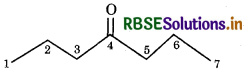
Write bond line formula for Isopropyl alcohol, 2.3-dimethyl butanal, Heptan-4-one
Answer:
Isopropyl alcohol:
2,3,- Dimethyl butanal:
Heptan-4-one:

Question 12.4.
Give the IUPAC names of the following compounds:
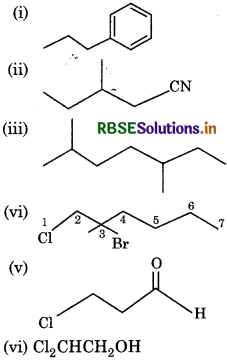
Answer:
(i) Propylbenzene
(ii) 3-Methylpentanitrile
(iii) 2,5-Dimethylheptane
(iv) 3-Bromo-1-chloropheptane
(v) 3-Chloropropanal
(vi) 2,2-Dichloroethanol
Question 12.5.
Which of the following represents the correct IUPAC name for the compound concerned?
(i) 2,2 dimethylpentane
or
2-Dimethylpentane
(ii) 2,4,7- Trimethyloctane
or
2,5,7 Trimethyloctane
(iii) 2- chloro-4-methylpentane
or
4-chloro-2-methylpentane
(iv) But-3-yne-1-ol or But-4-ol-1-yne
Answer:
(i) 2,2-Dimethyl pentane is correct.
Reason: The prefix 'di' indicates that the two identical subsitituents are present in parent chain.
(ii) 2, 4,7-Trimethyloctane is correct
Reason: 2 4, dorimethyloctane set is lower than 2, 5,7.
(iii) 2-chloro-4-methyl pentane is correct.
Reson : Alphabetical order of substituents.
(iv) But-3-yne-1-ol is correct
Reason: Lower number is given to principal functional F group i.e., alcholic group.
Question 12.6.
Draw formulae for the first five members of each homologous series beginning with the following compounds :
(i) H-COOH
(ii) CH3 COCH
(iii) H-CH=CH2
Answer:
(i) HCOOH Methanoic acid
CH3 - COOH : Ethanoic acid
COOH: Propanoic acid
CH2-CH2-CH2 - COOH: Butanoic acid
CH3 -CH2 -CH2 - CH2 - COOH : Pentanoic acid
(ii) CH, COCH3 : Propanone
CH3COCH2CH3 : Butan -2-one
CH3COCH2CH2CH3 : Pentan-2-one CH3COCH2CH2CH2CH3 : Hexan-2- one
CH,COCH,CH,CH,CH,CH3 : Heptan-2-one
(iii) H – CH=CH2 : Ethene
CH3-CH=CH2: Propene
CH3-CH2-CH=CH2; 1- Butene
CH3-CH2-CH2-CH=CH2 : 1 Pentene
CH3-CH2-CH2-CH2-CH=CH2 : 1- Hexene

Question 12.7.
Give condensed and bond line structural formulas and identify the functional groups (s) present, if any, for
(i) 2,2, 4- Trimethylpentane
(ii) 2-Hydroxy-1, 2,
3-propanetricarboxylic acid
(iii) hexanedial
Answer:
(i) 2, 2, 4-Trimethylpentane
Condensed formula
(CH3)2 CHCH2C(CH3)3
Bond line formula
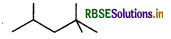
No functional group is present.
(ii) 2-hydroxy-1, 2,3-propanetricarboxylie acid
Condensed formula : (COOH) CH2C (OH ) (COOH)CH2 (COOH)
Bond line formula
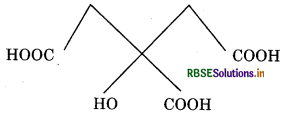
functional groups present in the given compound are- OOH and -OH.
(iii) Hexanedial: Condensed Formula
(CHO) (CH2)4 (CHO)
Bond line Formula

Functional group present in the given compound is - CHO.
Question 12.8.
Identify the funetional groups in the following compounds :
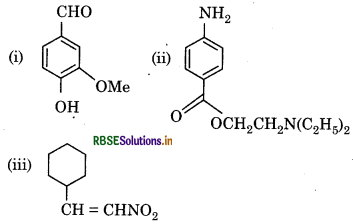
Answer:
(i) Aldehyde (-CHO)
Hydroxyl (-OH)
Methoxy (-OMe)
and CC double bond
(ii) Amino (– NH2)
Ketone (C=O), and
Diethylamine (N(C2H5)2
(iii) Nitro (-NO2) and
CC double bond
Question 12.9.
Which of the two O2NCH2 CH2O- 0r CH3 CH2O- is expected to be more stable and why?
Answer:
O2NCH2CH2O- is more than CH3CH2O becaus NO2 groups has -I-effect and hence it tends to dispers the -ve charge on the O- atom. In contrast, CH3CH2 ha +I-effect. It, therefor, tends to intensify the negativ charge and hence destabilizes it.
Question 12.10.
Explain why alkyl groups act as electron donors when attached to a л system.
Answer:
When an alkyl group is attached to a л system, acts as an electron-donor group by the process hyperconjugation. Consider an example of propene,
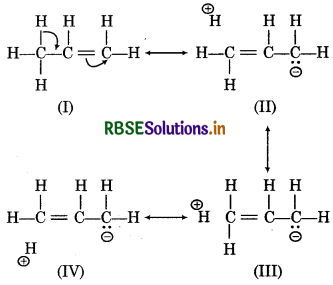
Question 12.11.
Draw the resonance structures for the following compounds. Show the electro shift using curved-arrow notation.
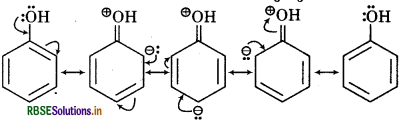
(i) C6H5OH
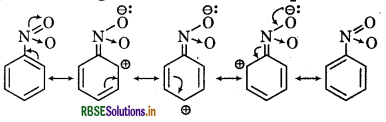
(ii) C6H5NO2
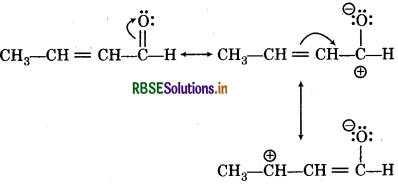
(iii) CH3CH = CHCHO
(iv) C6H5 - CHO
(v) C6H5 CH2
(vi) CH3CH = CH-CH2
Answer:
(i) Resonating sturtures of C6H5OH
(ii) Resonating strutures of C6H5NO2
(iii) Resonating sturtures of CH3-CH = CH-CHO
(iv) Resonating structures of C6H5CHO
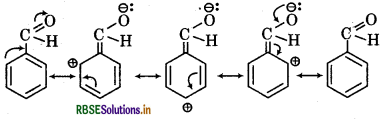
(v) Resonating structures of C6H5CH2
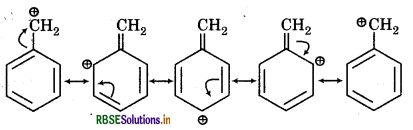
(vi) Resonating strutures of CH3-CH= CHCH2
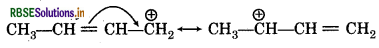
Question 12.12.
What are electrophiles and nucleophiles ? Explain with examples.
Answer:
Electrophiles: These are electron-deficient species which can accept an electron pair
e.g
H+, CI+, NO+2, AlCl3, BF, etc.
Nucleophiles: These are electron-rich species which can donate an electron pair.
e.g. Cl- Br- NH3, ROH etc.

Question 12.13.
Identify the reagents shown in underlined in the following equations as nucleophiles or elecrtrophiles.
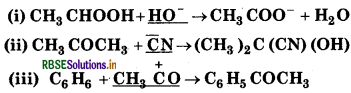
Answer:
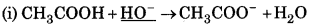
In this reaction, HO act as a nucleophile because it is electron-rich species.

In this reaction, CN acts as nucleophile because it is O electron-rich species.
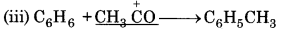
In this reaction, CH3 CO+ acts as electrophile because it is electron deficient species.
Question 12.14.
Classify the following reactions in one of the reaction type studied in this unit.
(i) CH, CH2 Br + HS- → CH3 CH2SH + Br-
(ii) (CH3)2 C = CH2 + HCl → (CH3)2 Cl C-CH3
(iii) CH, CH2 Br + HO- → CH2 = CH2 + H2O + Br-
(iv) (CH3)3 C-CH2OH + HBr → (CH3)2CBr CH2 CH2CH3 + H2O
Answer:
(i) Nucleophilic substitution reaction
(ii) Electrophilic addition reaction
(iii) Bimolecular elimination reaction
(iv) Nucleophilic substitution reaction with rearrangement
Question 12.15.
What is the relationship between the members for following pairs of structures ? Are they structural of geometerical isomers of resonance contributers?
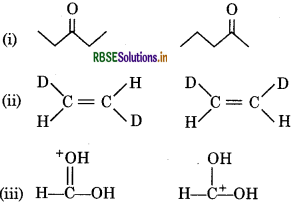
Answer:
(i) The given compounds have the same molecular formula but they differ in the position of the functional group, so they are structural isomers (actually position isomers as well as metamers).
(ii) The given compounds having the same molecular formula, the same constitution and the sequence of covalent bonds but with different relative position of their atoms in space, so they are called geometrical isomers.
(iii) The given compounds are resonance contributors because they differ in the position of electrons but not atoms.
Question 12.16.
For the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical carbocation and carbanion.
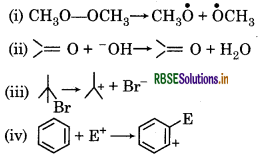
Answer:

It is an example of homolytic cleavage as one of the shared pair in a covalent bond goes with the bonded atom. The reaction intermediate formed is a free radical.
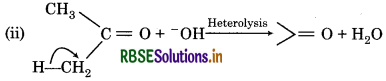
It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate formed is carbanion.
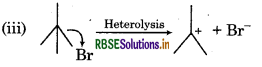
It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of the electrons remains with the bromine ion. The reaction intermediate formed is a carbocation.
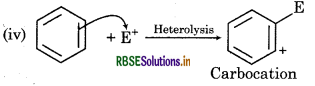
It is a heterolytic cleavage as bond breaks in such a manner that the shared pair of electrons remains with one of the fragments. The reaction intermediate formed is a carbocation.
Question 12.17.
Explain the terms inductive and electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids ?
(i) Cl3CCOOH > Cl2 CHCOOH > CICH2 COOH
(ii) CH3 CH,COOH > (CH3)2 CHCOOH > (CH3)3 CCOOH
Answer: Inductive Effect
The permanent displacement of bonding electrons towards a more electronegative element along a saturated chain in called inductive effect.
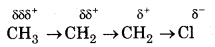
It is of two types:
(a) + I effect: When an atom or group of atoms releases bonding electrons towards more electronegative element then it is said to have + I effect.
e.g. CH3, -CH2 CH3 etc.
(b) - I effect: When an atom or group of atoms attract bonding electrons towards itself, then it is said to have - I effect
e.g. Cl-, Br, -NO2, etc.
Electromeric Effect: It involves the transfer of the shared pair of π-electrons to either of the two atoms attached to multiple bond in the presence of attacking agent. It is a temporary effect.
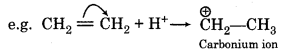
It is of two types:
(a)+ E effect: In this effect, the electrons are transferred towards the attacking reagent.
(b) E effect: In this effect, the electrons are transferred away from the attaking reagent.
(i) Cl,CCOOH > Cl3CHCOOH > ClCH2COOH
The order of acidity can be explained on the basis of inductive effect (I effect). As the number of chlorine atoms increases the -I effect increases, with the increase in I effect, the acid strength also increases accordingly.
(ii) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3 CCOOH The order of acidity can be explained on the basis of inductive effect (+ I effect). As the number of alkyl grups increases the + I effect, also increases with the increase in +I effect, the acid strength also increases accordingly.

Question 12.18.
Give a brief description of the principles of the following techniques taking an examples in each case.
(i) Crystallisation
(ii) Distillation
(iii) Chromatography
Answer:
(i) Crystallisation
This technique is one of the most important technique for the purification of solid organic compounds. It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent. In this technique the impure compound, to be purified is dissolved in a solvent in which it is sparingly soluble at roon temperature but it is soluble at high temperature. The solution so obtained is concentrated to get a nearly saturated solution. When the solution is cooled, pure compound crystallines out and is removed by filtration. The filtrate contains impurities and small amount of the compound. Repeated cystallisation becomes necessary for the purification of compounds having impurities of comparable solubilities.
(ii) Distillation:
This technique is used to separate volatile liquids from non-volatile impurities as well as the liquids having sufficient difference in their boiling points. For example, chloroform (Bp 334K) and aniline (Bp 47K) are easily separated from this technique.
Process In this technique, the liquid mixture to be separate is taken in a round bottom flask and heated carefully. On boiling first of all the vapours of lower boiling point are formed. The vapours are condensed by using a condenser and the liquid is collected in a receiver. The vapours of higher boiling point form later and the liquid can be collected separetely.
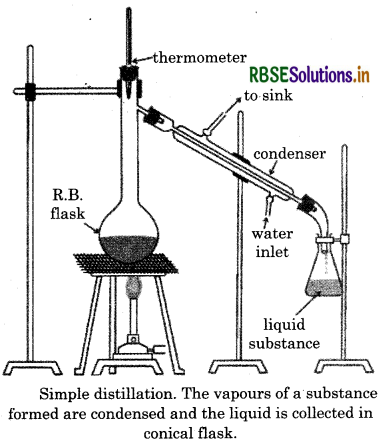
The vapours of a substance formed are condensed and the liquid is collected in conical flask.
(iii) Chromatography:
Chromatography is a laboratory technique for the separation of a mixture. The term chromatography is based on the Greek word chroma (means colour), since this technique was first used for the separation of coloured substances found in plants. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material
called the stationary phase. The various constituents of the mixture travel at diffferent speeds, causing them to separate.
On the basis of principle involved, Chromatography is categorized into classes:
- Adsorption chromatography
- Partition Chromatography
Question 12.19.
Describe the method, which can be used to separate two compounds with different solubilities in a solvents.
Answer:
Fractional crystallistion can be used to separate two compounds with different solubilites in a solvents. In this process, a hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out while the more soluble compound remains in the solution. The crystals are separate from the mother liquor and the mother liquor is again concentrated and the hot solution again allowed to cool when the crystals of the second compound are obtained. These crystals are again filtered and dried.
Question 12.20.
What is the difference between distillation under reduced pressure and steam distillation?
Answer:
|
Distillation |
Distillation under reduced pressuere |
Steam distillation |
|
This method is used to purify the compounds that are associated with non-volatile impurities which do not decompose on boiling. e.g. A mixture of chloroform and aniline is separated by this method. |
This method is used to purify liquid that tends to decompose on boiling. e.g glycerol is purified by this method. reduced pressure, it boils at 453 K without decomposition, as it decomposes at 593 K. |
This method is used to purify ar organic compound, which is steam volatile and immiscible in water. e.g. A mixture of water and aniline is separated by this method. |
Question 12.21.
Discuss the Chemistry of Lassaigne's test.
Answer:
Lassaigne's test is used to detect the pressence of elements: Nitrogen (N), Sulphur (S), Chlorine (Cl), Bromine (Br) and Iodine (I). This test involves following two steps:
- Preaparation of sodium fusion extract (SFE)
- Detection of elements using SFE.
1. Preparation of SFE or Lassaigne's Extract: A small amount of oganic substance is fused with small quantity of sodium metal in a fusion tube. The red hot fusion tube is then plugged into distilled water and the contents are boiled for a few minutes, the cooled and filtered. The filtrate obtained is called sodium fusion extract (SFE) or Lasaigne's extract. It is usually alkaline. If it is not alkaline, a few drops of NaOH solution may be added to make it alkaline.
2. Detection of Elements using SFE :
Thus obtained SFE is used to detect the presence of elements like N, S, Cl, Br and I.
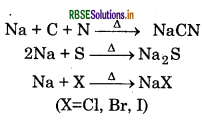
The elements in the organic compound react with sodium during fusion raction as follows:
Na + C + N → NaCN (if N is present)
2Na + S → Na2S (if S is present)
Na + S + C + N → NaSCN
(if both N and S are present and insufficient amount of Na is used)
2Na + X2 → 2NaX (if halogens are present)
Where (X = Cl, Br,I)
Hence SFE may conatin any of or all of ionic forms of respective elements.

Question 12.22.
Differentiate between the principle of estimation of nitrogen in an organic compound by
(i) Duma's method and
(ii) Kjeldahl's method.
Answer:
|
Distillation |
Distillation under reduced pressuere |
|
In this method a known amount of nitrogen containing organic compound is heated strongly with excess of copper oxide in an atmosphere of CO2 to produce free nitrgoen, which is collected over an aqueous solution of KOH. The volume of nitrogen produced is measured at room temperature and atmospheric pressure. |
In this method a known amount of nitrogen containing compound is heated with conc. H2SO4. The nitrogen present is quantitativeiy converted into ammonium sulphate. It is then distilled with excess of NaOH, ammonia NH3 gas is evolved which is passed into a known volume of H2SO4. The unused acid is estimated by volumetric analysis and the amount of ammonia produced is determined, So, the % of nitrogen can be estimated. |
Question 12.23.
Discuss the principle of estimation of halogen suphur and phosphorous present in an organic compound.
Answer:
Estimation of Halogens
Carius method : A known weight of organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding. Silver halide (AgX). It is filtered, washed dried and weighed. Let the mass of organic compound taken = m gm.
Mass of AgX formed = m1 g
1 mole of AgX contains 1 mol of X
Mass of halogen in m1 g of AgX = atomic mass of Xx m1 g /molecular mass of AgX
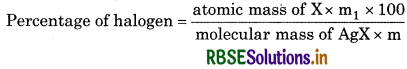
Estimation of Sulphur:
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidsed to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution in water.
The percentage of sulphur can be calculated from the mass of barium sulphate.
Let the mass of organic compound taken = mg and
the mass of barium sulphate formed = m.g
1 mol of BaSO4 = 233 g BaSO4 = 32 g sulphur
m1g. BaSO4 contains \(=\frac{32 \times m_1}{233} \text { g sulphur }\)
% of S = \(\frac{32 \times m_1 \times 100}{233 \times m}\)
Estimation of Phosphorus:
A known mass of organic compound is heated with fuming nitric acid where upon phosphours present in the compound is oxidised to phosphorus acid. It is precipitated as ammonium phosphomolybdate (NH4)3. PO4. 12M0O3, by adding ammonia and ammonium molybdate. Alternatively phosphoric acid may be precipitated as MgNH,PO by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated which on ignition yields Mg2P2O7. Let the mass of organic compound taken = mg and mass of ammonium phosphomolybdate = m1g
Molar mass of (NH4)3PO4.12MoO1 = 1877g
% of phosphorus = \(\frac{31 \times m_1 \times 100}{1877 \times m}\)
Question 12.24
Explain the principle of paper chromatography.
Answer:
Paper chromatography is based on continuous differential partitioning of compounds of a mixture between stationary and mobile phase. In this technique chromatography paper acts as stationary phase. A strip of this paper spotted at the base with the solution of the mixture is suspended in a suitable solvent or a mixture of solvents. The solvent acts as moblie phase. The solvent rises up the paper by capillary action and flows over the spot. The paper selectively retains various components on the basis of differing partition in the two phases. The paper strip so developed is called chromatogram. The spots of the separated coloured compounds are visible at different heights from the position of initial spot on the chromatogram. The sample shots can be seen under UV light or by use of suitable spray reagent.
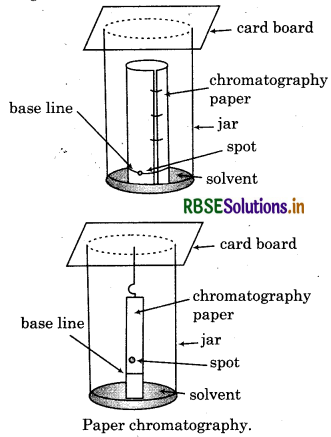
Chromatography paper in two different shapes.

Question 12.25.
Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
Answer:
Nitric acid is added to sodium extract before adding silver nitrate for testing halogen for the decomposition of NaCN to HCN and Na2S to H2S and to expel these gases so, if any nitrogen and sulphur present in the form of NaCN and Na2S then they are removed from two solution.
NaCN + HNO3 → Na2S + HCN
NaNO3 + HNO3 → 2 NaNO3 + H2S
Question 12.26.
Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur, and halogens.
Answer:
In organic compound, nitrogen, sulphur and halogens are covalently bonded. So they have to be first converted into ionic form for their detection. So organic compound is fused with metallic sodium to form Lassaigne's extract. The chemical equations take place.
Question 12.27.
Name a suitable technique of separation of the compounds from a mixture of calcium sulphate and camphor.
Answer:
Sublimation is used to separate the components from a mixture of calcium sulphate and camphor. The process involves the conversion of solid directly into vapour state without passing through the liquid state. As we known that, camphor is a sublimate compound and calcium sulphate is non-sublimate compound. So on heating camphor will sublime whereas calcium suphate will be left behind.
Question 12.28.
Explain why an organic liquid vaporises at a temperature below its boiling point in its steam distillation?
Answer:
It steam distillation, the organic liquid starts to boil when the sum of vapour pressure due to the organic liquid (p1) and the vapour pressure due to water (P2) becomes equal to atmospheric pressure (p), that is,
p = P1 + P2
since p1 < P2, organic liquid will vapourise at a lower temperature than its boiling points.
Question 12.29.
Will CCl4 give white precipitate of AgCl on heating it with silver ntirate ? Gives reason for your answer.
Answer:
CCl4 will not given the white precipitate of silver chloride on heating it with silver nitrate. This is due to h the fact that the chlorine atoms are covalently bonded to T carbon in CCl4. To get the precipitate, it should be present in ionic form and for this, it is necessary to prepare the Lassaigne's extract of carbon tetrachloride. N
Question 12.30.
Why is solution of potassium hydroxide used absorb carbon dioxide evolved? h during the estimation of carbon present in an organic compound?
Answer:
Carbon dioxide is acidic in nature and potassium hydroxide is a strong base. Hence, carbon dioxide reacts with potassium hydroxide to form potassium carbonate and water as follows:
2KOH + CO2 → K2CO3 + H2O
Hence, the mass of the U-tube containing KOH increases. This increase in the mass of U-tube gives the mass of CO2 produced. From its mass the percentge of carbon in the organic compound can be estimated.
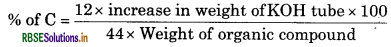
Question 12.31
Why is necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test ?
Answer:
Although the addition of sulphuric acid will precipitate lead sulphate, the addition of acetic acid will ensure a complete precipitation of sulphur in the form of lead sulphate due to common ion effect. Hence, it is necessary to use acetic acid for acidification of sodium extract for testing sulphur by lead acetate test.

Question 12.32.
An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0.20 g of this substance is subjected to complete combustion.
Answer:
% of carbon in organic compound = 69%
100 g of organic compound contains 69 g of carbon.
∵ 0.2 g of organic compound will contain = \(\frac{69 \times 0.2}{100}\)
Molecular mass of carbon dioxide, CO2 = 44g
∵ 12 g of carbon is contained in 44 g of CO2.
Therefore, 0.138 g of carbon will be contained in
\(\frac{44 \times 0.138}{12}\)
= 0.506 g of CO2
Thus, % of hydrogen in organic compound is 4.8.
e., 100 g of organic compound contains 4.8 g of ydrogen.
Therefore, 0.2 g of organic compound will contain
\(\frac{4.8 \times 0.2}{100}=0.0096 \mathrm{~g} \text { of } \mathrm{H}\)
Molecular mass of water H2O = 18g.
∵ 2 g of hydrogen is present in 18 g of water 0.0096 g of
hydrogen will be contained of water
\(=\frac{18 \times 0.0096}{2}\)
0.0864 g of water
Question 12.33.
A sample of 0.50 g of an organi compound was treated according t Kjeldahl's method. The ammonia evolve was absorbed in 50 ml of 0.5 M H2SO4 The residual acid required 60 mL of 0.5 N solution of NaOH for neutralisation. Fin the percentage composition of nitroge in the compound.
Answer:
Given, mass of organic compound = 0.50 g
60 mL of 0.50 M solution of NaOH was required b residual acid for neutralisation.
60 mL of 0.5 NaOH solution = \(\frac{60}{2}\)
H2SO4 = 30 mL of 0.5 M H2SO4
Acid consumed in absorption of evolved ammonia i (50 - 30) mL = 20 mL
Again, 20 mL of 0.5 M H2SO4 = 40 mL of 0.5 M NH2
Also, since 1000 mL of 1 m NH3 contains 14 g of nitrogen.
40 mL of 0.5 M NH3 will contain
= \(\frac{14 \times 40}{1000} \times 0.5\)
= 0.28 g of N
∵ Percentage of nitrogen in 0.50 g of organic compound
\(=\frac{0.28}{0.50} \times 100=\mathbf{5 6} \%\)
Question 12.34.
0.3780 g of an organic chloro compound gave 0.5740 g of silver chloride in Cariu estimation. Calculate the percentage o chlorine present in the compound.
Answer:
Given, Mass of organic compound is 0.3780 g.
Mass of AgCl formed = 0.5740 g
1 mol of AgCl contains 1 mol of Cl.
Thus, mass of chlorine in 0.5740 g of AgCl
\(\frac{35.5 \times 0.5740}{143.32}=\mathbf{0 . 1 4 2 1} \mathrm{g}\)
% of chlorine = \(\frac{0.1421}{0.3780}\) × 100 = 37.59%
Question 12.35.
In the estimation of sulphur by Carius method. 0.468 g of an organic sulphu compound afforded 0.668 g of barium sulphate. Find out the percentage of sulphur in the given compound.
Answer:
Given: mass of organic compound = 0.468 g
Mass of BaSO4 formed = 0.668 g
1 mol of BaSO4 = 233 g of BaSO4 = 32 g of sulphur
Thus, 0.668 g of BaSO4 contains = \(\frac{32 \times 0.668}{233}\)
= 0.0917 g of sulphur
% of sulphur = \(\frac{0.197}{0.468} \times 100\) = 19.59%
Question 12.36.
In the organic compound CH2 CH
CH2 - CH2 - C=CH, the pair of hybridised orbitals involved in the formation of C2 - C3 bond is :
(a) sp - sp2
(b) sp - sp3
(c) sp2 - sp3
(d) sp3 - sp3
Answer:
(b) sp - sp3

Question 12.37.
In the Lassaigne's test for nitrogen in an organic compound the prussian blue colour is obtained due to the formation of:
(a) Na4 [Fe(CN)6]
(c) Fe2[Fe(CN)6]
(b) Fe4 [Fe(CN)6]3
(d) Fe [Fe(CN)6]4
Answer:
(b) Fe4 [Fe(CN)6]3
Reason: FeSO4 + 2 NaOH → Fe(OH)2 + Na2SO4
6 NaCN + Fe(OH)2 → Na4[Fe(CN)6] + 2NaOH

Question 12.38.
Which of the following carbocation is most stable?
(a) (CH3)3 C + CH2
(b) (CH3)3 C +
(c) CH3 CH2 C + H2
(d) CH3 CHCH2 CH3
Answer: (b) (CH3)3 C+
Reason: (CH3)3 C is a tertiary carbocation which is most stable due to more + I effect as compared to other carbocations.

Question 12.39.
The best and latest technique for isolation, purification and separation of organic compound is:
(a) Crystallisation
(b) Distillation
(c) Sublimation
Answer:
(d) Chromatography
(d) Chromatography
Reason: Chromoatgraphy is the latest technique for separation and purification of organic compound.
Question 12.40.
The reaction:
CH3 CH2I + KOH (aq) → CH3 CH2 OH + KI is classified as:
(a) electrophilic substitution
(b) nucleophilic substitution
(c) elimination
(d) addition
Answer:
(b) Nucleophilic substitution.
Reason: In the reaction OH- substitutes I- so, it is an example of nucleophilic substitution reaction.
