RBSE Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Solutions Chapter 1 Some Basic Concepts of Chemistry
RBSE Class 11 Chemistry Some Basic Concepts of Chemistry InText Questions and Answers
Question 1.1.
Calculate the molecular mass of glucose (C6H12O6) molecule.
SolutIon:
Atomic mass of C = 12.011 u
Atomic mass of H = 1.008 u
Atomic mass’of O = 16.00 u
Molecular mass of C6H12O6
= 6 x (12.011 u) + 12 x (1.008 u) + 6 x (1600 u)
= 72.066 u + 12.096 u + 9600 u
= 180.162 u
Question 1.2.
A compound contains 4.07% hydrogen, 24.27% carbon and 71.65% chlorine. Its molar mass is 98.96 g. What are its empirical and molecular fomula?
Solution:
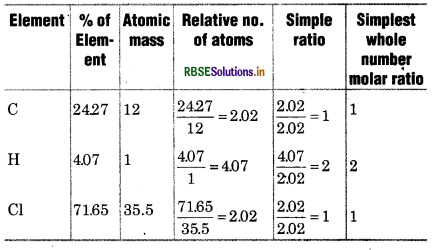
∴ Empirical formula of compound = C1H2Cl1
= CH2 Cl
Empirical formula mass = 12 x 2 (1) + 35.5
= 49.5
Moleular mass = 98.96
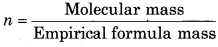
= \(\frac{98.96}{49.5}\) = 2
Molecular formula = n x Empirical formula
= 2 x CH2Cl
= C2H4Cl2

Question 1.3.
Calculate the amount of water (g) produced by the combustion of 16 g of methane.
Solution:
The balanced equation for the combustion of
methane (CH4) is
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
16g of CH4 = lmole of CH4
∵1 mol of CH4(g) gives 2 mol of H2O(g).
2 mol of water(H2O) = 2 x (+16)
= 2 x 18 = 36g
1 mol of H2O 18g H2Os = \(\frac{18 \mathrm{~g} \mathrm{H}_2 \mathrm{O}}{1 \mathrm{~mol} \mathrm{H}_2 \mathrm{O}}\) = 1
∴ 2 moles H2O x \(\frac{18 \mathrm{~g} \mathrm{H}_2 \mathrm{O}}{1 \mathrm{~mol} \mathrm{H}_2 \mathrm{O}}\) = 2 x 18gH2O = 36gH2O
Question 14.
How many moles of methane are required to produce 22 g CO2 after combustion?
Solution:
The chemical reection for combustion of methane is:
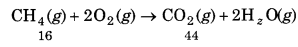
∵ 44g CO2 (g) is obtained from 16g CH4(g)
[1 mol CO2(g) is obtained from 1 mol of CH4(g)]
Number of moles of CO2(g)
= 22gCO2(g) x \(\frac{1 \mathrm{molCO}_2(g)}{44 \mathrm{~g} \mathrm{CO}_2(g)}\)
= 0.5 mol CO2(g)
∴ 0.5 mol CO2 (g) would be obtained from 0.5 mol CH4(g)
Hence, 0.5 moI of meihane is required to porduce 22g of CO2(g) after combustion.
Question 1.5.
50.0 kg of N2(g) and 10.0 kg of H2(g) are mixed to produce NH3(g). Celculate the amount of NH3 formed. Identify the limiting reagent in the production of NH3 in this reaction.
Solution:
A balanced equetion is
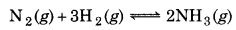
Number of moles of N2
= 5OkgN2 x \(\frac{1000 \mathrm{~g} \mathrm{~N}_2}{1 \mathrm{~kg} \mathrm{~N} \mathrm{~N}_2} \times \frac{1 \mathrm{~mol} \mathrm{~N} \mathrm{~N}_2}{28 \mathrm{~g} \mathrm{~N}_2}\)
= 1786 x 102 mol
Number of moles of H2
= 10kgH2 x \(\frac{1000 \mathrm{~g} \mathrm{H}_2}{1 \mathrm{~kg} \mathrm{H}_2} \times \frac{1 \mathrm{~mol} \mathrm{H}_2}{2.016 \mathrm{~g} \mathrm{H}_2}\)
= 496 x 103 mol
∵1 mol N2(g) requires 3 moles H2(g) for the reaction
∴ For 17.86 x 102 mol of N2,
No. of moles of H2(g)
= 1786 x 102molN2 x im
= 536 x 103 molH2
But we have only 4.96 x 103 mol H2. Therefore. dihydrogen is the limiting reagent in this case. Therefore, NH3(g) would be obtained only from that
amount of available dihydrogen i.e. 4.6 x 103 mol
∵ 3 mol H2 (g) gives 2 mol NH3 (g)
∴ 4.96 x 103 mol H2(g) will give
= 4.96 x 103 mol H2(g) x \(\frac{3 \mathrm{~mol} \mathrm{H}_2(g)}{1 \mathrm{~mol} \mathrm{~N}_2(g)}\)
= 3.30 x 103 mol NH3(g)
lmolNH3(g) = 17.OgNH3(g)
3.30 x 10 mol NH3(g) x \(\frac{17.0 \mathrm{~g} \mathrm{NH}_3(g)}{1 \mathrm{molNH}_3(g)}\)
= 3.30 x 103 x 17g NH3(g)
= 56.1 x 103g NH3(.g)
= 56.1 kg NH3(g)
Question 1.6.
A solution is prepared by adding 2 g of a substance ‘A’ to 18 g of water, calculate the mass percent of the solute.
SolutIon:
Mass of substance ‘A’ =2 g
Mass of water = 18g
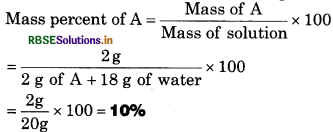

Question 1.7.
Calculate the molarity of NaOH in the solution prepared by dissolving its 4g in enough water to form 250 mL of the solution.
SolutIon:
Mass of NaOH = 4g
Molar mass of NaOH = 23 + 16 +1 =40
Volume of solution = 250 mL
Molarity = ?

Question 1.8.
The density of 3 M solution of NaCl is 1.25 g mL1. Calculete the molality of the solution.
Solution:
M = 3 mol L’
Molar mass of NaCI = 23 + 35.5 = 58.5
Mas8 of NaC1 in iL solution = 3 x 58.5 = 175.5 g
Mass of 1 L solution = 1000 x 125 = 1250 g
(∵Mass = volume x density)
Mass of water in solution = 1250 - 175.5
= 10745 g
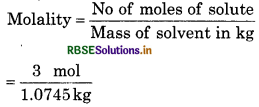
= 279 m
RBSE Class 11 Chemistry Some Basic Concepts of Chemistry Textbook Questions and Answers
Question 1.1.
Calculate the molar mass of the following:
(i) H2O
(ii) CO2
(iii) CH4
SolutIon:
(i) Molar mass of H2O
= 2 x (1.008u) + 16.00 u
= 2.016 u + 16.00 u
= 18.02 u
(ii) Molar mass of CO2
= 12.011 u + 2 x (16.00 u)
= 12.011 u + 32.00 u
= 44.O1u
(iii) Molar mass of CH4
=12.011 u + 4 x (1.008 u)
= 12.011 u + 4.032 u
= 16.0434 u

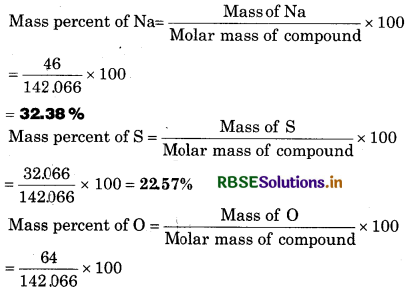
Question 1.2.
Calculate the mass percent of different elements present n sodium sulphate (Na2SO4).
Solution:
Molar mass of Na2SO4
= 2 x (23.00 u) + 32.066 u + 4 x (16.00 u)
= 46.00 u + 32.066 u + 6400u
= 142.066 u
Question 1.3.
Determine the empirical formula of an oxide of iron, vhich has 96.9% iron and 30.1% dioxygen mass.
Solution:
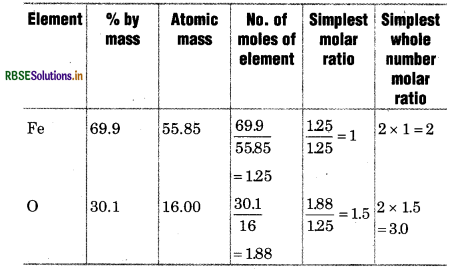
∴ Empirical formula of oxide of iron.
= Fe2O3.
Question 1.4.
Calculate the amount of carbon dioxide that could be produced when:
(1) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16g of dioxygen.
(iii) 2 molar of carbon are burnt in 16 g of dioxygen.
SolutIon:
Balanced equation is:
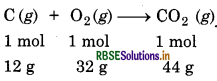
(i) From the above reaction
1 mol of carbon on burning in air produces
1 mol of CO2 i.e. 44g of CO2
(ii) When 1 mo1 of carbon is burnt in 16 g of dioxygen then 22g of CO will be produced. In this case, dioxygen
is a limiting reagent.
∵32 g of O2 gives 44 g of CO2
∵ 16 g of O2 will give = \(\frac{44}{32} \times 16\)
= 22 g of CO2
(iii) As dioxygen is a limiting reagent, therefore, 16 g of dioxygen can react only with 0.5 mol of carbon to produce
22 of CO2.

Question 1.5.
Calculete the mass of sodium acetate (CH3COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mo1-1.
Solution:
Volume of solution 500 mL
Molarity = 0.375 M
Molar mass of sodium acetate (M) = 82.0245 g mol-1
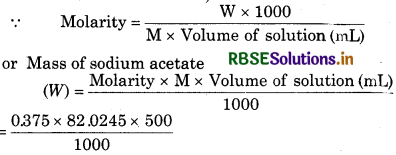
= 15.379 g
Question 1.6.
Calculate the concentration of nitric acid in moles per litre in a sample which has a density l.41g mU’ and the mass percent of nitric acid in it being 69%.
Solution:
Density of solution = 1.41 g mL-1
Mass % of HNO3 = 69%
69% of HNO3 means loo g of its solution contains 69 g HNO3
∴ Mass of solution =100 g
Mass (w) of HNO3 = 69 g
Moler mass (M) of HNO3 = L008 + 14.007 + 3 x 16.00
= 63.015g mol-1
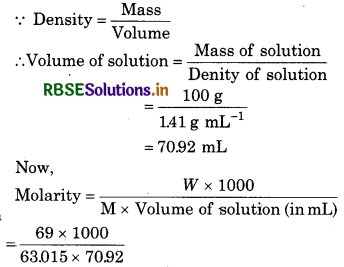
= 15.439 M
Question 1.7.
How much copper can be obtained from loo g of copper sulphate (CuSO4)?
Solution:
Mass of CuSO4 100 g
Molar mass of CuSO4 = 63.5 + 32 + 4 x 16
= 159.5g mol-1
1 mol of CuSO4 contains 1 mol of Cu.
∵159.5 g of CuSO4 forms = 63.5 g of Cu.
∵ 100 g of CuSO4 will form = \(\frac{63.5 \mathrm{~g}}{159.5 \mathrm{~g}}\)
= 39.81 g of Cu.

Question 1.8.
Determine the molecular formula of an oxide of iron, in which the mass percent of iron and oxygen are 69.9 and 30.1 respectively.
Solution:
∴ Empirical formula of oxide of iron = Fe2O3
Empirical formula mass = 2 x 55.85 + 3 x 16.00
= 159.1 g mol-1
\(=\frac{159.8}{159.7}=1\)
Molecular formula = n x Empirical formula
= n x Fe2O3
= 1 x Fe2O3
= Fe2O3
Question 1.9.
Calculate the atomið mass (average) of chlorine using the following data:
|
%Natural |
Abundence |
Molar mass |
|
35Cl |
75.77 |
34.9689 |
|
35Cl |
24.33 |
36.9659 |
Solution:
Fractional abundance òf 35Cl
\(=\frac{75.77}{100}\) = 0.7577
Molar mass of 35Cl = 34.9689
Molar mass Cl = 36.9659
∵ Average atomic mass of chlorine
= Fractional abundance òf 35Cl x Molar mass of 35C1 +
Fracttonal abundance of Cl x Molar mass Cl
= 0.7 577 x 34.9689 + 02423 x 36.9659
= 26.4959 + 8.9568
= 35.4527 amu
Question 1.10.
In three moles of ethane (C2H6), calculate the following:
(i) Number of moles of carbon atoms
(ii) Number of moles of hydrogen atoms
(iii) Number of molecules of ethane
Solution:
(i) ∵ 1 mol of ethane (C2 H6 )contain 2 mol of carbon atoms.
∴ 3 mol of C2H6 will contain = 2 x 3 = 6 mol of carbon atoms
(ii) ∵ 1 mol of ethane (C2 H6 )contains 6 mol of hydrogen atoms.
∴ 3 mol of C2H6 will contain = 6 x 3 = 18 mol of hydrogen atons.
(iii) ∵ 1 mol of ethane (C2 H6) contains 6.022 x 1023 molecules of ethane.
∴3 moles of C2H6 will contain
= 3 x 6.022 x 1023
= 18.066 x 1023 molecules
= 1.8066 x 10 molecules

Question 1.11.
What is the concentration of sugar (C12H22O11) in mol L-1 if its 20 g are dissolved in enough water to make a final volume up to 2L?
Solution:
Mass of C12H22O11(w) = 20 g
Volume of solution = 2L
Molar mass ofC12H22O11
M = 12 x 12.01 + 22 x 1.008 x 11 x 16.0O
= 34229
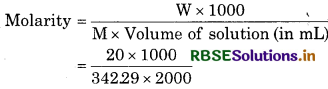
= 0.029 M
Question 1.12.
f the density of methanol is 0.793 kg L’, what is its volume needed for making 2.5 L
of its 0.25 M solution?
Solution: Denstiy of methanol = 0.793 kg L’
= 0.793 X 103g L-1
Molar mass of methanol (CH3OH)
= 1 x 12.01 + 4 x 1.008 + 16.00
= 32.042
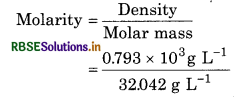
= 24.749 molL1
∴ Initial molarity (M1) = 24.749 M
Initial volume (V1) = ?
Final molarity (M2) = 025 M
Final volume (V2) = 2.5 L
∵ M1V1 = M2V2
24.749 x V1 = 0.25 x 2.5
\(V_1=\frac{0.25 \times 2.5}{24.749}\)
= 25.25 ML
Question 1.13.
Pressure is determined as force per unit area of the surface. The SI unit of pressure, pascal is as shown below:
1Pa = lNm-2
If mass of air at sea level is 1034 g cm2, calculate the pressure in pascal.
Solution:
Mass of air at sea level =1034 g cm-2
As we know that,
Acceleration due to gravity
g = 9.8 ms-2
Pressure of air = \(\frac{1034}{1000}\) x 9.8 x 100 x 100
= 1.012322 x 105 pa
Question 1.14.
What is the SI unit of mass? How is it defined?
Solution:
The S.J unit of mass is kilogram (kg). It is defined as the amount of matter present in a substance.

Question 1.15.
Match the following prefixes with their multiples:
|
Prefixes |
Multiples |
|
Micro |
106 |
|
deca |
109 |
|
mega |
10-6 |
|
giga |
10-15 |
|
femio |
10 |
Solution:
|
Prefixes |
Multiples |
|
Micro |
106 |
|
deca |
109 |
|
mega |
10-6 |
|
giga |
10-15 |
|
femio |
10 |
Question 1.16
What do you mean by significant figures?
Solution:
The total number of digits in a number, which are meaningful with certainty are called significant figures. e.g 3.016 has four significant figures.
Question 1.17.
A sample of drinking water was found to be severely contaminated with chloroform, CHCl3, supposed to be carcinogenic in nature.The level of contamination was 15 ppm (by mass).
(i) Express this in percent by mass.
(ii) Determine the molality of chloroform in the water sample.
Solution:
(i) 15 ppm means 15 parts in 106 parts
Mass % = \(\frac{15 \times 100}{10^6}\) = 1.5 x 103%
(ii) Molar mass of CHCl3 (M)
= 12.01 + 1.008 + 3 x 35.5
= 119.36gmol-1
1.5 x 10% means 1.5 x 103g CHCl3 is found in loo g sample.
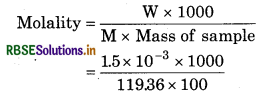
= 1.26 x 104m
Question 1.18.
Express the following in the scientific notation:
1. 0.0048
2. 234,000
3. 80088
4. 500.0
5. 6.0012
Solution:
- 0.0048 = 48 × 10-3
- 234,000 = 234 x 105
- 80088 = 008 x 103
- 5000 = 500 x 102
- 6.0012 = 6.0012
Question 1.19.
How many significant figures are present in the following?
|
(i) 0.0025 |
|
(ii) 208 |
|
(iii) 5005 |
|
(iv) 126,000 |
|
(v) 500.0 |
|
(vi) 2.0034 |
Solution:
|
Number |
Significant figures |
|
(i) 0.0025 |
2 |
|
(ii) 208 |
3 |
|
(iii) 5005 |
4 |
|
(iv) 126,000 |
3 |
|
(v) 500.0 |
4 |
|
(vi) 2.0034 |
5 |

Question 1.20.
Round up the following upto three significant figures:
1. 34.216
2. 10.4107
3. 0.04597
4. 2808
Solution:
- 34.216 = 34.2
- 10.4107 = 10.4
- 0.04597 0.0460
- 2808 = 2810 or 2810 x 103
Question 1.21.
The following data are obtained when dinitrogen and dioxygen react together to form different compounds:
|
Mass of dioxygen |
Mass of dinitrogen |
|
(i) 14 g |
16g |
|
(ii) 14 g |
32g |
|
(iii) 28 g |
32g |
|
(iv) 28 g |
80g |
(a) Which law of chemical combination is obeyed by the above experimental data?
(b) Fill in the blanks in the following conversions:
(i) 1 km = ................ mm = ............... pm
(ii) 1mg = ................ kg ............... ng
(iii) 1 mL.=................ L = ................ dm3
Solution:
|
Mass of dioxygen |
Mass of dinitrogen |
|
(i) 14 g |
16g |
|
(ii) 14 g |
32g |
|
(iii) 28 g |
32g |
|
(iv) 28 g |
80g |
The above experimental data obey law of multiple proprotions.
According to this law," when two elements combine to form more than one compound, then the masses of one of the elements which combine with fixed mass of other element are in the simple whole number ratio,"
(b) (i) 1 km = 10 mm = 1015 pm
(ii) 1 mg = 10 kg = 106 ng
(iii) 1 ml = 10-3L= 10-3 dm3
Question 1.22.
If the speed of light is 3.0 x 10 ms1, calculate the distance covered by light in 2.00 ns.
Solution:
Speed of light = 3.0 x 10 ms-1
Time = 2.00 ns
= 2 × 10 9 8
∵ Distance = speed x time
= 30 x 10 ms 1 x 2 x 10-9 s
= 60 × 10-1 m
= 0.60 m
Question 1.23.
In a reaction,
A + B2 → AB2
Identify the limiting reagent, if any, in the following reaction mixtures:
(i) 300 atoms of A + 200 molecules of B
(ii) 2 mol A + 3 mol B
(iii) 100 atoms of A + 100 molecules of B
(iv) 5 mol A + 2.5 mol B
(v) 2.5 mol A + 5 mol B
Solution:
A + B2 → AB2
(i) In above rection,
∵1 atom of A reacts with 1 molecule of B.
∴200 atoms of A will react with 200 moleculers of B. Hence, in this case, 'B' is the limiting reagent, as 'A' is in excess.
(ii) In above reaction,
∵1 mol of A reacts with 1 mol of B
∴2 mol of A will react with 2 mol of B.
Hence, in this case, 'A' is the limiting reagent, as 'B' is in. excess.
(iii) In above reaction,
∵1 atom of A reacts with 1 molecule of B.
∴100 atoms of A will react with 100 molecules of B. Hence, in this case there is no limiting reagent.
(iv) In above reaction,
∵1 mol of 'A' reacts with 1 mol of B
∴2.5 mol of 'A' will react with 2.5 mol of B.
Hence, in this case, 'B' is limiting reagent, as 'A' is in excess.
(v) In above reaction,
∵1 mol of 'A' reacts with 1 mol of 'B'.
∴2.5 mol of 'A' will reacts with 2.5 mol of 'B'.
Hence, In this case, 'A' is limiting reagent, as, 'B' is in excess.

Question 1.24.
Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation:
Ng (g) + H2(g) → 2 NH3 (g)
(i) Calculate the mass of ammonia produced if 2.00 10 g dinitrogen reacts with 1.00 x 103 g of dihydrogen.
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
Solution:
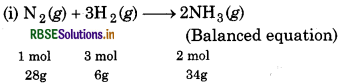
∵ 28g of N2 react with 6g of Hg
∴ 1g of N reacts with g of H 2
∴ 2000g of Ng will react with = \(\frac{6}{28} \times 2000\)
= 428.57 g of H 2
Since H2 is in excess, so, N2 is the limiting reagent. N2 limits the amount of ammonia produced.
∵ 28g of N2 produces 34g of NH3.
∴ 2000g of N2 will produce = \(\frac{34}{28}\) x 2000
= 2428.57 g of NH3
(ii) Hydrogen (H2) will remain unreacted because it is in excess.
(iii) Amount of H2 remain unreacted
= 1000 - 428.57 = 571.43 g
Question 1.25.
How are 0.50 mol Na2CO3 and 0.50 M Na2CO3 different?
Solution:
Molar mass of Na2CO3 = 2 x 23 + 12 × 3 × 16 = 106 g mol-1
1 mol of Na2CO3 means 106 g of Na2CO3
0.50 mol of Na2CO3 means = 0.50 x 106 g
= 53 g of Na2CO3
0.50 M Na2CO3 means 0.50 mol i.e., 53g of Na2CO3 are present in 1 litre of the solution.
Question 1.26.
If 10 volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour would be produced?
Solution:
The balanced equation is,
2H2(g) + O2(g) → 2H2O(g)
Hence, the above equation shows that 10 volumes of water vapour[ (H2O(g)] will be produced.
Question 1.27.
Convert the following into basic units:
(i) 28.7 pm
(ii) 15.15 pm
(iii) 25365 mg 1pm = 10-12 m
Solution:
(i) ∵ 1 pm = 10-12 m
∴ 28.7 pm = 287 x 10-12 m
(ii) 15.15 pm = 15.15 x 10-12 m
= 15.15 x 10-11 m
(iii) ∵ 1mg = 10-6 Kg
∴ 25365 mg = 253265 x 10-6 kg
= 2.5365 x 10-2 kg
Question 1.28.
Which one of the following will have the largest number of atoms?
(i) 1g Au(s)
(ii) 1g Na(s)
(iii) 1g Li(s)
(iv) 1g of Cl2(g)
Solution:
(i) Atomic mass of Au = 197
∴ Number of atoms in 1 g Au(s) = \(\frac{1}{197}\) x 6.022 × 1028
= 3.057 x 1021 atoms
(ii) Atomic mass of Na = 23
∴ Number of atoms in 1g Na(s) = \(\frac{1}{23}\) x 6.022 x 1023
= 2.618 x 1022 atoms
(iii) Atomic mass of Li = 7
∴ Number of atoms in 1 g Li(s)
= \(\frac{1}{7}\) x 6022 × 1023
= 8.6042 × 1022 atoms
(iv) Molar mass of Cl2 = 2 x 35.5 = 71 Number of atoms in 1g of Cl2 (g)
= \(\frac{1}{71}\) × 6022 × 1023
= 8.48 x 1021 atoms
Therefore, 1g of Li(s) will have the largest number of atoms.

Question 1.29.
Calculate the molarity of a solution of ethanol in water, in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
Solution:
Molarity means number of moles of solute in one litre of solution.
1L of ethanol = 1 L of water
Number of moles of H2O in 1 litre water
= \(\frac{1000 \mathrm{~g}}{18}\)
= 55.55 mol
For a binary solution
X1 + X2 = 1
Хн2о + Хс2н5он = 1
∴Хн2O = 1 - Хс2н5он
= 1 - 0.040
= 0.96
\(\begin{aligned} X_{\mathrm{H}_2 \mathrm{O}} & =\frac{n_{\mathrm{H}_2 \mathrm{O}}}{n_{\mathrm{H}_2 \mathrm{O}}+n_{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}} \\ 0.96 & =\frac{55.55}{55.55+n_{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}} \end{aligned}\)
or 53.328 + 0.96 nc2H2OH = 55.55
or
0.96 nc2H2OH = 55.55 - 53.328
= 2.222
\(n_{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}=\frac{2.222}{0.96}\)
= 2.3145
Molarity = 2.3145 mol L-1
Question 1.30.
What will be the mass of one 12C atom in g?
Solution :
1 mole of 12 C atoms = 6.022 x 1023
= 12 g of carbon
∵ 6022 x 1023 atoms of 12 C have mass = 12 g
∴ 1 atom of 12 C will have mass
= \(\frac{12}{6.022 \times 10^{23}} g\)
= 1.9927 x 10-23 g atoms

Question 1.31.
How many significant figures should be present in the answer of the following calculation?
\(\text { (i) } \frac{0.02856 \times 298.15 \times 0.112}{0.5785}\)
(ii) 5 x 5.364
(iii) 0.0125 + 0.7864 + 0.0215
Solution:

= 1648511
= 1.65
It is due to the fact that 0.112 has only three significant figures, so, final result should have three significant figures.
(ii) 5 × 5364 = 26.82
26.82 should have 4 significant figures.
(iii) 0.0125 + 0.7864 + 00215
= 0.8204
= 0.820
It is due to the fact that 0.0215 or 0.0125 has three significant figures, so, final result should have only three significant figures.
Question 1.32.
Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
|
Istope |
Isotopic Molar mass |
Abudance |
|
36Ar |
35.96755 g mol-1 |
0.377% |
|
38Ar |
37.96272 g mol-1 |
0.063% |
|
40Ar |
39.9642 g mol-1 |
99.600% |
Solution:
Average molar mass of argon
= (0,00337 x 3596755) + (0.00063)
= 37.96272) + (0.9960 × 39.9624)
= 0.121 + 0.024 + 39.803
= 39.948 g mol-1
Question 1.33.
Calculate the number of atoms in each of the following:
(i) 52 moles of Ar
(ii) 52 u of He
(iii) 52 g of He
Solution:
(i) ∵ 1 mol of Ar contains 6.022 x 1023 atoms
∴ 52 mol of Ar will contain = 52 x 6.022 × 1023 atoms
= 313.144 x 1023 atoms
= 3.1314 x 1025 atoms
(ii) 4 u of He contains 1 atom of He
∴52 u of He will contain = x 52 atoms = 13 atoms
(iii) 1 mol of He = 4 g of He = 6.022 x 1023 atoms
= \(\frac{6.022 \times 10^{23}}{4} \times 52 \text { atoms }\)
= 7.8286 x 1024 atoms

Question 1.34.
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Calculate. (i) empirical formula, (ii) molar mass of the gas and (iii) molecular formula. C + O2 → CO2
Solution:
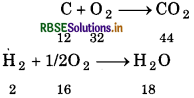
(i) ∵44g of CO2 contains 12g of carbon:
∴ 3.38 g of CO2 will contain
= \(\frac{12}{44}\) x 3.38 g
= 0.9218 g of Carbon
∵ 18g of H2O contains 2g of hydrogen
∴ 0.690 g of H2O will contain
= \(\frac{2}{18}\) x 0.690 g
= 0.0767 g of hydrogen
Total mass of compound = 0.9218 + 0.0767
= 0.9985 g
% of C = \(\frac{0.9218}{0.9985}\) x 100
= 92.318
= 92.32%
% of H = \(\frac{0.0767}{0.9985}\) x 100 = 7.68%
∴ Empirical formula of compound = CH
(ii) ∵ 10.0 L of the given gas weigh 11.6 g at STP
∴ 22.4 L of the given gas will weigh
\(=\frac{11.6 \times 22.4}{10}\)
= 25984 g at STP
∴ Molar mass = 25984 g mol-1 = 26 g mol-1
(iii) Empirical formula mass of CH = 12 + 1 = 13
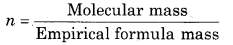
= \(\frac{26}{13}\) = 2
∴ Molecular formula = n x Empirical formula
= 2 × CH
= C2H2
Question 1.35.
Calcium carbonate reacts with aqueous HCl to give CaCl, and CO2 according to the reaction,
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
Solution:
Volume of HCl = 25 mL
Molarity of HCl = 0.75 M
Molecular mass of HCl (m) = 1 + 35.5 = 36.5 g mol1
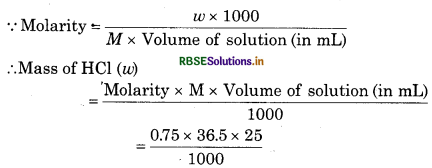
= 0.6844 g
For the reaction,
CaCO3(s) + 2HCl (aq) → CaCl2 (aq) + CO2(g) + H2O(l)
100 g 2 x 36.5 = 73 g
According to balanced chemical equation,
∵ 73 g HCl reacts completely with 100 g of CaCO3
∴ 0.6844 g HCl will react with
= \(\frac{100}{73}\) x 0.6844 = 0.9375 g

Question 1.36.
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction
4HCl(aq) + MnO2 (s) → 2H2O(l) + MnCl2 (aq) + Cl2(g)
How many grams of HCl reacts with 5.0 g of manganese dioxide ?
Solution:
For the reaction,
4HCl(aq) + MnO 2 (s) → 2H2O(l) + MnCl2(aq) + Cl2(g)
= 146.0 g
According to above balanced chemical equation, 87g of MnO2 reacts with 146 g of HCl
∴5.0 g of MnO2 will react with
= \(\frac{146}{87} \times 5\)
= 8.39 g of HCI

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter