RBSE Class 11 Chemistry Notes Chapter 7 Equilibrium
These comprehensive RBSE Class 11 Chemistry Notes Chapter 7 Equilibrium will give a brief overview of all the concepts.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Chapter 7 Notes Equilibrium
→ Equilibrium: Equilibrium is the particular state of a process after which there is no any physical or chemical change occurs with the passage of time.
→ Equilibrium Mixture: Mixture of reactants and products at equilibrium is called equilibrium mixture.
→ Standard Melting Point or Standard Freezing Point: At the melting point, the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard pressure.
→ Boiling Point: The boiling point is the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure of the liquid and the liquid is converted to vapour.
→ Saturated Solution: A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent. Additional solute will not dissolve in a saturated solution.
→ Henry’s Law: “At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
→ Chemical Equilibrium: Chemical equilibrium is the point at which the concentrations of reactants and products do not change with time.

→ Law of Mass Action: According to Law of Mass Action, at constant temperature, the rate of a chemical reaction is directly proportional to the active mass of the reactant present at,that time. If more than one reactant takes part in the reaction then the rate of the reaction is proportional to the product of the active masses of the reactants.
→ Rate of Reaction: The rate of reaction can be defined as the change in the concentration of any one of the reactants or products per unit time.
→ Active Mass: Active mass or ionic concentration is the amount of substance in gram moles which is converted to products in unit time.
→ Homogeneous Equilibrium: When the reactants and products are present in same phase in a reaction at equilibrium; such type of equilibrium is called as homogeneous equilibrium.
→ Heterogeneous Equilibrium: When the reactants and products are not present in same phase in a reaction at equilibrium; such type of equilibrium is called heterogeneous equilibriums
→ Equilibrium Constant: Equilibrium constant has constant value at a fixed temperature and at this stage all the macroscopic properties such as concentration, pressure, etc. become constant. For a gaseous reaction, equilibrium constant is expressed as Kp and is written by replacing concentration terms by partial pressures in Kc expression.
→ The value of equilibrium constant

- • The factors which affect equilibrium constant are (i) Temperature (ii) Stoichiometry (iii) Method to express equilibrium constant.
- • Kp = Kc(RT)Δn
- Δn = 0, then Kp = Kc
- Δn = + ve, then Kp > Kc
- Δn = - ve, then Kp < Kc
→ Le-Chatelier’s Principle: Le-Chatelier’s principle states that the change in any factor such as temperature, pressure, concentration, etc. will cause the equilibrium to shift in such a direction so as to reduce or counteract the effect of the change. It can be used to study the effect of various factors such as temperature, concentration, pressure, catalyst and inert gases on the direction of equilibrium and to control the yield of products by controlling these factors.
→ Electrolytes: All substances that conduct electricity in aqheous solutions are called electrolytes.
Acids, bases and salts are electrolytes and the conduction of electricity by their aqueous solutions is due to anions and cations produced by the dissociation or ionization of electrolytes in aqueous solution.

→ Strong electrolytes: The strong electrolytes are completely dissociated.
→ Weak electrolytes: In weak electrolytes, there is equilibrium between the ions and the unionized electrolyte molecules.
→ Non-electrolytes: Non-electrolyte is a compound whose melts or aqueous solutions do not conduct electricity. Example: - Aqueous solution of sugar, urea, etc.
→ Degree of Ionization: The degree of dissociation (sometimes also degree of ionization), is a way of representing the strength of an acid. It is defined as the ratio of the number of ionized molecules and the number of molecules dissolved in water.
→ Arrhenius Concept: According to Arrhenius, acids give hydrogen ions while bases give hydroxyl ions in their aqueous solutions.
→ Bronsted-Lowry Concept: Bronsted-Lowry defined an acid as a proton donor and a base as a proton acceptor. When a Bronsted-Lowry acid reacts with a base, it produces its conjugate base and a conjugate acid corresponding to the base with which it reacts. Thus a conjugate pair of acid-base differs only by one proton.
→ Lewis Concept: Lewis further generalised the definition of an acid as an electron pair acceptor and a base as an electron pair donor.
→ Protogenic: Protogenic compounds are those which give proton in non aqueous media.All acids are protogenic. Examples of protogenic acids: NH4,
H30+ , ROH+ , RNH3, H2O, NH3, HCl, H2S04, OH- etc.
→ Proton Loving Solvent: All compounds which accept protons are proton loving solvents. All bases are proton loving. Examples are: PO43-, Cl-, HSO4 ROH, ROR, RNH2 etc.
→ Amphiprotic: Amphiprotic describes a substance that can both accept and donate a proton or H + . An amphiprotic molecule has characteristics of both an acid and a base and can act as either.

→ Aprotic: In general terms, any solvent that contains a labile H+ is called aprotic solvent. The molecules of such solvents readily donate protons (H+) to reagents. Conversely, aprotic solvents cannot donate hydrogen. Example, BF3, ZnCl2, SO2 etc.
→ Ampholyte: Ampholyte is a substance that can act as either an acid, in the presence of a strong base, or a base, when in the presence of a strong acid. It contains both —COOH and —NH2 group. Example, NH2 —CH2 —COOH.
→ The expressions for ionization (equilibrium) constants of weak acids (Ka) and weak bases (Kb) are developed using Arrhenius definition. The degree of ionization and its dependence on concentration and common ion are discussed.
→ The pH scale (pH = -log[H+]) for the hydrogen ion concentration (activity) has been introduced and extended to other quantities (pOH = - log[OH-]); pKa = - log[Ka]; pKb = log[Kb] and pKw = - log[Kw] etc). The ionization of water has been considered and we note that the equation: pH + pOH = pKw is always satisfied.
→ The salts of strong acid and weak base, weak acid and strong base, and weak acid and weak base undergo hydrolysis in aqueous solution.
→ Buffer Solution: The solution which resist a change in pH on addition of small amount of acid or base is called buffer solution. Acidic buffer is made of strong acid and weak base (CH3COOH + CH3COONa) whereas basic buffer is made of weak acid and strong base (NH4 OH + NH4 Cl).
→ Generally, phenolphthalein indicator is used for acid-base titrations.
→ The degree of dissociation of an electrolyte (weak) is suppressed by the addition of another electrolyte (strong) containing a common ion. This is termed as common ion effect. It is used in qualitative analysis.
→ Some Important Facts and Formulae
- Equilibrium: The condition of any process in which there is no change in proportion.
- Both physical and chemical equilibrium are dynamic in nature.
- In a reversible process, equilibrium can be established in any direction.
- There is no effect of catalyst on equilibrium in a reversible process.
- Equilibrium can be established in a closed container only because in a closed container, reactants and products cannot go out and equilibrium can be obtained.
→ According to Law of mass action,
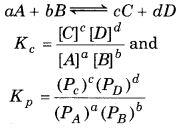
→ Relation between Kp and Kc.
Kp = Kc(RT)Δn
According to Ostwald dilution Law,
α = \(\sqrt{\frac{k}{C}}\)
For weak acids [H+] = \(\sqrt{K_a C}\)
For weak bases [OH-] = \(\sqrt{K_b C}\)
Ionic product of water Kw = [H3O+] [OH-]
= 10-14 (at 291 K)
pH = - log[H+]
Henderson’s equation for pH
pH = pKa + log\(\frac{\text { [salt] }}{\text { [acid] }}\) [For acidic buffer]
pH = pKb + log\(\frac{\text { [salt] }}{\text { [base] }}\) [For basic buffer]
For salts of strong acid and weak base.
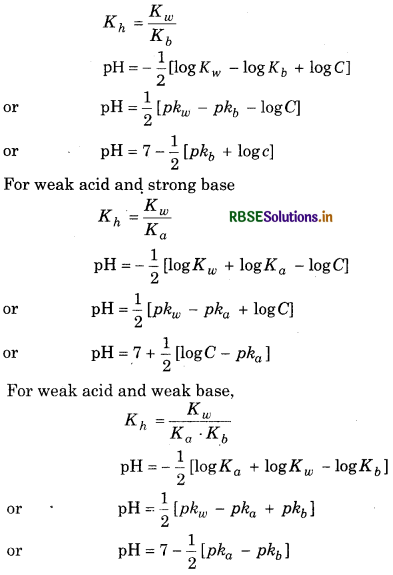
→ The relation between solubility and solubility product for monovalent electrolytes such as AgCl, AgBr, Agl, AgNO3, NaBr, Nal etc.
Ksp = s2 (mol2L-2)
or s = \(\sqrt{K_{s p}}\)
→ The relation between solubility and solubility product of divalent electrolytes each as CdS, MnS, CaCO3, BaCO3, MgCO3 etc.
Ksp = s2 [mol2L-2]
or s = \(\sqrt{K_{s p}}\)

→ If ionic product < Ksp, salt will be soluble.
→ If ionic product > Ksp, salt will be precipitated.
- For neutral solution pH = 7
- For basic solution pH > 7
- for acidic solution pH < 7
→ Ka × Kb = Kw
pKa + pKb = pkw = 14
For neutral solution, (H3O+] =[OH-] and [H3O+] = 1CT7 M.
For acidic solution, [H3O+] >[OH-] and [H3O+] > 10-7 M.
For basic solutions, [H3O+] < [OH-] and [H3O+] <10-7 M.
ΔG = -2.303 RT log K ,
K = e ΔG°/RT
K = 10-ΔG °/2-303RT
ln K = TΔH°/RT

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter