RBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons
These comprehensive RBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons will give a brief overview of all the concepts.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Chapter 13 Notes Hydrocarbons
→ Hydrocarbons: Hydrocarbons are the compounds of carbon and hydrogen only.
→ Paraffins: saturated hydrocarbons are inactive in general state because they can not react with acid and other reagent. So they are called paraffins.
→ Natural Gases: Mixture of gases on the surface of petroleum.
Example: Mixture of methane, ethane, propane, butane etc. It is called natural gase.
→ isomerism in Alkanes: Alkanes show the chain and position isomerism.
→ ‘Boiling point of Unbranched and Branched Alkanes: Alkanes have the high value of boiling point than isomers of branched alkanes because shape of molecule of branched alkanes are symmetric that why surface area is less and ' intermolecular forces are weaker. So boiling point of branched alkanes are less.

→ Alkanes: Due to saturated nature they shows the substitution reactions which are completed by free radical mechanism. Examples: halogenation, sulphonation, nitration, alkylation etc.
→ Alkanes show the substitution and other reaction like combustion, oxidation, thermo reduction, isomerisation, aromatization etc.
→ Pyrolysis or Cracking: Higher alkanes are heated on high temperature. It decomposes into lower alkanes and alkenes. This process is called pyrolysis or cracking.
→ Conformation: Spatial arrangements of atoms which can be converted into one another by rotation around a C—C single bond are called conformations. They are formed by free rotation of C—C single bond. In ethane one case is eclipsed conformation and staggered conformation.
→ Hydrocarbons which contains C = C are called alkenes. These are also called olefins.
→ In alkenes C—C bond is σ-bond and sp2 -hybridised.
→ In alkene isomers increasing order of stability depands upon increasing order of branching. In cis and trans form, trans form is more stable.
→ Increase of branching in alkenes increase the stability order is due to hyperconjugation, n -orbitals in double bond and a-bonds are hybridised due to resonance.
→ Alkenes shows the addition, substitution, oxidation polymerisation and isomerisation reactions.
→ Electrophilic Addition Reactions: In this type of reactions alkenes are added with H2, X2, HX
→ In Electrophilic Addition Reactions In electrophilic addition reactions first electrophilic attacks on > C = C < double bond and forms intermediate. After this nucleophile attacks and forms products.
→ Markownikoffs Rule: The addition occurs through polar mechanism when polar molecules are added with unsymmetrical alkene. So in polar molecule the electropositive part is attached with that carbon which is less reactive.
→ Peroxide or Karasch Effect: Addition of alkenes in presence of peroxide is completed due to free radical mechanism and its product is against Markownikoff rule.
→ Hydrogenation: Additions of Alkenes and alkyne with H2 in presence of catalyst Pt, Pd and Ni and form alkanes is called hydrogenation.
→ Decarboxylation: Sodium salts of carboxylic acid are heated with sodalime (NaOH + CaO) alkanes are formed. C02 gas is evolved from carboxylic acid. This process is called decarboxylation.
→ Alkynes: Hydrocarbons which contains C ≡ C are called alkynes.
→ Structure of Alkynes: Every carbon is sp -hybridised and forms one σ-bond and two π-bond.
→ Acidic Character of Acetylene: Hydrogen or alkynes are acidic in nature because sp hybridised carbon atom has high electronegativity and attract the e- of bond and easily donate it.
→ Order of acidic character due to hybridised carbon atoms is as follows:
sp > sp2 > sp3
HC ≡ CH > CH2 =CH2 > CH3 —CH3

→ Aromaticity of Benzene: In benzene addition of 6 carbon and 6 hydrogen 1 - 1 hydrogen is left on each carbon. That why 6 e group in a ring is called aromatic character. Due to this benzene show aromaticity.
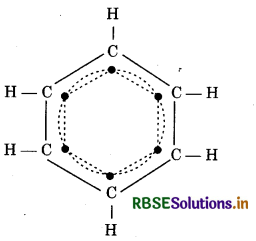
→ Huckel’s Rule: Cyclic and planar compounds which have (An + 2) e in ring show the aromatic character.
→ Arenes show the electrophillic substitution reaction. Example: halogenation, nitration, sulphonation, friedel craft reaction etc.
→ Carcinogenecity and Toxicity: That aromatic compounds which have more than two benzene rings are toxic in nature. These compounds are formed by incomplete combustion of coal and petroleum which enter the human body due to many biochemical reactions.
→ Some Important Facts
- Alkyne reduced into alkenes by Lindlar’s catalyst.
- Alkane of even number is not formed in high quantity by Wurtz reaction.
- Alkene show the geometrical isomerism.
- In geometrical isomerism cis and trans isomers are present.
- Br2 which is dissolved in CCl4 is used in test of unsaturation.
- Bayer’s reagent gives the unsaturation test.
- Alkenes take part in electrophilic addition reaction.
- Alkynes give both electrophilic and nucleophilic types of reactions.
- By ozonolysis position of double bond can be determined in alkenes.
- Terminal alkynes are weakly acidic in nature.
- Arenes take part in electrophilic substitution reactions.
- Ortho and para directing groups are as follow:
—OH, —NH2, —Cl, —Br, —OR, —R etc. - Meta directive groups are:
—CHO, —COOH, —NO2, —C = N, —SO3H etc.

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter