RBSE Class 11 Chemistry Notes Chapter 12 Organic Chemistry – Some Basic Principles and Techniques
These comprehensive RBSE Class 11 Chemistry Notes Chapter 12 Organic Chemistry – Some Basic Principles and Techniques will give a brief overview of all the concepts.
RBSE Class 11 Chemistry Chapter 12 Notes Organic Chemistry – Some Basic Principles and Techniques
→ Vital Force Theory: According to this theory, organic compounds are formed by vital forte.
→ Organic Chemistry: The branch of Chemistry which deals with the study of organic compounds is called Organic Chemistry.
→ Kekule’s Principle: According to Kekule’s principle, carbon is tetravalent. Carbon combines with other carbon atoms to form open and closed ' chain structures. It can form triple bond.
→ Van’t Hoff Le Bel’s Principle: According to Van’t Hoff Le Bel’s, four valencies of carbon are directed towards four corners of tetrahedral.
→ In carbon, sp3 -, sp2 - and s/>hybridisation is formed. Most common examples are methane, ethene and ethyne respectively.
→ Qualitative analysis of organic compounds is generally done by Lassaigne’s method.

→ In quantitative analysis, carbon and hydrogen are estimated by Liebig method, Nitrogen is estimated by Duma’s or Kjeldahl’s method and halogens by Carius method. Sulphur and phosphorus are estimated by oxidising them to sulphuric and phosphoric acids respectively.
→ Organic compounds are classified on the basis of their structure and functional groups.
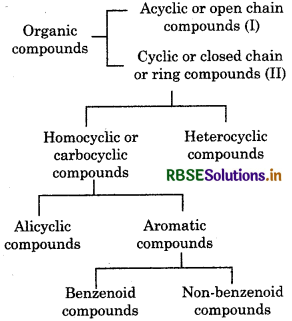
→ Homologous Series: In a homologous series, successive members differ from each other by -CH2 and have same functional group.
→ Functional Group: The functional group may be defined as an atom or group of atoms joined in a specific manner which is responsible for the characteristic chemical properties of the organic compounds.
→ Covalent bond may be cleaved in heterolytic or homolytic fashion. A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate.
→ The inductive, resonance, electromeric and hyperconjugation effects may help in the polarisation of a bond making certain carbon atom or other atom positions as places of low or high electron densities.
→ Inductive Effect: The polarization of a σ-bond due to electron withdrawing or electron donating effect of adjacent groups or atoms is called inductive effect. When a covalent bond is formed between atoms of different electronegativity, the electron density is more towards the more electronegative atom of the bond. Such a shift of electron density results in a polar covalent bond. Bond polarity leads to various electronic effects in organic compounds.
→ Electromeric Effect: It is a temporary effect. The organic compounds having a multiple bond (a double or triple bond) show this effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of n-electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent. The effect is annulled as soon as the attacking reagent is removed from the domain of the reaction. It is represented by E and the shifting of the electrons is shown by a curved arrow (↷).
→ Hyperconjugation or No-bond Resonance:
The delocalization of cs-electrons or lone pair of electrons into adjacent n-orbital or p-orbital is called hyperconjugation. It occurs due to overlapping of a-bonding orbital or the orbital containing a lone pair with adjacent Tt-orbital or p-orbital. It is also known as “no bond resonance” or “Baker-Nathan effect”.

→ Resonance: When one Lewis structure is unable to describe completely the bonding that takes place between atoms in a molecule, resonance structures are used.
→ Resonance Effect: The electron-withdrawing or releasing effect attributed to a substituent through delocalization of p or π-electrons, which can be visualized by drawing various canonical forms, is known as mesomeric effect or resonance effect. It is symbolized by M or R.
→ Free Radicals: Free radicals are chemical species that contain a singly occupied orbital. They are neutral and tend to be highly reactive. These are formed by homolytic cleavage of covalent bonds. These are neutral species.
→ Carbonium Ions: A species having a carbon atom possessing sextet of electrons and a positive charge is called a carbocation (earlier called carbonium ion). The CH3+ ion is known as a methyl cation or methyl carbonium ion. Carbocations are classified as primary, secondary or tertiary depending on whether one, two or three carbons are directly attached to the positively charged carbon.
→ Carbanion: The heterolytic cleavage can also give a species in which carbon gets the shared pair of electrons. For example, when group Z attached to the carbon leaves without electron pair, the methyl anion is formed. Such a carbon species carrying a negative charge on carbon atom is called carbanion. Carbanions are also unstable and reactive species. It has eight electrons in outermost shell.
→ Carbene: A carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. These are very reactive and converted to products rapidly after the formation.
→ Nitrene: A nitrene (R-N:) is the nitrogen analogue of a carbene. The nitrogen atom has only 5 valence electrons and is therefore considered an electrophile. A nitrene is a reactive intermediate and is involved in many chemical reactions.
→ Reagent and Substrate: A substrate is typically the chemical species being observed in a chemical reaction, which is organic in nature and reacts with a reagent to generate a product.
→ Electrophile: Positively charged or neutral species, which are deficient of electrons and can accept a pair of electrons, are called electrophiles. These are also called electron loving (philic) species or electron seeking.
→ Nucleophile: A nucleophile is a reagent containing an atom having unshared or lone pair of electrons. As a nucleophile is electron rich it seeks electron deficient sites i.e., nucleus (nucleus loving). According to Lewis concept of acids and bases, nucleophiles behave as Lewis bases.
→ Types of Organic Reaction: Organic reactions can be broadly classified into following types; substitution, addition, elimination and rearrangement reactions.

→ Addition Reactions: Additions are
characteristic of compounds with multiple bonds. Ethene, for example, .reacts with bromine by an addition. In an addition all parts of the attacking reagent appear in the product; two molecules appearance one.
→ Displacement or Substitution Reactions:
Substitutions are the characteristic reactions of saturated compounds such as alkanes and alkyl halides and of aromatic compounds (even though they are unsaturated). In a substitution, one group replaces another.
→ Elimination Reaction: Eliminations are the opposite of additions. In an elimination one molecule loses the elements of another small molecule. Elimination reactions give us a method for preparing compounds with double and triple bonds.
→ Rearrangement Reactions: In a rearrangement reaction a molecule undergoes a reorganization of its constituent parts.
