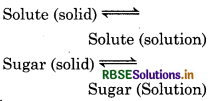RBSE Class 11 Chemistry Important Questions Chapter 7 Equilibrium
Rajasthan Board RBSE Class 11 Chemistry Important Questions Chapter 7 Equilibrium Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Chapter 7 Important Questions Equilibrium
Multiple Choice Questions:
Question 1.
A reversible reaction is one which:
(a) proceeds in one direction
(b) proceeds in both directions
(c) proceeds spontaneously
(d) all the statements are wrong
Answer:
(b) proceeds in both directions

Question 2.
In any chemical reaction, equilibrium is supposed to be established when:
(a) Mutual opposite reactions undergo
(b) Concentration of reactant and resulting products are equal
(c) Rate of mutual reactions become equal
(d) The temperature of mutual opposite reactions become equal
Answer:
(c) Rate of mutual reactions become equal
Question 3.
When the rate of forward reaction becomes equal to the backward reaction, this state is termed as:
(a) Chemical equilibrium
(b) Reversible state
(c) Equilibrium
(d) All of these
Answer:
(a) Chemical equilibrium
Question 4.
In the chemical reaction \(\mathrm{N}_2+3 \mathrm{H}_2 \rightleftharpoons 2 \mathrm{NH}_3\) at equilibrium point, state whether:
(a) Equal volumes of N2 and H2 are reacting
(b) Equal masses of N, and H2 are reacting
(c) The reaction has stopped
(d) The same amount of ammonia is formed as is decomposed into N2 and H2
Answer:
(d) The same amount of ammonia is formed as is decomposed into N2 and H2
Question 5.
In a reaction the rate of reaction is proportional to its active statement is known as:
(a) Law of mass action
(b) Le-Chatelier's principle
(c) Faraday law of electrolysis
(d) Law of constant proportions
Answer:
(a) Law of mass action
Question 6.
In the reversible reaction  the concentration of each C and D at equilibrium was 0.8 mole/litre, then the equilibrium constant Kc will be:
the concentration of each C and D at equilibrium was 0.8 mole/litre, then the equilibrium constant Kc will be:
(a) 6.4
(b) 0.64
(c) 1.6
(d) 16.0
Answer:
(d) 16.0
Question 7.
On a given condition, the equilibrium concentration of HI, H2 and I2 are 0.80, 0.10, and 0.10 mole/litre. The equilibrium constant for the reaction imm will be:
(a) 64
(b) 12
(c) 8
(d) 0.8
Answer:
(a) 64
Question 8.
In which of the following, the reaction proceeds towards completion:
(a) K = 103
(b) K = 10-2
(c) K = 10
(d) K = 1
Answer:
(a) K = 103
Question 9.
Unit of the equilibrium constant for the reversible reaction  is :
is :
(a) mol-1 litre
(b) mol-2 litre
(c) Mol litre-1
(d) None of these
Answer:
(d) None of these

Question 10.
Concentration of a gas is expressed in the following terms in the equilibrium constant :
(a) Number of molecules per litre
(b) Number of grams per litre
(c) Number of gram equivalent per litre
(d) Number of molecules equivalent per litre
Answer:
(a) Number of molecules per litre
Question 11.
In a chemical equilibrium, the rate constant of the backward reaction is 7.5 × 10-4 and the equilibrium constant is 1.5. So, the rate of forward reaction is:
(a) 5 x 10-4
(b) 2 × 10-3
(c) 1.125 x 10-3
(d) 9.0 × 10-4
Answer:
(a) 5 x 10-4
Question 12.
In a chemical equilibrium  , when one mole each of the two reactants is mixed, 0.6 moles each of the products are formed. The equilibrium constant calculated as:
, when one mole each of the two reactants is mixed, 0.6 moles each of the products are formed. The equilibrium constant calculated as:
(a) 1
(b) 0.36
(c) 2.25
(d) 4/9
Answer:
(c) 2.25
Question 13.
For N2 + 3H2 → 2NH3 + heat:
(a) Kp = Kc (RT)
(b) kc = Kp(RT)-2
(c) Kp = Kc
(d) Kp = Kc
Answer:
(b) kc = Kp(RT)-2
Question 14.
A reaction attains equilibrium when the free energy change is :
(a) Positive and large
(b) Zero
(c) Negative and large
(d) Negative and small
Answer:
(b) Zero

Question 15.
In a reversible reaction, the catalyst:
(a) increases the activation energy of the backward reaction
(b) increases the activation energy of the forward reaction
(c) decreases the activation energy of both forward and backward reaction
(d) decreases the activation energy of forward reaction
Answer:
(c) decreases the activation energy of both forward and backward reaction
Question 16.
When in any system at equilibrium state pressure, temperature and concentration is changed then the equilibria shifted to such a direction which neutralizes the effect of change. This is known as:
(a) First law of thermodynamics
(b) Le-Chatelier's principle
(c) Ostwald's law
(d) Hess's law of constant heat summation
Answer:
(b) Le-Chatelier's principle
Question 17.
The pH of neutral water at 25°C is 7.0. As the temperature increases, ionization of water increases, however, the concentration of H+ ions and OH ions are equal. What will be the pH of pure water at 60°C?
(a) Equal to 7.0
(b) Greater than 7.0
(c) Less than 7.0
(d) Equal to zero
Answer:
(c) Less than 7.0
Question 18.
The ionization constant of an acid, Ka, is the measure of strength of an acid. The K, values of acetic acid, hypochlorous acid and formic acid are 174 × 10-5, 3.0 × 10-8 and 18 × 10-4 respectively. Which of the following orders of pH of 0.1 mol dm-3 solutions of these acids is correct?
(a) acetic acid > hypochlorous acid > formic acid
(b) hypochlorous acid > acetic acid > formic acid
(c) formic acid > hypochlorous acid > acetic acid
(d) formic acid > acetic acid > hypochlorous acid
Answer:
(d) formic acid > acetic acid > hypochlorous acid
Question 19.
Acidity of BF3 can be explained on the basis of which of the following concepts?
(a) Arrhenius concept
(b) Bronsted-Lowry concept
(c) Lewis concept
(d) Bronsted-Lowry as well as Lewis concept
Answer:
(c) Lewis concept
Question 20.
Which of the following will produce a buffer solution when mixed in equal volumes?
(a) 0.1 mol dm-3 NH4OH and 0.1 mol dm-3 HCl
(b) 0.05 mol dm-3 NH4OH and 0.1 mol dm-3 HCl
(c) 0.1 mol dm-3 NH4OH and 0.05 mol dm-3 HCl
(d) 0.1 mol dm-3 CH3COONa and 0.1 mol dm-3 NaOH
Answer:
(c) 0.1 mol dm-3 NH4OH and 0.05 mol dm-3 HCl
Question 21.
The following equilibrium exists in aqueous solution, imm if dil. HCl is added, without change in temperature, the :
(a) concentration of CH3COO will increase
(b) concentration of CH3COO will decrease
(c) the equilibrium constant will increase
(d) the equilibrium constant will decrease
Answer:
(b) concentration of CH3COO will decrease

Question 22.
For two acids A and B, pKa = 1.2, pKb = 2.8 respectively in value, then which is true?
(a) A and B both are equally acidic
(b) A is stronger than B
(c) Bis stronger than A
(d) Neither A nor Bis strong
Answer:
(b) A is stronger than B
Question 23.
The conjugate base of NH-2 is:
(a) NH3
(b) NH2-
(c) NH4+
(d) N-3
Answer:
(b) NH2-
Question 24.
In the following reaction HC2O-4 + PO43- → HPO2-4 + C2O2-4. Which are the two Bronsted bases?
(a) HC2O4- and PO3-4
(b) HPO42- and C2O42-
(c) HC2O-4 and HPO42-
(d) PO43- and C2O42-
Answer:
(d) PO43- and C2O42-
Question 25.
On addition of ammonium chloride to a solution of ammonium hydroxide:
(a) Dissociation of NH4, OH increases,
(b) Concentration of OH- increases.
(c) Concentration of OH- decreases.
(d) Concentration of NH4+ and OH- increases.
Answer:
(c) Concentration of OH- decreases.
Question 26.
Let the solubility of an aqueous solution of Mg(OH)2 be x, then its Ksp is:
(a) 4x3
(b) 108x5
(c) 27x4
(d) 9x
Answer:
(a) 4x3
Question 27.
The solubility in water of a sparingly soluble salt AB2 is 1.0 × 10-5 mol L-1. Its solubility product will be :
(a) 4 × 10-15
(b) 4× 10-10
(c) 1 x 10-15
(d) -1 x 10-10
Answer:
(a) 4 × 10-15
Question 28.
The pK of a weak acid (HA) is 4.5. The pOH of an aqueous buffered solution of HA in which 50% of the acid is ionized is:
(a) 4.5
(b) 2.5
(c) 9.5
(d) 7.0
Answer:
(a) 4.5
Question 29.
The pH of 10-7 M NaOH is :
(a) 7
(b) between 7 and 8
(c) between 9 and 10
(d) greater than 10
Answer:
(c) between 9 and 10

Question 30.
A base dissolved in water yields a solution with a hydroxyl ion concentration of 0.05 mo litre1. The solution is:
(a) Basic
(b) Acidic
(c) Neutral
(d) Either (b) or (c)
Answer:
(a) Basic
Question 31.
The pH of a soft drink is 3.82. Its hydrogen ion concentration will be:
(a) 1.96 x 10-2 mol/l
(b) 1,96 × 10-3 mol/l
(c) 1.5 x 10-4 mol/l
(d) 1.96 × 10-1 mol/l
Answer:
(b) 1,96 × 10-3 mol/l
Question 32.
Blood pH is controlled by concentration of H2CO3 and HCO3. In presence of NaHCO3 pH of blood is :
(a) Increased
(b) Decreased
(c) No change
(d) Statement is wrong
Answer:
(a) Increased
Question 33.
The dissociation constant of two acids HA1, and HA2 are 3.14 × 10-4 and 1.96 × 10-5 respectively. The relative strength of the acids will be approximately :
(a) 1 : 4
(b) 4 : 1
(c) 1 : 16
(d) 16 : 1
Answer:
(d) 16 : 1
Question 34.
The solubility product of AgCl is 1.44 × 10-4 at 100°C. The solubility of silver chloride in boiling water may be :
(a) 0.72 × 10-4 M
(b) 1.20 x 10-2 M
(c) 0.72 × 102 M
(d) 1.20 × 10-4 M
Answer:
(d) 1.20 × 10-4 M
Question 35.
The unit of ionic product of water Kw is:
(a) mol-1L-1
(b) mol-2L-2
(c) mol-2L-1
(d) mol2L-2
Answer:
(c) mol-2L-1
Very Short Answer Type Questions:
Question 1.
What is the effect on equilibrium constant on addition of catalyst?
Answer:
No effect.
Question 2.
What is active mass?
Answer:
Active mass is the number of moles dissolved in one litre of solution.

Question 3.
If partial pressure is written in atmosphere, then what will be the value of constant R?
Answer:
If partial pressure is in atmosphere; then R = 0.0831 litre atm K-1mol-1
Question 4.
Give an example of gas-solution at equilibrium in a day to day life.
Answer:
Cold drinks
Question 5.
A gas is kept in closed vessel at equilibrium, (atm), if helium is introduced into it; what happens at the same temperature?
Answer:
No effect.
Question 6.
If equilibrium constant K < 1; what does it indicate ?
Answer:
The reaction does not proceed in forward direction.
Question 7.
Can Le-Chatelier's principle applicable to homogeneous as well as heterogeneous equilibria?
Answer:
Yes.
Question 8.
What will happen to the concentration when equilibrium is attained?
Answer:
Concentration becomes constant.
Question 9.
Le-Chatelier's principle is applicable only for which type of conditions?
Answer:
System in equilibrium.
Question 10.
If for a reaction pressure increases tenfold, what will happen with K,?
Answer:
K, will remain unchanged.
Question 11.
Why NaOH is a strong base?
Answer:
NaOH is a strong base because it is completely ionized in its aqueous solution to give OH- ions.
Question 12.
What is an electrolyte?
Answer:
An electrolyte is a substance which on dissolving with water gives ions.

Question 13.
What will be the conjugate acid of NH2?
Answer:
NH3.
Question 14.
What will be the conjugate base of a strong acid?
Answer:
Weak base.
Question 15.
What is Lewis acid?
Answer:
Lewis acid is that substance which accepts electron pair from other. (Lewis base)
Question 16.
What will be the unit of the ionic product of the water (Kw)?
Answer:
Mol2L-2.
Question 17.
What is solubility product?
Answer:
Solubility product is the ionic product of an electrolyte in its saturated solution.
Question 18.
Write the solubility product of SnS2.
Answer:
[Sn 4+] [S2-12]
Question 19.
What will be the aqueous solution of sodium acetate?
Answer:
Sodium acetate (CH3COONa) is a salt of weak acid and strong base. Hence its aqueous solution is alkaline.
Question 20.
Write the formula of two Lewis acids.
Answer:
BF, AlCl3 AICl
Question 21.
What is the effect on equilibrium on addition of catalyst?
Answer:
On addition of catalyst, forward and backward reactions become faster to the same extent and equilibrium is attained quickly.
Question 22.
What are the units of equilibrium constant?
Answer:
Units in terms of concentration = (Units in terms of partial pressures where [An = Total number of moles of products - Total number of moles of reactants]
Question 23.
If in an exothermic reaction; the temperature is increased, what will happen to the equilibrium constant ?
Answer:
In exothermic reaction, with increase of temperature, Kp increases much more than K. Hence, K decreases.

Question 24.
What do you conclude for
(a) Q = K
(b) Q < K
(c) Q > K ?
Answer:
(a) If Q = K; then reaction is in equilibrium.
(b) If Q > K; reaction will proceed in backward direction.
(c) If Q < K; reaction will proceed in forward direction.
Question 25.
What are the conditions for getting maximum yield of NH3 by Haber's process ?
Answer:
Conditions:
- High concentration of N2
- Low temperature
- High pressure and H2
Question 26.
Raising the temperature of an equilibrium system favours which type of reaction?
Ans.
Raising the temperature of an equilibrium system favours the endothermic reaction.
Question 27.
In N2 + 3H2 → 2NH3 reversible reaction, increase in pressure will favour in which direction?
Answer:
Forward direction.
Question 28.
For N2 + 3H2 → 2NH3 + heat, what will be the relation between K, and Ke ?
Answer:
For the given reaction; ∆n = 2 - (3+1) = 2 - 4 = -2
∴ Kp = K2 [RT]2-
Question 29.
The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2.0 × 10-15 and 6 × 10-17 respectively. Which salt is more soluble? Explain.
Answer:
AgCN → Ag+ + CN
Because, its solubility product is more than the other.
Question 30.
Why the aqueous solution of FeCl3 is acidic?
Answer:
FeCl3 is a salt of weak base Fe(OH)3 and strong acid HCl. So, aqueous solution of FeCl3 is acidic.
Question 31.
What will be the Ka for H2O2?
Answer:
H2O2 is a volatile liquid. It is slightly acidic in nature. Its pKa value is approximately 10-12.
Question 32.
What does a pH = 7 signifies?
Answer:
pH = 7 signifies a neutral solution.
Question 33.
Due to which mechanism, the pH of blood is maintained constant ?
Answer:
Due to the buffer mechanism, the pH of blood maintained constant.
Question 34.
What will be the pH of normal KOH?
Answer:
It is strong base. So, its pH will be highly basic.
Question 35
What is the pH of HCI (107 M)?
Answer:
HCl is a strong acid, so, [H] = 10 -12 M
pH = - log [H + ] = -log [10-12] = 12.
Question 36.
pH of water is 7. When a substance Y i dissolved in water, the pH becomes 13. So substance Y is a salt of .................
Answer:
If we mix any substance into the solution, then th value of the pH is increased. So, this substance is a sal of weak acid and strong base.
Question 37.
If S and Ksp are solubility and solubility product respectively of a sparingly solubl binary electrolyte, find the relation between them?
Answer:
Because electrolyte is binary, So, Ksp =S' S = S2
Question 38.
Why pH of boiling water is less than 7?
Answer:
The pH of boiling water is 6.56. It is due to greate dissociation of H2O into H and OH-.
Question 39.
Why buffers cannot withstand the addition of large amounts of acids or alkalies?
Answer:
Buffer cannot withstand the addition of large amounts of acids or alkalies. The addition of 1 mol/litre of H or OH is about the maximum that any buffer car be expected to withstand.
Question 40.
If the value of pKa is negative, what it shows?
Answer:
If the value of pKa is negative, it shows that the acid is completely ionized i.e., acid is strong.
Question 41.
At which time, buffer capacity of a buffer is maximum. Explain.
Answer:
Buffer capacity of a buffer is maximum when the concentration of weak acid and its salt or weak base and its salts are equal i. e., when pH = pKa or pOH = pKb·
Short Answer Type Questions:
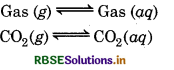
Question 1.
How many types of equilibrium are found in physical process?
Answer:
There are five types of equilibrium in physical process:
- Solid-Liquid
- Liquid-Gas
- Solid-Gas
- Solid-Saturated solution of solid in a liquid
- Gas-Saturated solution of gas in a liquid
Question 2.
Explain solid-vapour equilibrium with an example.
Answer:
In nature, some solids after heating do not melt and directly change into the vapour and after cooling directly change into solid. The direct changing from solid to vapour is called sublimation and from vapour to solid is called diposition.
Example: - 12 (solid)
I2 (vapour)

Question 3.
What is Le-Chatelier's principle?
Answer:
Le-Chatelier's principle is "change in any of the factors that determine the equilibrium conditions of a system will shift the equilibrium in such a manner to reduce or counteract the effect of the change."
Question 4.
What are the applications of equilibrium constants?
Answer:
Applications of equilibrium constants are:
- To predict the extent of reaction
- To predict the direction of reaction
- To calculate equilibrium constant
Question 5.
What is equilibrium? Explain with example.
Answer:
Equilibrium is the particular state of a process after which there is no any physical or chemical change takes place with the passage of time.
Example:
When water is heated in a closed container, molecules which gain more heat will gain high kinetic energy. Due f to this high kinetic energy, these molecules escape the boundary of the water and reached to the ceiling of the container. After sometime, these vapour molecules due to condensation changes into water drops and return to the liquid. In this process, after sometime, the number of molecules leaving the liquid become almost equal to the number of molecules returning to the liquid; which we can say the system attained an equilibrium stage.
Question 6.
0.2 molar solution of formic acid is ionized 3.2%. What will be its ionization constant?
Answer:
We Know that Ka = Ca2
So, Ka = 0.2 × Ka = 0.2 × (0.032)2 = 2.048 × 10-4
Question 7.
At 450 K, Kp = 2.0 × 1010/bar for the given reaction at equilibrium 2SO2(g) + O2(g) 2SO3 (g); what is the K, at this temperature?
Answer:
For the given reaction, ∆n 2 - 3 = -1.
Kp = Kc(RT)" or
Kc = Kp × RT
(∆n = -1)
= (2.0 × 1010 bar -1) (0.0831 L bar K-1) (450K)
= 7.48 x 1011 L mol-1
Question 8.
The solubility of a sparingly soluble salt AB2 in water is 1.0 × 101 mol-1. What will be its solubility product?
Ans.
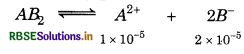
K sp = [1 × 10-5] [2 × 10-5 ]2 = 4 × 10-15
Question 9.
What are the effects of addition of inert gas at the equilibrium?
Answer:
Effects of addition of inert gas at the equilibrium:
- If the volume of the reaction is kept constant (i.e., in a closed vessel), addition of inert gas won't change the concentration of reactant or product and equilibrium state remain intact.
- If the pressure of the reaction is kept constant, ( addition of inert gas makes the volume high. So, at equilibrium, the concentration of each reactant and product will increase. So, dissociation takes place with addition of inert gas.
Question 10.
What are the general characteristics of chemical equilibrium?
Answer:
The general characteristics of chemical equilibrium are:
- At equilibrium state, Rate of forward reaction = Rate of backward reaction
- At equilibrium, concentrations of both reactants and products are same.
- Equilibrium is always reversible and dynamic in nature.
- At equilibrium state; free energy change, AG = 0.
- Equilibrium state always achieved in only closed vessel.

Question 11.
What are the factors affecting equilibria?
Answer:
Factors affecting equilibria :
- Addition of catalyst
- Addition of some inert gas
- Change in concentration of reactant or product Change in the temperature of the system Change in the pressure of the system
Question 12.
What is the effect of change of temperature, explain with suitable examples?
Answer:
The change of temperature hinders the state of e equilibrium and value of the equilibrium constant t changes.
In exothermic reaction; if temperature increases, the equilibrium constant decreases.
In endothermic reaction; if temperature increases, equilibrium constant increases.
Example: N2(g) + 3H2 (g) → 2NH3(g) + 92.38 kJ/mol.
Here forward reaction is exothermic and backward reaction is endothermic. If temperature is increased, then according to Le-Chatelier's principle, the equilibrium will shift to the side that absorbs heat, i.e., in the backward direction. Similarly, decrease in temperature will shift the equilibrium in the forward reaction.
Question 13.
How can we predict the extent of reaction through equilibrium constant?
Answer:
The numerical value of the equilibrium constant gives an idea of the amounts of reactants and products:
If the value of the equilibrium constant is high i.e., > 103 :
- The reaction will favour forward direction.
- Concentration of reactants > Concentration of products
- If the value of the equilibrium constant is moderate i.e., between 10-3 to 103 :
- So, the appreciable concentration of reactants and products are present.
- If the value of the equilibrium constant is low i.e.,< 10
The reaction will favour backward direction :
Here, Concentration of reactants > Concentration of products i.e., reaction proceeds at a very small extent in he forward direction.
Question 14.
The aqueous solution of sugar does not conduct electricity. However, when sodium chloride is added to water, it conducts electricity. How will
will you explain this statement on the basis of ionization and how is it affected by concentration of sodium chloride?
Answer:
It can be explained through following ways:
- Sugar being a non-electrolyte does not ionize in water whereas NaCl ionizes completely in water and produces Nat and Cl ions which help in the conduction of electricity.
- When concentration of NaCl is increased, more Nat and Cl ions will be produced.
- Hence, conductance increases.
Question 15.
Explain the terms
(i) Solubility product
(ii) Common ion effect.
Answer:
(i) Solubility product: Solubility product of an -lectrolyte at a specified temperature may be defined as he product of the molar concentrations of ions in a saturated solution, each concentration raised to the power equal to the number of ions produced on lissociation of one molecule of the electrolyte.
(ii) Common ion effect: If a strong electrolyte is added o weak electrolyte having a common ion is added which onizes almost completely, the ionization of the weak lectrolyte is further suppressed. Similarly, if a soluble alt having a common ion is added, if the solution of a paringly soluble salt (like AgCl, etc) the solubility of a -paringly soluble salt further decreases.
Question 16.
What is the effect of pH on solubility?
Answer:
The solubility of a salt of weak acid increases if the solution is made more acidic (i.e., pH is increased). For example, the solubility equilibrium of CaF2 may be represented as :
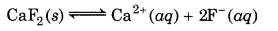
On making the solution more acidic, H+ ions will combine with the F ions. As a result, equilibrium will shift forward,i.e., solubility will increase.

Question 17.
What is the effect of change of pressure at the equilibrium? Explain with suitable example.
Answer:
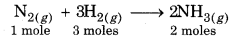
In this reaction, the forward reaction is accompanied by a decrease in the total number of moles of reactants. If the pressure of the system is increased, then according to the Le-Chatelier's principle, the equilibrium will shift in that direction where pressure decreases i.e., decrease in number of moles taken i.e., in the favour of formation of ammonia. Thus, higher the pressure, better is the yield of ammonia. If pressure is decreased, the equilibrium will shift in the direction where pressure increases i.e., increase in number of moles occur i.e., in backward direction. So, a decrease in pressure will form a dissociation of NH3 to form N2 and H2.
Question 18.
Describe the effect of: (a) addition of H2 (b) addition of CH3OH, (c) removal of CO (d) removal of CH3OH, on the equilibrium of the reaction:
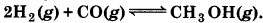
Answer:
Effects will be following according to the Le-Chatelier's principle:
(a) Equilibrium will shift in the forward direction.
(b) Equilibrium will shift in the backward direction.
(c) Equilibrium will shift in the backward direction.
(d) Equilibrium will shift in the forward direction.
Question 19.
A mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant, K, for the reaction N2(g) + 3H2(g) → 2NH3 (g) is 1.7 x 102. Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
Answer:
The reaction is: N2(g) + 3H2 (g) → 2NH3 (g)
Qc = 2.38 × 103. As Qc # Ke, the reaction mixture is not in equilibrium.
As Qc > Ke, the reaction will be in the backward direction.
Question 20.
How we predict the direction of reaction through reaction constant ?
Answer:
For a reaction; aA + bB → cC + dD
From the law of chemical equilibrium
Concentration ratio = \(\frac{[C]^c[D]^d}{[A]^a[B]^b}\)
It is also called reaction quotient and denoted by Qc or Q.
If Qc = Ke; then reaction is in equilibrium.
Qc > K.; reaction will proceed in backward direction.
Qc < Ke; reaction will proceed in forward direction.
Question 21.
Calculate the approximate pH of a 0.100 M aqueous H2S solution. K1 and K2 for H2S are 1.00 × 10-7 and 1.30 × 10-13 and 1.30 × 10-13 respectively at 25°C.
Answer:
K2 << K1. Hence H+ ions are mainly form first dissociation, i.e.,
H2S → H+ + HS-
Hence,
K1 = 1.00 × 10-7
X = or [H+] = 10-4
pH = 4.

Question 22.
pH of a solution of a strong acid is 5.0. What will be the pH of the solution obtained after diluting the given solution 100 times?
Answer:
pH = 5 means [H+] = 10-5 M.
On diluting 100 times, [H+] = 10-7 M.
This should give pH = 7 but it cannot be so because solution is acidic and pH should be less than 7. The reason is that [H+] from H2O cannot be neglected.
Thus, total [H+] = 107 M (from HCl) + 10-7 M
(from H2O) = 2 × 10-7 M
pH = -log (2 × 10-7) = 7 - 0.3010 = 6.699.
Question 23.
The solubility product of Al(OH); is 2.7 × 10-11. Calculate its solubility in g L-1 and also find out pH of this solution. (Atomic mass of Al = 27u)
Answer:
Suppose the solubility is S mol L-1. Then
\(\mathrm{Al}(\mathrm{OH})_3 \rightleftharpoons \mathrm{Al}^{3+}+3 \mathrm{OH}^{-}\)
Ksp = Sx (3S)3 = 27S4
∵ 27 S4 = 2.7 × 10-11 or
S4 = 10-12 or S = 10-3 mol L-1
Molar mass of Al(OH)3 = 27 + 3(16 + 1) = 78.
∵ Solubility of Al(OH)3 in g L-1 = 10-3 × 78
Long Answer Type Questions
Question 1.
Explain briefly the dynamic nature of equilibrium?
Answer:
Chemical equilibrium is the state in which the concentration of forward reaction is equal to the concentration of the backward reaction.
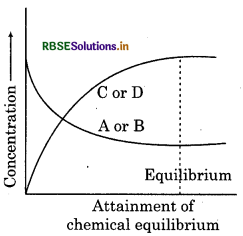
- The dynamic nature of chemical reaction can be understood in the synthesis of ammonia by Haber's process. In this process, the reaction starts with definite amount of N2 and H2 at a particular temperature. When after reaction they attain equilibrium, the concentrations of N2, H2 and NH become constant. In this process, Haber also came to the conclusion about the left concentration of N2, H2 and NH3 after reaction that equilibrium, the concentration of all the molecules was same.
- If we take deuterium (D2) in place of hydrogen, the same picture comes on the place as in the case of dihydrogen.
- If the above two reactions, are mixed, after sometime it is found that the concentration of NH3 and H2 are same except all new forms of ammonia (i.e., NH3, NH2D, NHD2, ND3) and all forms of hydrogen (i.e., H2, HD, D2) are present. This shows the equilibrium, but still the reaction is going on i.e., equilibrium is dynamic in nature.
Question 2.
What is the chemical reaction? Describe the types of chemical reaction.
Answer:
A chemical reaction is a reaction in which the amount of reactant decreases and amount of product increases.
Chemical reaction is of two types:
(i) Reversible reaction:
A reaction in which reactant and product are formed with reaction in themselves in the same condition is called reversible reaction.
Examples:
\(\begin{aligned} & \mathrm{PCl}_5(g) \rightleftharpoons \mathrm{PCl}_3(g)+\mathrm{Cl}_2(g) \\ & \mathrm{H}_2(g)+\mathrm{I}_2(g) \rightleftharpoons 2 \mathrm{HI}(g) \end{aligned}\)
(ii) Irreversible reaction: A reaction in which entire amount of reactant is changed into product and no reaction occurs from product side is called irreversible reaction.
Examples: 2Mg(g) + O2(g) → 2MgO(s)
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)

Question 3.
What Equilibrium 399 are the homogeneous equilibria? Explain with and heterogeneous examples.
Answer:
Homogeneous Equilibria: When the reactants and products are present in same phase in a reaction at equilibrium; such type of equilibrium are called homogeneous equilibria.
N2(g) + 3H2(g) → 2NH3 (g).
\(\begin{gathered} K_c=\frac{\left[\mathrm{NH}_3\right]^2}{\left[\mathrm{~N}_2\right]\left[\mathrm{H}_2\right]^3} \\ K_p=\frac{\left(\mathrm{P}_{\mathrm{NH}_3}\right)^2}{\mathrm{P}_{\mathrm{N}_2} \times \mathrm{P}_{\mathrm{H}_2}^3} \end{gathered}\)
Heterogeneous Equilibria: When the reactants and products are not present in same phase in a reaction at equilibrium; such type of equilibrium is called heterogeneous equilibria.
H2O(l) → H2O(g)
\(K_c=\frac{\left[\mathrm{H}_2 \mathrm{O}(g)\right]}{\left[\mathrm{H}_2 \mathrm{O}(l)\right]}\)
[H2O(l)] = 1
Kc = [H2O (g)]
Also
Kp = PH2O(g)
Question 4.
How can we calculate the equilibrium concentration of a particular reaction? Explain with suitable example.
Answer:
If we know the initial concentration of reactants; hen we will able to know the concentration of reactants and products at equilibrium through following steps:
Step-1: Write the balanced equation for the reaction.
Step-2 : Assume x as the amount of reactant reacted or product formed.
Step-3 Calculate equilibrium concentration of each reactants and products.
Step-4: Write the expression for K, and Ke, substitute equilibrium concentrations and calculate x
Step-5 Check the result by substituting calculated -alue of reactants and products to get the values of K, or Xp.
Example : Consider a reaction

Find the value of x, then put this value and find the oncentration of each reactant and product. Put the value of molar concentration at the equation of K, and heck the answer.
Question 5.
What is the effect of change of concentration at equilibrium? Explain with example.
Answer:
Consider a reaction; X + YA + B. This reaction is in equilibrium. If we add some amount of X or Y in the reaction; then according to the Le-Chatelier's principle, the total amount of X or Y starts decreasing. It means the equilibrium shifts in forward direction. If we add some amount of A or B, then same phenomenon takes place and equilibrium will shift in backward direction.
We take a reaction: H2(g) + I2 (g) → 2HI(g). If we add some amount of H2(g) in this reaction; the equilibrium gets disturbed. To find again equilibrium; the reaction will proceed forward to reduce the amount of H2(g) and increases the amount of HI. Example: We sweat more in a humid day. In a humid day, the air is already in saturation with water vapours. Due to this the water that comes out to the body doesn't vapourize. This results more sweat in a humid day.
Question 6.
What are strong and weak electrolytes? Define the term 'degree of dissociation' and derive how the degree of ionization is related to the concentration of the solution of electrolyte?
Answer:
Strong electrolyte: A strong electrolyte is a substance which dissociates completely into ions in aqueous solution; so, they are good conductors of electricity. Example : NaOH, KOH, HCl, NaCl, etc. Weak electrolytes: A weak electrolyte is a substance which dissociates partially in aqueous solution and hence conducts very less electricity.
Example: NH, OH, CH3COOH, etc.
Degree of dissociation: The fraction of the total number of molecules which dissociates into ions is called the degree of dissociation or ionization and is represented by a.
i.e., a = degree of dissociation or ionization.
lonization of weak electrolytes:
When a weak electrolyte (acetic acid) is dissolved in water, it dissociates partially into H3O+ and CH3COO ions. So, at the equilibrium,
CH3COOH + H2O → CH3COO- + H3O+
Now, from the law of chemical equilibrium,
\(\mathrm{K}=\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]\left[\mathrm{H}_3 \mathrm{O}^{+}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]\left[\mathrm{H}_2 \mathrm{O}\right]}\)
In dilute solution, [H2O] is constant. The product of K and constant [H2O] is denoted as Ka.
Ka = K × [H2O]
If 'C represents the initial concentration of the acid in moles L-1 and 'a' is the degree of dissociation, then equilibrium concentration of the ions (CH3COO- and H2O) is equal to Ca and the undissociated acetic acid
will be C (1 - α). So,
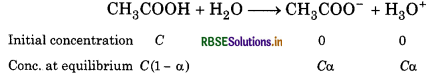
Substituting the values of the concentrations in the equation, then
\(K_a=\frac{\mathrm{C} \alpha \times \mathrm{C \alpha}}{\mathrm{C}(1-\alpha)}=\frac{C \alpha^2}{1-\alpha}\)
In case of weak electrolyte, the value of 'a' is very small;
so, 1 - α = 1.
Hence, Kα = Ca2 or, a = \(\sqrt{\frac{K_a}{\mathrm{C}}}\)
If C = 1/V (Where V = Volume of the solution in liters)
then,
\(\alpha=\sqrt{K_a \cdot V}\)
Similarly for weak base like NH4OH, we have
\(\alpha=\sqrt{K_a \cdot V}\)
So, for a weak electrolyte, the degree of ionization is inversely proportional to the square root of molar concentration or directly proportional to the square root of volume containing one mole of the solute.
Question 7.
What are acids and bases according to
(i) Arrhenius concept
(ii) Bronsted-Lowry concept
(iii) Lewis concept?
Answer:
(i) Arrhenius Concept of Acids and Bases: According to Arrhenius; those substances which give H+ ions, when dissolved in water are called acids while those which ionize in water to furnish OH ions are called bases.
Examples:
Acids: HCl, HNO3 H2SO4
HCl → H+ + Cl-
Bases: NaOH, KOH
NaOH → Na+ + OH-
(ii) Bronsted-Lowry Concept of Acids and Bases: An acid is defined as a substance which has the tendency to give a proton (H) and a base is defined as a substance which has a tendency to accept a proton.
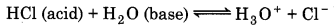
(Here HCl is acid, because they donate a proton to water)
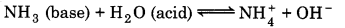
(Here NH3 is base, because they accept a proton from water.)
(iii) Lewis Concept of Acids and Bases : According to the Lewis "an acid is a substance which accepts a pair of electron and a base is the substance which donates a pair of electron." So, the acid is electron pair acceptor or electrophile and the base is electron pair donor or nucleophile. Examples,
Lewis acids: BF3, BCl3, AICl3, etc.
Lewis bases: CN-, Cl- etc.
Question 8.
Briefly explain the terms - Salt hydrolysis, Hydrolysis constant and Degree of the hydrolysis. Derive expression for calculation of pH and degree of hydrolysis for salt of weak acid and strong base.
Answer:
Salt hydrolysis: It is defined as the process in which a salt reacts with water to give back the acid and base.
Salt + Water → Acid + Base
Hydrolysis constant: For the hydrolysis of a salt BA
BA + H2O → HA + BOH
Now, from the law of chemical equilibrium,
\(\mathrm{K}=\frac{[\mathrm{HA}][\mathrm{BOH}]}{[\mathrm{BA}]\left[\mathrm{H}_2 \mathrm{O}\right]}\)
Since concentration of water is taken as constant, so,
\(K\left(\mathrm{H}_2 \mathrm{O}\right)=\mathrm{K}_h=\frac{(\mathrm{HA})(\mathrm{BOH})}{(\mathrm{BA})}\)
Kh = K[H2O] = where Kh is hydrolysis constant.
Degree of hydrolysis: The degree of hydrolysis of a salt is defined as the fraction of the total salt which is hydrolysed, i.e., h = degree of hydrolysis
Salts of weak acid and strong base:
(a) Hydrolysis constant: For the salt BA, the hydrolysis will be represented as :
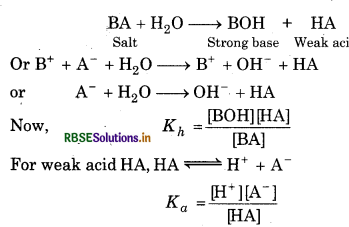
Ionic product of water, Kw = [H+][OH-]
So, = 1 or, Kh = Kw/Ka
(b) Degree of hydrolysis: From the equation,
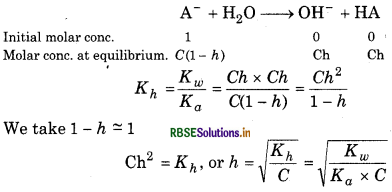
(c) pH: In this case, we have,
\(\left[\mathrm{OH}^{-}\right]=C h=\frac{h}{V} \therefore\left[\mathrm{H}^{+}\right]=\frac{K_w}{\left[\mathrm{OH}^{-}\right]}\)
Now, putting the value of h,
\(\left[\mathrm{H}^{+}\right]=\frac{K_w}{C h}=\frac{K_w}{C} \sqrt{\frac{K_a C}{K_w}}=\sqrt{\frac{K_w \cdot K_a}{C}}\left[\because h \sqrt{\frac{K_w}{K_a C}}\right]\)
Now,pH = = -log[H+] = [log Ka + log Kw - log C]
= [pKa + pKw + logC]

Question 9.
What are the general properties of equilibrium of physical process?
Answer:
For equilibrium, system should be closed to any particular temperature.
- Rate of forward and backward reactions should be at the same rate.
- All physical properties of the system should be remain constant.
- For attaining equilibrium of physical processes, all the parameters should have a constant value at a particular temperature. Such qualities are given below :
|
Process |
Conclusion |
|
Liquid - Vapour |
Constant at giventemperature |
|
Solid - Liquid |
Melting point is fixed at constant pressure |
|
|
Concentration of solute in solution is constant at given temperature. |
|
|
[gas(aq)/gas(g)] is constant at a given temperature [CO2 (aq)]/[CO2(g)] is constant at a given temperature. |
• The magnitude of all such quantities explains all the events before reaching the equilibrium.
Numerical Problems:
Question 1.
The value of Kc for the reaction, imm is 2.0 × 10 at 25°C. If the equilibrium concentration of O2 in air at 25°C is 1.6 × 10-2, what is the concentration of O3?
Answer:
[O3]2 = Kc [O2]3 = (20 × 10-50) (1.6 × 10-2)3
= 8.192 × 10-56
[O3] = 2.86 × 10-28 M
Question 2.
At 450 K, K2 = 20 × 1010/bar for the given reaction at equilibrium
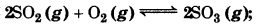
what is the Kc at this temperature?
Answer:
For the given reaction, ∆n = 2 - 3 = -1.
Kp = K(RT)-1 or, Kc = Kp(RT)
= (2.0 × 1010 bar-1) (0.0831 L bar K-1) (450 K)
= 7.48 × 1011 L mol-1
Question 3.
Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.
Answer:
pH of solution A = 6.
Therefore, concentration of [H] ion concentration of solution A = 10-6 mol L-1 pH of solution B = 4.
Therefore, concentration of [H+] ion concentration of solution B = 10-4 mol L-1
On mixing one litre of each solution, total volume
= 1L + 1L = 2L
Amount of H+ ions in 1L of solution A = Concentration X volume V
= 10-6 mol x 1L
Amount of H+ ions in 1L of solution B = 104 mol × 1L
∴ Total amount of H+ ions in the solution formed by mixing solutions A and B is
= (10-6 mol + 10-4 mol)
This amount is present in 2L solution.
∴ Total [H+] = 5 x 10-5 mol L-1
pH = -log[H+] = - log(5 × 10-5)
= - [-log 5 + 5 log10]
= 4.3

Question 4.
Calculate the volume of water required to dissolve 0.1g lead (II) chloride to get a saturated solution. (K„ of PbCl2 = 3.2 × 10-8 atomic mass of Pb = 207 u).
Answer:
K sp of PbCl2 = 3.2 × 10-8
Let S be the solubility of PbCl2.
imm
K sp = [Pb2+] [Cl]2 = (S) (2S)2 = 4S3
s3 = mol L-1
= 8 × 10-9 mol L-1
S = 2 × 10-3 mol L-1
Molar mass of PbCl2 = 278.
∴ Solubility of PbCl2 in g L
= 2 × 10-3 × 278 g L-1
= 0.556 g L-1
To get saturated solution, 0.556 g of PbCl2 is dissolved in 1L of water.
0.1 g PbCl is dissolved in L= 0.1798 L water.
To make a saturated solution, dissolution of 0.1g PbCl2 in 0.1798 L = 0.2 L of water will be required.
Question 5.
Two moles of PCl5 were heated to 327°C in a two litres closed vessel and when equilibrium was achieved, PCl5 was found to be 40% dissociated into PCl3 and Cl2. Calculate the equilibrium constants Kp and K. for this reaction.
Answer:
PCl5 dissociates as:
\(\mathrm{PCl}_5 \rightleftharpoons \mathrm{PCl}_3+\mathrm{Cl}_2\)
Initial amount of PCl5 = 2 moles
Now, PCl5 was 40% dissociated at equilibrium
So, PCl5 dissociated at equilibrium = 40/100 × 2 = 0.8 mole.
∴ Amounts of PCl5, PCl3 and Cl2 at equilibrium will be PCl5 = 2 - 0.8 = 1.2 mole,
PCl3 = 0.8 mole, Cl2 = 0.8 mole
Since the volume of the vessel is 2 liters, therefore, the molar concentration at equilibrium will be
[PCl5] = 0.6 mol L-1, [PCl3] = 0.4 mol L-1
and [Cl2] = 0.4 mol L-1
\(K_c=\frac{\left(\mathrm{PCl}_3\right)\left(\mathrm{Cl}_2\right)}{\left(\mathrm{PCl}_5\right)}=\frac{0.4 \times 0.4}{0.6}\)
= 0.267 mol L-1
Here; ∆n = 2 - 1 = 1 mol
∴ Kp = Kc (RT)
But, T = 327 + 273 = 600K, R = 0.0821L atm K-1 mol-1
∴ Kp = 0.267 × 0.0821 × 600 = 13.15 atm.
Competitive Exam Questions:
Question 1.
AIF3 is soluble in HF only in the presence of KF, it is due to the formation of:
(a) K [AIF3H]
(b) K3 [AlF3H3]
(c) K3 [AIF6]
(d) AlH3
Answer:
(c) K3 [AIF6]
Question 2.
200 mL of a very strong acidic solution whose pH value is 2.0, is added to 800 mL of any other acidic solution whose pH value is 3.0, then the resultant pH of the solution will be
(a) 2.55
(b) 2.97
(c) 2.40
(d) 2.10
Answer:
(a) 2.55
Question 3.
0.01 mol of NaOH is added to 1 L buffer solution in which 0.1 M acetic acid and 0.1 M sodium acetate is present. If the pKa value of acetic acid is 4.76, then pH value of solution will be:
(a) 4.76
(b) < 4.76
(c) 7.6
(d) > 4.76
Answer:
(d) > 4.76
Question 4.
Two insoluble salts MY and NY3 have same K value 6.2 × 10-13 at room temperature which of the following statement is true with respect of MY and NY3
(a) MY and NY both have some molar solubility in water.
(b) The malor solubility of MY is less than NY3 water.
(c) MY and NY salts are more soluble in 0.5 M KY in comparis on to pure water.
(d) There is no effect in the solubility of MY and NY3 on addition of KY salt in their solution.
Answer:
(b) The malor solubility of MY is less than NY3 water.
Question 5.
For the aqueous solution of 0.10 M pyridine (C5H5N+) K1 = 1.7 × 109. The percentage of pyridine for the formation of pyridinium ion [C5 H5 N+] is:
(a) 1.6%
(b) 0.0060%
(c) 0.013%
(d) 0.77%
Answer:
(c) 0.013%
Question 6.
The solubility of AgCl(s) with K in 0.1 M NaCl solutions is :
(a) Zero
(b) 1.26 × 10-5 M
(c) 1.6 x 10-9 M
(d) 1.6 × 10-11 M
Answer:
(c) 1.6 x 10-9 M

Question 7.
Boric acid is an acid, beacuse its molecules:
(a) Are joined with protons of water molecule.
(b) Give H+ ion in water.
(c) release proton
(d) Accept OH- ion from water and release proton.
Answer:
(d) Accept OH- ion from water and release proton.
Question 8.
Which fluoro compound behave strongly as lewis base?
(a) SiF4
(b) BF3
(c) PF3
(d) CF4
Answer:
(c) PF3
Question 9.
Which of the following conditions are favourable for the formation of ammonia by Haber's process.
N2(g) + 3H2(g) → 2 NH3 (g) + 92.3 kJ ?
(a) on increasing temperature
(b) on increasing pressure
(c) on decreasing temperature
(d) by removing NH3
Answer:
(a) on increasing temperature
Question 10.
Which curve shows exothermic reaction?
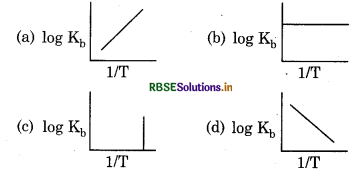
Answer:
Question 11.
1 mol of A and 0.5 mol of B are close in a 3 L vessel. After certain condition following equilibrium is established.
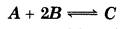
At quilibrium, 0.3 mol B is found back at room temprature. What will be the value of equilibrium constant K?
(a) 11.1
(b) 1.11
(c) 0.01
(d) 2.50
Answer:
(a) 11.1
Question 12.
At temperature 298 K, the equilibrium constant for the reaction A + B → C + D is 100. If initial concentration of all the four is 1 M then equilibrium concentration of D (mol/L) will be:
(a) 0.818
(b) 1.818
(c) 1.182
(d) 0.182
Answer:
(b) 1.818
Question 13.
At 300 temperature imm (g), the value of ∆G° is -690.9R. What will be the value of equilibrium constant at this temperature (Here R = gas constant)?
(a) 10 atm-1
(b) 10 atm
(c) 10
(d) 1
Answer:
(a) 10 atm-1
Question 14.
At 7800 K temperature the reaction imm have equilibrium constant (K) 1.80 × 10-3 and R 8.314 JK-1 mol-1. Then what will be the value of K at this temperature?
(a) 3.09 × 10-7 mol/L
(b) 5.07 × 10-8 mol/L
(c) 8.18 x 10-9 mol/L
(d) 9.24 × 10-10 mol/L
Answer:
(a) 3.09 × 10-7 mol/L
Question 15.
If equilibrium constant for the reaction imm is K1 and for the reaction imm it is K2 then:
(a) K1 = K2
(b) K2 = √K1
(c) K1 = 2K2
(d) K1 = 1/2K2
Answer:
(b) K2 = √K1

Question 16.
If equilibrium constant for a particular reaction is 1.6 × 1012 the equilibrium system will have :
(a) mostly reactant
(b) mostly product
(c) equal amount of reactants and products
(d) all reactants
Answer:
(b) mostly product
Question 17.
Ksp value for Ag2CrO4, AgCl, AgBr and AgI is 1.1× 10-12, 1.8 × 10-10, 5.0 × 10-13, 8.3 × 10-17 respectively. At last which salt will be precipitated of solution of AgNO3 is added to equal moles of NaCl, NaBr, Nal and Na2CrO4?
(a) AgCl
(b) AgBr
(c) Ag2CrO4
(d) AgI
Answer:
(c) Ag2CrO4
Question 18.
Which of the pair is not acidic buffer?
(a) HClO4 and NaClO4
(b) CH3COOH and CH3COONa
(c) H2CO3 and Na2CO3
(d) H3PO4 and Na3PO4
Answer:
(a) HClO4 and NaClO4
Question 19.
The pH value of the solution formed by mixing equal volume of 0.1 M NaOH and 0.01 M HCl is:
(a) 12.65
(b) 2.0
(c) 7.0
(d) 1.04
Answer:
(a) 12.65
Question 20.
The increasing order acidity of diprotic acidsin aqueous solution is:
(a) H2Te < H2S < H2Se
(b) H2Se < H2Te < H2S
(c) H2S < H2Se < H2Te
(d) H2Se > H2S < H2Te
Answer:
(c) H2S < H2Se < H2Te
Question 21.
Which of the following salt will give highest value of a pH in water?
(a) Na2CO3
(b) CuSO4
(c) KCl
(d) NaCl
Answer:
(a) Na2CO3
Question 22.
The conditions which yield maximum amount of ammonia by Haber's process:
N2 + 3H2 → 2 NH3 + Q k Cal are:
(a) high temperature, high pressure and high concentration of reactants
(b) high temperature, low pressure and low concentration of reactants
(c) low temperature and high pressure
(d) low temperature low pressure and less concentration of hydrogen
Answer:
(c) low temperature and high pressure
Question 23.
For an exothermic reaction at temperature T1 and T2 the equilibrium constant is k, and k, respectively. If we consider that heat of reaction constant at the temperature limit T1 and T2 then which is correct?
(a) kp = Kp
(b) Kp = 1/Kp
(c) kp > kp
(d) kp < kp
Answer:
(c) kp > kp

Question 24.
For which of the reactions, the value of k and ke will be equal?
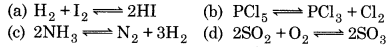
Answer:
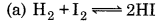
Question 25.
For the reaction IMM then the value of x will be.
(a) -1
(b) 1/2
(c) 1/2
(d) 1
Answer:
(b) 1/2
Question 26.
Thermal decomposition of CaCO3 (solid) is done at different condition,
\(\mathrm{CaCO}_3(s) \rightleftharpoons \mathrm{CaO}(s)+\mathrm{CO}_2(g)\)
The correct statement for the reaction is:
(a) The value of AH depends on temperature
(b) Equilibrium constant (K) for CaCO3 do not depend on initial result.
(c) The value of K at constant temperature depends on pressure of CO2.
(d) AH does not depend on effect of catalyst (if present)
Answer:
(a) The value of AH depends on temperature
Question 27.
Which has low probability to behave as Lewis base?
(a) OH-
(b) H2O
(c) NH3
(d) BF3
Answer:
(d) BF3
Question 28.
In which of the following the solubility of AgCl will be minimum?
(a) 0.001 M AgNO3
(b) Pure water
(c) 0.01 M CaCl2
(d) 0.01 M NaCl
Answer:
(c) 0.01 M CaCl2
Question 29.
Which is incorrect for the value of solubility product?
(a) COS > CuS
(b) NiS > PbS
(c) Fe(OH)3 > Fe(OH)2
(d) Ni(OH)2 > Cr(OH)3
Answer:
(c) Fe(OH)3 > Fe(OH)2
Question 30.
The value of Ksp for CaCO3 and CaC2O4 is 4.7× 10-9 and 1.3 × 10-9 at 25°C respectively. If the mixture of these two are washed with water then the concentration of Ca2 ion in water will be?
(a) 5.831 x 10-5 M
(b) 6.856 × 10-5 M
(c) 3.606 × 10-5 M
(d) 7.746 × 10-5 M
Answer:
(d) 7.746 × 10-5 M
Question 31.
At 100°C temperature kw of water is 55 times in comparision to the value of kw of water at 25°C, then pH value of neutral solution will be: (log 55 = 1.74)
(a) 7.00
(b) 7.87
(c) 5.13
(d) 6.13
Answer:
(d) 6.13
Question 32.
At 298 L the value of kp (Ag2CrO4) is 1.1× 10-12. The solubility of Ag2CrO4 will be in 0.1 M AgNO3 in mol L-1:
(a) 1.1 x 10-11
(b) 1.1 × 10-10
(c) 1.1 × 10-12
(d) 1.1 × 10-9
Answer:
(b) 1.1 × 10-10
Question 33.
For diprotic acid, first and second ionisation constants will be:
(a) ka1 = ka2
(b) ka1 > ka2
(c) ka2 = 1/ka1
(d) ka2 > ka1
Answer:
(b) ka1 > ka2
Question 34.
The correct order of basicity is:
(a) ClO- < ClO2 < ClO3 < ClO4
(b) ClO4 < ClO-3 < ClO-2 < ClO-
(c) ClO-3 < ClO4 < ClO-2 < ClO-
(d) ClO-2 < ClO- < ClO3 < ClO-4
Answer:
(a) ClO- < ClO2 < ClO3 < ClO4
Question 35.
What will be the number of moles of Ca(OH)2, which on addition to water give pH = 10.65 and the volume of solution will be 250 mL ?
(a) 5.6 x 10-5
(b) 6.5 × 10-5
(c) 4.5 × 10-5
(d) 5.4 × 10-5
Answer:
(a) 5.6 x 10-5

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter