RBSE Class 11 Chemistry Important Questions Chapter 6 Thermodynamics
Rajasthan Board RBSE Class 11 Chemistry Important Questions Chapter 6 Thermodynamics Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Chapter 6 Important Questions Thermodynamics
Multiple Choice Questions:
Question 1.
Which reaction shows the enthalpy of formation of CO2(g)?
(1) CH4(g) + 2O2(g) → CO2(g) + 2H2O
(2) CO(g) + 1/2O2(g) → CO2(g)
(3) C(graphite) + O2(g) → CO2(g)
(4) CO(g) + H2O(g) → CO2(g) + H2(g)
Answer:
(3) C(graphite) + O2(g) → CO2(g)
Question 2.
The correct order is :
(1) 1 cal > 1 joule > 1 erg
(2) 1 erg > 1 joule > 1 cal
(3) 1 erg > 1 cal > 1 joule
(4) 1 joule > 1 cal> 1 erg
Answer:
(1) 1 cal > 1 joule > 1 erg

Question 3.
The standard condition is:
(1) 0°C and 1 atm
(2) 20°C and 1 atm
(3) 25°C and 1 atm
(4) 0°K and 1 atm
Answer:
(3) 25°C and 1 atm
Question 4.
Value of ∆H for Cl(g) + e- → Cl-(g) is:
(1) positive
(2) negative
(3) zero
(4) infinite
Answer:
(2) negative
Question 5.
When q is given to system and work done by system is w then first law of Thermodynamics will be:
(1) ∆E = q + w
(2) ΔΕ = q - w
(3) ΔΕ = - q + w
(4) ΔΕ = - q - W
Answer:
(2) ΔΕ = q - w
Question 6.
Which statement is correct for CO(g) + 1/2O2(g)
(1) ΔH = ΔE
(2) ΔΗ < ΔΕ
(3) ΔH > ΔE
(4) None of the above
Answer:
(2) ΔΗ < ΔΕ
Question 7.
Which statement is incorrect?
(1) Work is a state function.
(2) Temperature is a state function.
(3) Work appears at boundary of system.
(4) State change is constant completely.
Answer:
(1) Work is a state function.
Question 8.
Standard molar enthalpy of formation of O2 is:
(1) equal to zero
(2) equal to molar enthalpy of combustion of C (gas)
(3) equal to total enthalpy of formation of CO and O2
(4) equal to standard molar enthalpy of combustion of C (graphite)
Answer:
(4) equal to standard molar enthalpy of combustion of C (graphite)
Question 9.
The change in enthalpy for a reaction does not depend upon :
(1) states of reactants and products
(2) nature of reactants and products
(3) on different intermediate reaction
(4) change in enthalpy of initial and final state of reaction
Answer:
(3) on different intermediate reaction
Question 10.
Any system is considered thermodynamically reversible if:
(1) there is exchange of system and surrounding to each other
(2) there is no limit between system and surroundings
(3) surroundings is always balanced in system
(4) system converts into surroundings spontaneously
Answer:
(3) surroundings is always balanced in system

Question 11.
Which one is true for closed system?
(1) System can exchange energy but not matter with surroundings.
(2) System can exchange both energy and matter with surroundings.
(3) System can neither exchange energy nor matter with surroundings.
(4) System can exchange only matter with surroundings.
Answer:
(1) System can exchange energy but not matter with surroundings.
Question 12.
In adiabatic process, the temperature of exchange :
(1) is constant
(2) decreases
(3) increases
(4) decreases or increases
Answer:
(4) decreases or increases
Question 13.
Hess's law is used to calculate :
(1) enthalpy of reaction
(2) entropy of reaction
(3) work done
(4) all of the above
Answer:
(1) enthalpy of reaction
Question 14.
Reaction N2 + 3H2 → 2NH is completed at constant temperature and pressure. If ΔH and ΔU are enthalpy and internal energy of reaction, then which one is correct?
(1) ΔH > ΔU
(2) ΔH < ΔU
(3) ΔΗ = ΔΥΟ
(4) ΔH = 0
Answer:
(2) ΔH < ΔU
Question 15.
A thermodynamic quantity is :
(1) quantity used in measurement of thermal changes
(2) quantity which depends upon state of system
(3) quantity used in thermodynamics
(4) quantity which follows thermodynamic laws
Answer:
(1) quantity used in measurement of thermal changes
Question 16.
At a certain pressure, Q heat is absorbed by any system. The value of Q will be equal to :
(1) energy change of system (U)
(2) enthalpy change of system (ΔH)
(3) volume change of system (ΔV)
(4) None of the above
Answer:
(2) enthalpy change of system (ΔH)
Question 17.
In any chemical reaction, at constant pressure, the value of heat (Q) will be equal to :
(1) internal energy of product internal energy of reactant
(2) internal energy of reactant of product internal energy
(3) enthalpy of product enthalpy of reactant
(4) enthalpy of reactant- enthalpy of product
Answer:
(3) enthalpy of product enthalpy of reactant
Question 18.
Which of the following variables determine the state of system?
(1) pressure and volume
(2) pressure, temperature and volume
(3) pressure, concentration temperature,
(4) pressure and concentration volume and
Answer:
(3) pressure, concentration temperature,
Question 19.
A weightless piston expands volume ΔV at constant temperature, if pressure of piston is variable then work done by piston will be:
(1) W = PV
(2) W = ΔPAV
(3) W = 0
(4) None of these
Answer:
(4) None of these
Question 20.
Which one is false for spontaneous process?
(1) spontaneous processes are unidirectional.
(2) spontaneous processes happen instantaneously/very fast.
(3) spontaneous proceses are reversible.
(4) In spontaneous process, internal energy of system decreases.
Answer:
(2) spontaneous processes happen instantaneously/very fast.
Question 21.
Heat exchange of a chemical reaction at constant. temperature and pressure is known as:
(1) internal energy
(2) enthalpy
(3) entropy
(4) Gibb's free energy
Answer:
(2) enthalpy
Question 22.
If entropy is considered as thermodynamic parameter, then it will determine the spontaneity of which process?
(1) ASgys + ASsurr > 0
(2) ASgys - ASsurr > 0
(3) ASgys > 0
(4) ASgurr > 0
Answer:
(1) ASgys + ASsurr > 0
Question 23.
Chemical reaction is completed spontaneously in the direction in which:
(1) decrease in free energy occurs
(2) there is no useful work done
(3) increase in free energy occurs
(4) none of these
Answer:
(1) decrease in free energy occurs

Question 24.
The change in enthalpy (AH) for a reaction depends upon :
(1) initial state
(2) final state
(3) both initial and final states
(4) none of these
Answer:
(3) both initial and final states
Very Short Answer Type Question:
Question 1.
What will be nature of enthalpy change for the reaction, SO2 + 1/2O2 → SO3?
Answer:
It will be low because heat of combustion is always released.
Question 2.
Enthalpy is an extensive property. Suppose that ∆,H is the enthalpy for the reaction A→ B. While enthalpies of other A intermediate paths are ∆, H1, ∆, H2, ∆, H3 etc. Then what is the relation among them?
Answer:
According to Hess's law
∆rH = ∆rH1 + ∆H2 + ∆H2
Question 3.
HU + PV gives the relation between Cp and Cv. 'Calculate the relation between Cp and Cv for 10 moles of ideal gas?
Answer:
Cp - Cv = nR
= 10 × 8.314 = 83.14 J
Question 4.
What is the primary requirement for fuel to be a good quality?
Answer:
For a good quality of fuel, it should have high calorific value.
Question 5.
Why heat of NaCl solution is positive?
Answer:
∆H solution = ∆Hion - ∆Hhydration
:: ΔΗ hyd is negative and ∆Hion is positive. Since value of of NaCl is greater than ΔHionisation of NaCl greater than ∆Hhydration.
Question 6.
Does temperature remains constant for an isolated, system?
Answer:
The temperature of isolated system remains constant if there is no physical or chemical reactions in the system.
Question 7.
Why air of atmosphere becomes colder when it goes to upward?
Answer:
In upper layer of atmosphere, the pressure is low. Hence, on moving upward the air expands in which air works by. spending its internal energy. So its temperature decreases and it becomes colder.
Question 8.
When an ideal gas expands in vaccum then heat is neither released nor absorbed. Why?
Answer:
In ideal gas, there is no intermolecular force. S Hence, when volume of gas expands then neither energy is absorbed nor released.
Question 9.
What will be change in internal energy if work done by system?
Answer:
If work is done by the system then due to increase n volume, the internal energy will be decrease.

Question 10.
When ∆S = ∆H/T is valid?
Answer:
It is valid only when system is in equilibrium i.e., ∆G = 0
Question 11.
Why ∆U = 0 for isothermal expansion of ideal gas?
Answer:
Since internal energy and change in internal nergy are related to temperature. In isothermal rocess, the temperature remains constant. So, AU will zero.
Question 12.
The molar enthalpy of vaporisation of acetone is less than water. Why?
Answer:
Because water contains strong hydrogen bond.
Question 13.
Hess's law is supplementry of first law of thermodynamics. How?
Answer:
The first law of thermodynamics says that neither eat can be created nor be destroyed and according to Hess's law, there is no change in heat during direct or ndirect reactions".
Question 14.
Identify the exothermic and endothermic reactions out of the following:
(i) Lighting of match box.
(ii) Melting of ice.
(iii) When melted metal becomes solid.
(iv) Reaction of potassium with water.
(v) Evaporation of ether.
Answer:
(i) Lighting of match box - Exothermic
(ii) Melting of ice - Endothermic
(iii) When melted metal becomes solid - Exothermic
(iv) Reaction of potassium with water - Exothermic
(v) Evaporation of ether - Endothermic.
Question 15.
Which is most stable form of carbon?
Answer:
Graphite.
Question 16.
On combustion of 12 g carbon, diamond and graphtie, the absorbed amounts of heat are different. Why?
Answer:
The enthalpy of reaction depends upon physical tate of reactant. Since these are in different forms so nthalpy of these will be different.
Question 17.
The reactions completed at constant pressure are more important than reactions at constant volume. Why?
Answer:
Because spontaneous reactions furnished a atmospheric pressure.
Question 18.
What exchange occurs between system and surroundings?
Answer:
Both work and heat are exchanged between systen and surroundings.
Question 19.
Why, increase in enthalpy of solid to liquid i less than increase in enthalpy of liquid t gas?
Answer:
Because in gaseous state, the disorderness i molecule is more than liquid. So increase in entrop during solid → liquid is less than that of increase i enthalpy during liquid to gas.
Question 20.
Does negative enthalpy is criteria spontaneity of any reaction ?
Answer:
No, there are so many reactions in which ∆H i positive, but these are spontaneous. For example vaporisation of water, dissolution of NaCl, NH4Cl water.

Question 21.
Hotness of steam is isobaric process isothermal process and why?
Answer:
Hotness of steam is isobaric process becaus pressure remains constant when steam becomes ho after getting heat.
Question 22.
Why temperature of gas increased to compression?
Answer:
Because during compression work increase internal energy of molecules of gas. So temperature gas is increased on compression.
Question 23.
The standard molar entropy of H2O(l) is 70 JK mol-1. Then the value of standard molar entropy of H2O(s) will be more or less than this value?
Answer:
H2O(s) molecules are less disordered. So standar molar entropy of H2O(s) will be less than standard mola entropy of H2O(l).
Question 24.
The increase in enthalpy of surroundings i equal to decrease in enthalpy of system. Does both have equal temperature if both are i equilibrium?
Answer:
Yes, if both systems and surroundings are i thermal equilibrium then both will have temperature.
Short Answer Type Questions:
Question 1.
What is the difference between homogeneous and heterogeneous systems?
Answer:
Homogeneous System: When all the substances present in system are in same phase or having same chemical composition, then it is said to be homogeneous system, e.g., aqueous solutions of salt.
Heterogeneous System: When substances present in system are in two or more different phases then system is said to be heterogeneous. e.g. Mixture of ice and water.
Question 2.
One mole sample of monoatomic ideal gas is taken in cyclic process as for compression and expansion, which is shown in figure. What will be value of ∆H for whole process?
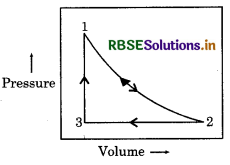
Answer:
For cyclic process, total enthalpy is zero
i.e. ΔΗ cyclic = 0
Question 3.
Explain enthalpy of sublimation.
Answer:
It is the enthalpy change when 1 mole of solid converts directly to vapour. For example:
I2(g) → I2(g) ;
ΔH = + 62.4 kJ
It is denoted by ΔHs.
Enthalpy of sublimation is the sum of enthalpy of fusion s and enthalpy of vaporisation.
ΔΗs = ΔΗf + ΔΗ.
Question 4.
Give the formula of heat capacity at constant volume (C) and heat capacity at constant pressure (C).
Answer:
1. Heat capacity at constant volume
\(C_v=\left[\frac{q_{\mathrm{v}}}{T_2-T_1}\right]_{\mathrm{v}}=\left[\frac{q_v}{\Delta T}\right]_{\mathrm{v}}\)
2. Heat capacity at constant pressure
\(C_p=\left[\frac{q_p}{T_2-T_1}\right]_{\mathrm{v}}=\left[\frac{q_p}{\Delta T}\right]_p\)
Question 5.
What will be work done on ideal gas in closed cylinder when it is compressed at external pressure text ? It is shown in following figure
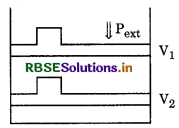
Answer:
As given Pext = constant, then it is an irreversible compression.
Wirr = pext ∆v
= pext (V1 - V2)
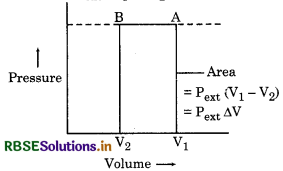
It can be determined by P - V graph. The work done here is area of ABV2V1.

Question 6.
What is difference between quantitative and specific properties?
Answer:
- Quantitative Properties: Those properties of system which depend upon the quantity or magnitude of substances present in system, are called quantitative properties.
- Specific Properties: Those properties of system which depend upon the nature of substance present in system. These properties are independent of quantity or magnitude.
Question 7.
What will be effect on internal energy of system if:
(a) Work done on system?
(b) Work done by system?
Answer:
(a) When any work is done on system then its internal energy decreases. The internal energy of system depends upon physical state pressure, temperature etc. The internal energy of system changes H according to work done on surroundings.
(b) If work is done by any system then internal energy increases. It is possible only when change in energy did not transform into heat.
Question 8.
The equal amount of heat is given to equal mass of helium and oxygen gas. In which temperature will rise more and why?
Answer:
Helium is monoatomic gas while oxygen is diatomic gas. The given heat will increase the kinetic energy of molecules in helium while heat given to oxygen will enhance the rotational and vibrational kinetic A energy i.e., given heat will be used for various tasks. Hence, the temperature of helium will be more as s compound to temperature of oxygen.
Question 9.
For a certain reaction, enthalpy graph is given below. It is possible to determine the spontaneity of reaction through this curve?
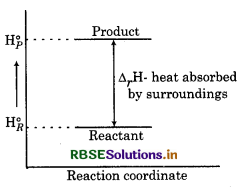
Answer:
No, only enthalpy is not pontaneity of any reaction since this reaction is dothermic i.e., the value of energy is increasing. The cher factors like entropy etc. are also considered for etermining the spontaneity of reaction.
Long Answer Type Questions:
Question 1.
The current flows in immersed resistance in water. Considering the resistance as system, Explain :
(a) Does heat enters in resistance from surroundings?
(b) Does heat enters into water?
(c) Does work done?
(d) Supposing that the state of resistance is constant, then apply first law of this system.
Answer:
(a) No, heat does not enters to resistance from urroundings.
(b) Yes, heat enters to water.
(c) Yes, work is done on resistance (system).
(d) According to first law
∆U = q + W
q = 0 (for resistance)
W = +ve
∆U = 0 + W
∆U = W
For water, w = 0 hence, ∆U = q
S0,
W = q
It is proved that work is equal to heat transferred to water.
Question 2.
What do you mean by free expansion of system? Is internal energy of system remains constant during free expansion?
Answer:
If in any system (like gas) the expansion carried ut in such a way that heat neither enters from urroundings nor heat goes to surroundings i.e., xpansion is adiabatic. If there is no work done on ystem or by the system then it is called free expansion. uppose, there is a pot divided in two parts. It is covered ith asbestos. One part of pot is filled with gas and other part is empty. When partition is removed suddenly then gas enters to empty part fastly and expands freely. If initial and final energies of gas are U; and Uf, then according to first law of thermodynamics,
U f - Ui = q + W
Since pot is heat resistant and process is sudden so heat neither enters nor released (q = 0) besides this gas expands in vacuum, so no work is done (w = 0). Hence,
Uf - Ui =0
Uf = U i
Therefore in free expansion, initial and final energies are same i.e., internal energy remains unchanged.

Question 3.
Show that change in entropy (∆S) is a state function.
Answer:
Suppose a fixed amount of substance is filled in cylinder which is associated with frictionless and weigh- less piston. Both are heat resistant and working as an isolated system.
Then change in entropy can be shown as below:
1. Expansion : Suppose initial volume V1 expands to final volume V2 at temperature T. It is done at isobaric and reversible process.
The change in entropy for reservoir (∆S res)
\(\Delta S_{\text {res }}=\frac{-q_{\text {rev }}{ }^{\circ}}{T}\)
Entropy change for system ∆Ssys = \(\frac{q_{\text {rev }}}{T}\)
Total entropy change = (∆S tot) =
\(\begin{aligned} & =\Delta S_{(\mathrm{sys})}+\Delta S_{(\text {res })} \\ & =\frac{q_{\mathrm{rev}}}{T}-\frac{q_{\mathrm{rev}}}{T}=0 \end{aligned}\)
2. Contraction: Suppose at temperature T, the volume of any system is contracted from V2 to V1. Here heat released is q.
So.
\(\begin{aligned} & \Delta S_{\text {(sys) }}=\frac{-q_{\mathrm{rev}}}{T} \\ & \Delta S_{\text {(res) }}=\frac{q_{\mathrm{rev}}}{T} \end{aligned}\)
Total entropy change AS (tot)
\(\begin{aligned} & =\Delta S_{\mathrm{sys}}+\Delta S_{\mathrm{res}} \\ & =-\frac{q_{\mathrm{rev}}}{T}+\frac{q_{\mathrm{rev}}}{T} \end{aligned}\)
Hence for a complete cycle, total change in entropy (∆S1 + ∆S2 = 0). Since, there is no change observed in entropy so it is state function.
Question 4.
With the help of curve, show the change in enthalpy for following process.
(i) Throw a stone from ground to roof.
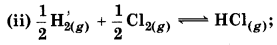
In which process, enthalpy change shows the spontaneity of process?
Answer:
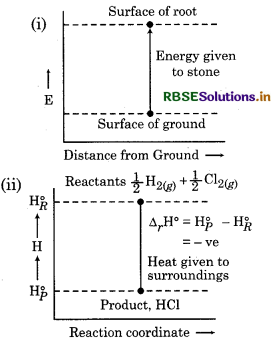
In process (i) energy is increasing while in process (ii) energy is decreasing. So in process (ii), enthalpy change shows the spontaneity.
Question 5.
1 mole of monoatomic gas expands from state-1 to state-2 as shown in figure. Calculate the work done at 298 K from state 1 to state 2.
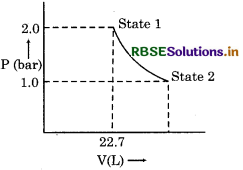
Answer:
W = -2.303 nRT log P2/P1
= - 2.303 × 1 × 8.314 × 298 log 2/1
= - 2.303 × 8.314 × 298 × 0.3010
= - 1717.46 J
= - 1.72 kJ
Numerical Questions:
Question 1.
Enthalpy of formation of methane at constant pressure and 300 K is -78.84 kJ. What will be enthalpy of formation at constant volume?
Answer:
Formation of methane given by following equation
C(s) + 2H2(g) → CH4 (g); Δf H = - 78.84 kJ
Δηg = 1 - 2 = -1 mol
R = 8.314 × 10-3 kJ mol-1K-1
ΔΗ = - 78.84 kJ
ΔU =?
ΔΗ = ΔU + Δηg RT
- 78.84 = ΔU + (-1 × 8.314 × 10-3 × 300)
ΔU = (-78.84) - (-1× 8.314 × 10-3 × 300)
ΔU = (-78.84) + (2.494)
Δu = - 76.346 kJ
Internal energy = 76346 kJ
Question 2.
Assuming water vapour as ideal gas at 100°C and 1 bar pressure on vaporization of 1 mol of water, the difference was obtained 41 kJ mol-1. Calculate the change in internal energy, When,
(i) 1 mol water is vaporized at 100°C and 1 bar pressure.
(ii) 1 mol water is converted into ice.
Answer:
(i) H2O (l) → H2O (g)
For change,
ΔH = ΔU + ΔngRT
ΔU = ΔH - ΔngRT
Substituting the values,
ΔU = 41.0 - (1 × 8.314 × 10-3 × 373)
= 41 - 3.1011
= 37.898 kJ mol-1
(ii) H2O (l) → H2O (s)
Here change in volume is minimal. Hence,
PΔV = Δn RT = 0
In this condition,
ΔH = ΔU
ΔU = 4100 kJ mol-1

Question 3.
5.0 mol of ideal gas is compressed reversibly upto its half of the volume at 25°C and 1 atm pressure. Calculate the work done and heat.
Answer:
T = 25 + 273 = 298 K
Given,
n = 5 mol R = 8.314 JK-1mol-1
\(V_2=\frac{V_1}{2}\)
W = - 2.303 nRT log V2/V1
= - 2.303 × 5 × 8.314 × 298 × log \(\frac{\frac{V_1}{2}}{V_1}\)
= - 2.303 × 5 × 8.314 × 298 × log 1/2
= 2.303 × 5 × 8.314 × 298 × log 2
= 2.303 × 5 × 8.314 × 298 × 0.3010
= 8587.3 J
W = 859 kJ
Process is isothermal so ΔU = 0
From first law of thermodynamics,
Δυ = q + W
q = -W
q = -859 kJ
Question 4.
5 mol ideal gas is heated from 27°C to127° C at constant pressure.
(i) Calculate the work done in expansion.
(ii) If gas is expanded from 2.0 atm to 1.4 atm at 30°C then calculate the work done.
Answer:
(i) Suppose that constant pressure is P Hence, WP (V2 − V1)
Gas is ideal then,
PV = nRT
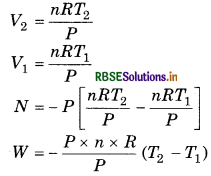
= - 5 x 8314(400 - 300)
= - 5 x 8.314 × 100
W = - 4157 J
W = -4.157 kJ
(ii) Work done in isothermal reversible expansion
W = -2.303 nRT log P1 /P2
= - 2.303 × 5 × 8.314 × 303 log 2/1.4
= -2.303 × 5 × 8.314 × 303 log 20/14
= - 2.303 × 5 × 8.314 × 303 log 1.4286
= -2.303 × 5 × 8.314 × 303 × 0.1549
= - 4493.6 J
= - 4.4936 kJ
Question 5.
In a swimming pool, 1× 105 L water is filled Calculate the amount of heat required to increase temperature of water from 20°C to 25°C. Specific heat capacity of water is 4.184 J/°C.
Answer:
Required heat = Mass specific heat capacity × ∆T
Mass of water W = density x volume
= \(\frac{1 \mathrm{~g}}{\mathrm{~cm}^3}\) 105 × 103 cm3
W = 108 g
Specific heat capacity (C) = 4184 J/°C g
∆T = 25 - 20 = 5°C
q = M x S x ∆T
Required heat = 108 × 4.184 x 5°
= 2.092 × 109 J
Question 6.
At constant volume, specific heat of gas is 0.321 Jg-1. If molar mass of gas is 45 g mol-1 then what will be atomicity of gas?
Answer:
Cv = Cs × molar mass = 0.321 × 45
Cv = 14.445 J mol-1
R = 8.314J mol-1
Cp = Cv + R = 14.445 + 8.314
= 22.759 J mol-1
Atomicity = \(\frac{C_p}{C_v}\)
= 1.6
So atomicity of gas = 1
Question 7.
Complete combustion of 2.5 g octane is carried out in bomb calorimeter in excess o oxygen. In calorimeter, observed increase temperature is 6.75 K. If heat capacity o calorimeter is 8.93 kJ K-1, calculate internal energy and heat exchanged.
Answer:
Heat taken by calorimeter
qv = Cv∆T
= 8.93 × 6.75
qv = 60.2775 kJ
(C8H18) molar mass of octane
= 8 × 12 + 18 × 1
= 96 + 18 = 114 mol-1
− ∆U = Сv ∆T M/m
- ∆U = 8.93 × 6.75 × 114/2.5
∆U = - 2748.654 kJ mol-1
Question 8.
781 k cal heat is released when 1 mol of benzene oxidised completely at 298 and 1 atm pressure. Calculate the enthalpy of reaction at constant volume.
Answer:
Oxidation of benzene is given by following equation.

ΔΗ = - 781 kcal
No. of moles of reactant = 15/2
No. of moles of product = 6
\(\Delta n_g=6-\frac{15}{2}=-1.5\)
ΔΗ = -781 kcal
ΔΗ = -781000 cal
T = 298 K
R = 1.987 cal K-1mol-1
ΔU =?
ΔU = ΔH - ΔngRT
= - 78100 - (- 1.5) × 1.987 × 208
= - 77480.56 = - 77.480 kcal.
Question 9.
At 300 K and constant volume, 7.8 g benzene releases 327 kJ heat on complete combustion. Calculate the enthalpy of combustion at constant pressure for benzene.
Answer:
Enthalpy of combustion of 7.8 g benzene = - 327kJ
Heat released by 1 g of benzene = imm = 3270 kJ
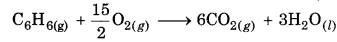
ΔU = - 3270 kJ; Δng = 6 - 7.5 = (- 1.5)
R = 8.314 × 10-3 kJ K-1mol-1, T = 300 K
ΔH = AU + ΔngRT
= - 3270 + (- 1.5 × 8.314 × 10-3 × 300)
= -3270 - 3.7413
ΔH = -3273.741kJ

Question 10.
Calculate the enthalpy of reaction at constant pressure and 25°C. Also give the difference in enthalpy of reaction at constant volume. 2C6H6(g) + 15O2(g) → 12CO2 (g) + 6H2O(l)
Answer:
2C6H6(g) + 15O2(g) → 12CO2(g) + 6H2O(l)
ΔH - ΔU = PΔV
Δη = Δηg RT
Δηg = 12 - 15 = - 3
R = 8.314 × 10-3 kJ deg-1 mol-1
T = 273 + 25 = 298 K
ΔH - Δ = (-3) × 8.314 × 10-3 × 298
= - 7.432 kJ
Question 11.
Combustion of glucose is given by following equation
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l); ∆c Ho = 2900 kJ mol Calculate the released heat by combustion of 1.8 g of glucose.
Answer:
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
1 mol (180 g) ∆H = - 2900 kJ mol-1
Heat released from 1 mol of glucose = 2900 kJ
Hence, amount of heat released from 1.8 g of glucose
\(=\frac{2900}{180} \times 1.8=\mathbf{2 9} \mathbf{k} \mathbf{J}\)
Question 12.
The values of AH for following reaction at 25°C are given as:
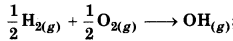
∆H = 10.6 k cal
H2(g) → 2H(g); ∆H = 104.18 k cal
O2(g) → 2O(g); ∆H = 118.32 k cal
Calculate the bond enthalpy of O-H from above data.
Answer:
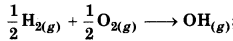
∆H = 10.6 k cal
H2(g) → 2H(g); ∆H = 104.18 k cal
O2(g) → 2O(g); ∆H = 118.32 k cal
Average bond entlapy (O - H)

= 105.95 k cal
Question 13.
Standard enthalpy of formation of CCl4(g), H2O(g), CO2(g) and HCI at 298 K are 25.5, 57.8, -94.1 and 22.1 kcal mol-1 respectively. Calculate the A, H® for following reaction. CCl4(g) + 2H2O(g) → CO2(g) + 4HCl(g)
Answer:
∆rH = ∑∆f H(p) - ∑∆f H(R)
= [4 × ∆f H(HCl) + ∆f H(CO2) ] - [ ∆f H(CCl4) + 2 x ∆f H(H2O)]
= [(4 × - 22.1) + (-94.1)] - [25.5 + 2 × 57.8]
= [-88.4 - 94.1] - [25.5 + 115.6]
= - 182.5 - 141.1
= - 3236 kcal mol-1
Question 14.
Calculate the bond enthalpy of Cl-Cl bond from following data:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g); ∆r, H = -100.3 kJ mol-1
Bond enthalpy of C-H, C-Cl and H-Cl are 413, 326 and 431 kJ mol-1respectively.
Answer:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g);
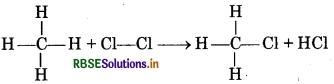
Hence, ∆r,H = Σ Bond enthalpy of reactants - Σ Bond enthalpy of products
∆rH = (4 × ∆H c - H + ∆HCl-Cl) - (3 × ∆HC-H + ∆Hc-cl + ∆HH-Cl)
- 100.3 = [4 × 413 + ∆HCl-Cl] - [3 × 413 + 326 + 431]
- 100.3 = [1652 + ∆HCl-Cl] - [1239 + 326 + 431]
- 100.3= [1652 + ∆HCl-Cl] - [1996]
∆HC-Cl = 100.3 - 1652 + 1996
= - 1752.3 + 1996
∆HC-Cl = + 243.7 kJ mol-1
Question 15.
For the reaction, HCN(g) + 2H2(g) → CH3NH2(g); ∆rHo =-150 kJ. Calculate bond enthalpy of C = N if bond enthalpies of C-H, H-H, N-H and C-N are 414, 435, 369 and 293 kJ mol-1 respectively.
Answer:
HCN(g) + 2H2(g) → CH3NH2(g);
or
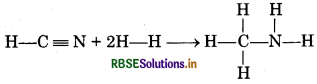
∆r H0 = Σ Bond enthalpies of reactants - Σ Bond enthalpies of products
\(\begin{aligned} &=\left[\left(\Delta H_{\mathrm{C}-\mathrm{H}}^0+\Delta H_{\mathrm{C}=\mathrm{N}}^{\ominus}\right)+\left(2 \times \Delta H_{\mathrm{H}-\mathrm{H}}^0\right)\right] \\ &-\left[3 \times \Delta H_{\mathrm{C}-\mathrm{H}}^0+\Delta H_{\mathrm{C}-\mathrm{N}}^0+2 \times \Delta H_{\mathrm{N}-\mathrm{H}}^0\right] \end{aligned}\)
- 150= [414 + ∆HC=N + 2 × 435]
- [3 × 414 + 293 + 2 × 369]
- 150= [414 + ΔΗC=N + 870] - [1242 + 293 + 738]
- 150 = [1284 + ΔΗC=N] - [2273]
- 150 = - 989 + ΔΗC=N
ΔΗC=N = 989 - 150 = 839 kJ mol-1

Question 16.
Determine the enthalpy of formation of dry Al2Cl6 from following data:
(i) 2Al(s) + 6HCl(aq) → Al2 Cl6(aq) + 3H2(g); ΔΗ = -1004 kJ
(ii) H2(g) + Cl2(g) → 2HCl(g); ΔH = - 183.9 kJ
(iii) HCl(g) + aq → HCl(aq); ΔH = - 732 kJ
(iv) Al2 Cl6(s) + aq → Al2 Cl6(aq); ΔH = 6430 kJ
Answer:
2Al(s) + 3Cl2(g) → Al2Cl6(aq) ; ΔH = ?
In equation (ii) multiply by 3 and add to equation (i)
3H2(g) + 3Cl2(g) → 6HCl(g); ΔΗ = - 183.9 × 3 = - 551.7 kJ
2Al(s) + 6HCl(aq) → Al2Cl6(aq) + 3H2(g); ΔH = - 1004.0 kJ
On addition
2Al(s) + 6HCl(aq) + 3H2(g) + 3Cl2(g) → 6HCl (g) + Al2Cl6(aq) + 3H2(g)
ΔH = (-551.7) + (- 1004.0) = - 1555.7 kJ ......... (v)
In equation (iii) multiply by 6 and add to equation (v)
6HCl (g) + aq → 6HCl(aq); ΔΗ = 73.2 × 6 = - 439.2 kJ
Al(s) + 6HCl(aq) + 3H2(g) + 3Cl2(g) → 6HCl (g) Al2Cl6(aq) + 3H2(g);
ΔΗ = - 1555.7 kJ
On addition,
2Al(s) + 3Cl2(g) + aq → Al2Cl6(aq);
ΔΗ = - (1555.7 + 439.2)
or
2Al (s) + 3Cl2(g) + aq → Al2Cl6(aq);
ΔΗ = - 1994.9 kJ ....... (vi)
Subtract eq. (iv) from eq. (vi), we have
2Al(s) + 3Cl2(g) + aq → Al2Cl6(aq);
ΔH = − 1994.9 kJ
Al2Cl6(s) + aq → Al2Cl6 (aq); ΔH = 643.0 kJ
On subtraction,
2Al(s) + 3Cl2(g) → Al2Cl6(s) ;
ΔH = - 1351.9 kJ
Enthalpy of formation for Al2 Cl6(s) = 13519 kJ
Question 17.
Calculate the entropy for following reversible process if 1 mol a-tin converts to B-tin at 1 atm pressure and 15°C temperature. (ΔHtrans (Sn) = 2100 J mol-1)
Answer:
Δtrans H(Sn) = 2100 J mol-1
Ttrans(Sn) = 15 + 273 = 288
Δtrans S = \(\frac{\Delta_{\text {trans }} H}{T_{\text {trans }}}\)
= 7.29 JK-1mol-1
Question 18.
Following data are known for MgSO4 ΔH = 7.80 kJ mol-1; ΔS = 7.0 JK-1mol-1. Calculate its melting point.
Answer:
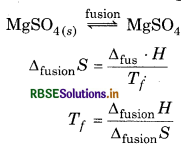
Δfusion H = 7.8 kJ mol-1
= 7.8 × 103 J mol-1
= 7800 J mol-1
Δfusion S = 7.0 JK -1mol -1.
\(T_f=\frac{\Delta_{\text {fus }} \cdot H}{\Delta_{\text {fus }} \cdot S}\)
= 1114.3 K
question 19.
(a) In a cylinder of cooking gas, 11.2 kg butane is filled. The chemical equation of combustion of butane is
 ∆cH = - 2658 kJ mol-1
∆cH = - 2658 kJ mol-1
If a family requires 15000 kJ heat daily for cooking food, then when will the cylinder be finish?
(b) Assume that due to incomplete combustion, 30% gas becomes waste then in this condition how many days a cylinder can be used?
Answer:

∆cH = - 2658 kJ mol-1
Molar mass of butane = 4C + 10H
= 4 × 1210 × 1 = 58 g
According to equation,
∵ 58 g butane gives 2658 kJ heat
∴11.2 kg (11200 g) butane will give =
\(\frac{2658}{58}\) × 11200
= 513269 kJ
∵ Family requires heat/day = 15000 kJ
∴ No. of days to finish cylinder = \(\frac{513269}{15000}\)
= 34.2≈ 34 days
(b) According to question, only 70% butane is utilized in combustion
∴ 70% butane will give heat = \(\frac{513269 \times 70}{100}\)
= 359283.3 kJ
Now, no. of days to finish cylinder = \(\frac{359283.3}{15000}\)
= 23.9 24 days.
Question 20.
Calculate change in free energy per mol for following reactions :
(i) CaCO3(s) → CaO(s) + CO2(g) at 298K;
∆H = + 177.9 kJ; ∆S = 160.4 JK-1
(ii) 2NO2(g) → N2O4(g) at 298K; ∆H = - 57.2 kJ;
Answer:
(i) Given, ∆H = 177.9 × 103 J
∆S = 160.4 JK-1
T = 298 K
∆G = ∆H - T∆S
= 177900 - 298 × 160.4
= 177900 - 47799.2
= 130100.8 J
= 130.1kJ.
(ii) Given ∆H = - 57.2 × 103 J; ∆S = - 175.6 JK-1;
∆G = ∆H - T∆S
= - 57200 - (- 175.6 × 298)
= - 57200 + 52328.8
= - 4871.2 J
= - 4.87 kJ
Question 21.
For a reaction, equilibrium constant is 10 at 300 K. Calculate the ∆G°. (R = 8.314 JK-1mol-1)
Answer:
∆G° = - RT In K = -2.303 RT log Kp p
R = 8.314 JK -1 mol-1
T = 300 K
Kp = 10
∆G° = - 2.303 × (8.314 JK-1 mol-1) × (300 K) log 10
= - 5744.14 J mol-1
= - 5.744 kJ mol-1

Question 22.
Comment on thermodynamics of NO(g) and NO2(g) on the basis of following reactions:
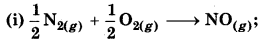
∆r H = 90 kJ mol-1
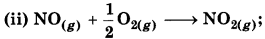
∆r H = 74.0 kJ mol-1
Answer:
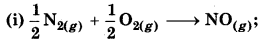
∆r H = 90 kJ mol-1
Since, ∆r H® is +ve. So, thermodynamically NO is not table. So it is an endothermic process.
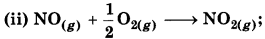
∆r H = - 74.0 kJ mol-1
Since ∆r H is negative. So it is thermodynamically table. The oxidation of NO to NO, is an exothermic process.
Question 23.
Calculate the equilibrium constant at 298 K.
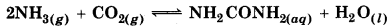
At given temperature ∆r G = - 13.6kJ mol1.
Answer:
As we know that,
\(\log K_c=\frac{\Delta_r G^{\ominus}}{2.303 R T}=\frac{\left(-13.6 \times 10^3 \mathrm{~J} \mathrm{~mol}^{-1}\right)}{2.303\left(8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1} \times 298^{\prime}\right)}\)
Kc = antilog 2.38
= 2.4 × 102
Question 24.
The enthalpy of combustion for CO2 is - 393.5 kJ mol-1. Calculate the heat released during the formation of 35.2 g CO2 from carbon and oxygen.
Answer:
C(s) + O2(g) → CO2(g); ∆H = -393.5 kJ mol-1
Free energy released in the formation of 44g CO2
= 393.5 kJ mol-1
∴ Energy released in the formation of 35.2 g CO2
\(=\frac{393.5 \times 35.2}{44}=314.8 \mathrm{~kJ}\)
Question 25.
N2(g) + 3H2(g) → 2NH3; ∆r H = - 92.4 kJ mol-1. Calculate the enthalpy of formation of gas?
Answer:
Standard enthalpy of formation of NH3 gas:
\(\Delta_f H_{\mathrm{NH}_3(g)}^{\ominus}=\frac{-92.4}{2}\)
= - 46.2 kJ mol-1
So standard enthalpy of formation of NH3 gas is 46.2 kJ mol-1.
Competitive Exam Questions:
Question 1.
If E° cell is negative for any given reaction then correct relation for value of AG° and keg will be:
(a) ∆G° 0; kea <1
(b) ∆G° > 0; keq <1
(c) ∆G° > 0; keq >1
(d) ∆G° < 1; keq >1
Answer:
(b) ∆G° > 0; keq <1
Question 2.
The correct thermodynamic conditions spontaneous reactions at all temperatures are:
(a) ∆H < ∆S = 0
(b) ∆H > 0 and S > 0
(c) ∆H <0 and ∆S > 0
(d) ∆H < 0 and ∆S < 0
Answer:
(c) ∆H <0 and ∆S > 0

Question 3.
If bond enthalpies of H-H, Br-Br and H-Br are 433, 192 and 364 kJ mol-1 respectively. Then for reaction H2(g) + Br2(g) → 2HBr(g) AH° will be:
(a) +261 kJ
(b) -103 kJ
(c) -261 kJ
(d) +103 kJ
Answer:
(b) -103 kJ
Question 4.
Enthalpies of combustion of carbon and CO are -393.5 and -283 kJ mol1 respectively. The enthalpy of formation of CO will be:
(a) -676.5 kJ
(b) 676.5 kJ
(c) 110.5 kJ
(d) -110.5 kJ
Answer:
(d) -110.5 kJ
Question 5.
Combustion of CH4 is given as CH4(g) + 2O2(g) → CO2(g) + 2H2O(g). What will be the number of moles to form 11.0 g CO2 after combustion?
(a) 0.02
(b) 0.20
(c) 0.25
(d) 0.50
Answer:
(c) 0.25
Question 6.
For a sample of ideal gas the change in pressure is Pi to pf at isothermal process. The change in entropy will be :
(a) AS = RT ln \(\left[\frac{P_i}{P_f}\right]\)
(b) AS = nR In \(\frac{P_f}{P_i}\)
(c) AS = nR ln \(\left(\frac{P_i}{P_f}\right)\)
(d) AS = nRT In \(\left(\frac{P_i}{P_f}\right)\)
Answer:
(c) AS = nR ln \(\left(\frac{P_i}{P_f}\right)\)
Question 7.
Which statement is correct for entropy?
(a) Value of entropy is zero at 0°C for crystalline substance.
(b) Entropy is +ve for crystalline substance at absolute temperature.
(c) Entropy is zero at absolute temperature for all crystalline substances.
(d) Entropy is zero at absolute zero temperature for pure crystalline substances.
Answer:
(d) Entropy is zero at absolute zero temperature for pure crystalline substances.
Question 8.
The enthalpy of combustion of carbon into CO2 is -393.5 kJ/mol. The amount of heat released to form 35.2 g CO2 from carbon and oxygen is :
(a) -315 kJ
(b) +315 kJ
(c) -630 kJ
(d) -3.15 kJ
Answer:
(a) -315 kJ
Question 9.
Following reaction is carried out at 398 K,
2NO(g) + O2(g) 2NO2(g)
At 298 K, Gibb's free energy of formations of NO (g) is 86.6 kJ/mol. What will be standard Gibb's free energy of NO2(g) at 298 K? (Kp = 1.6 × 1012)
(a) R (298) ln (1.6 × 1012) - 86600
(b) 86600 + R (298) In (1.6 × 1012)
(c) 86600 - \(\frac{\ln \left(1.6 \times 10^{12}\right)}{R(298)}\)
(d) 0.5 [2 × 8,6600 - R (298) In (1.6 × 1012)]
Answer:
(d) 0.5 [2 × 8,6600 - R (298) In (1.6 × 1012)]

Question 10.
Which statement is correct for reversible process in equilibrium state?
(a) ∆G = 2.303 RT log k
(b) ∆G° = - 2.303 RT log k
(c) ∆G° = 2.303 RT log k
(d) ∆G = 2.303 RT log k
Answer:
(b) ∆G° = - 2.303 RT log k
Question 11.
Which of the following statement is correct for spontaneous adsorption of gas?
(a) ∆S is +ve so AH should be -ve
(b) ∆S is +ve so AH should be highly +ve
(c) ∆S is +ve so AH is highly +ve
(d) ∆S is +ve so AH should be -ve
Answer:
(d) ∆S is +ve so AH should be -ve
Question 12.
For the process H2O(l) → H2O(g) at 100°C and 1 atmosphere pressure, the correct choice is:
(a) ∆S sys > 0 and ∆Sgurr > 0
(b) ∆S sys > 0 and ∆Ssurr < 0
(c) ∆S sys < 0 and ∆Ssurr > 0
(d) ∆S < 0 and ∆Ssurr < 0
Answer:
(b) ∆S sys > 0 and ∆Ssurr < 0
Question 13.
For the reaction
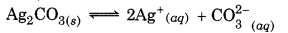
What will be value of K sp of Ag2CO3 of Ag2CO3(s) in water at 25°C (AG° = + 63.3 kJ, R = 8.314 JK-1 mol-1)
(a) 2.9 × 10-3
(b) 7.9 × 10-2
(c) 3.2 × 10-26
(d) 8.0 x 10-12
Answer:
(d) 8.0 x 10-12
Question 14.
For the reaction, C2H5OH (l) + 3O2(g) → 2CO2(g) + 3H2O(l), on complete combustion of ethanol in bomb calorimeter at 25°C, heat released is 1364.47 kJ mol-1. What will be enthalpy of combustion (∆CH)?
(a) 1366.95 kJ mol-1
(b) - 1361.95 kJ mol-1
(d) - 1350.50 kJ mol-1
(c) - 1460.50 kJ mol-1
Answer:
(a) 1366.95 kJ mol-1
Question 15.
At constant temperature of 37.0°C, 0.04 mol of ideal gas filled in piston expands reversibly from 50.0 mL to 375 mL and in this, 208 J heat is absorbed. What will be value of q and w for the process?
(a) q = + 208 J, w = - 208 J
(b) q = - 208 J, w = - 208 J
(c) q = - 208 J, w = + 208 J
(d) q = + 208 J, w = + 208 J
Answer:
(a) q = + 208 J, w = - 208 J
Question 16.
Pressure-volume work done by ideal gas system at constant volume (where E internal energy of system), is:
\(\text { (a) }-\frac{\Delta P}{P}\)
(b) zero
(c) - VAP
(d) - ΔΕ
Answer:
(b) zero
Question 17.
For isothermal expansion of an ideal gas, the correct combination of thermodynamic parameter will be:
(a) ΔU = 0, Q = 0, W ≠ 0, ΔH ≠ 0
(b) ΔU ≠ 0, Q ≠ 0, W ≠ 0, ΔH ≠ 0
(c) ΔU = 0, Q ≠ 0, W = 0, ΔH ≠ 0
(d) ΔU = 0, Q ≠ 0, W ≠ 0, ΔH ≠ 0
Answer:
(d) ΔU = 0, Q ≠ 0, W ≠ 0, ΔH ≠ 0
Question 18.
Mixing of two different ideal gases under isothermal reversible condition will be lead to:
(a) increasing Gibb's free energy of system
(b) no change in entropy of system
(c) increasing entropy of system
(d) increasing enthalpy of system
Answer:
(c) increasing entropy of system
Question 19.
Entropy change (dS) is defined as :
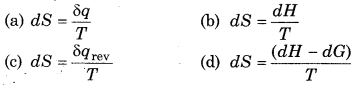
Answer:
\(\text { (c) } d S=\frac{\delta q_{\mathrm{rev}}}{\boldsymbol{T}}\)
Question 20.
On the basis of following equations, the heat of dimerization of NO2 will be :
(i) N2 + 2O2 → 2NO2; ΔH = 67.9 kJ
(ii) N2 + 2O2 → N2O4; ΔH = 9.3 kJ
(a) +77.2 kJ
(b) -77.2 kJ
(c) -58.6 kJ
(d) +58.6 kJ
Answer:
(c) -58.6 kJ
Question 21.
When 5L a gas mixture of methane and propane under go combustion completely at 0°C and 1 atm pressure, 16 L oxygen is utilized at same temperature and pressure. The amount of heat released in this combustion will be:
[ΔΗ comb CH4 = 890 kJ mol-1, ΔH comb C3H8 = 2220 kJ mol-1]
(a) 38
(b) 317
(c) 477
(d) 32
Answer:
(b) 317
Question 22.
Three thermochemical reactions are given below:
1. C(graphite) + O2(g) → CO2(g); Δ,H° = X kJ mol-1
2. C(graphite) + 1/2O2(g) → CO2(g) ΔH = Y KJ mol-1
3. CO(g) + 1/2 O2(g) → CO2(g) ΔH = Z KJ mol-1
What will be correct relation on the basis of these equations?
(a) Z = X + Y
(b) X = Y + Z
(c) Y = 2Z - X
(d) X = Y = Z
Answer:
(b) X = Y + Z
Question 23.
Standard enthalpy of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ/mol, -300 kJ/mol and -1300 kJ/mol respectively. The standard enthalpy of combustion of per gram of glucose at 25°C is :
(a) +2900 kJ
(b) -2900 kJ
(c) -16.11 kJ
(d) +16.11 kJ
Answer:
(c) -16.11 kJ
Question 24.
The equal volume of two monoatomic gases A and B are mixed at same temperature and pressure. What will be ratio of?
(a) 0.83
(b) 1.50
(c) 3.3
(d) 1.67
Answer:
(d) 1.67

Question 25.
Enthalpy change of vaporization of 1.0 kg of liquid water at 0°C and 1 atm pressure will be
(a) 2367 kJ kg-1
(b) -2367 kJ kg-1
(c) -2367 kJ mol-1
(d) -2367 kJ g-1
Answer:
(a) 2367 kJ kg-1
Question 26.
Which one is false?
\(\text { (a) } \frac{\Delta G_{\text {sys }}}{\Delta S_{\text {total }}}=-T\)
(b) for isothermal process, Werv = -nRT In Vf/Vi
(c) \(\ln K=\frac{\Delta H^{\circ}-T \Delta S^{\circ}}{R T}\)
(d) \(K=e^{-\Delta G \% R T}\)
Answer:
(c) \(\ln K=\frac{\Delta H^{\circ}-T \Delta S^{\circ}}{R T}\)
Question 27.
In following reactions, which has ∆S° = +ve and on increasing temperature, ∆G° decreases rapidly :
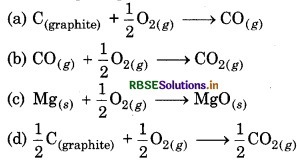
Answer:
\(\text { (c) } \mathrm{Mg}_{(s)}+\frac{1}{2} \mathrm{O}_{2(g)} \longrightarrow \mathrm{MgO}_{(s)}\)
Question 28.
Standard enthalpy of vaporisation of water at 100°C is 40.66 kJ mol-1. The internal energy of vaporisation of water at same temperature. (in kJ mol-1) is :
(a) + 37.56
(b) - 43.76
(c) + 43.76
(d) + 40.66
Answer:
(a) + 37.56
Question 29.
A reaction is spontaneous at 298 K but non spontaneous at 350 K what is true for this?

Answer:
\((\mathrm{e})+\quad+\quad-\)
Question 30.
What will be entropy change for 2 mol of ideal gas in which volume expands from 10 dm3 to 100 dm3 in isothermal reversible process at25° C ?
(a) 38.3 kJ mol-1 K-1
(c) 32.3 kJ mol-1 K-1
(b) 35.8 kJ mol-1K-1
(d) 42.3 kJ mol-1 K-1
Answer:
(a) 38.3 kJ mol-1 K-1

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter