RBSE Class 11 Chemistry Important Questions Chapter 5 States of Matter
Rajasthan Board RBSE Class 11 Chemistry Important Questions Chapter 5 States of Matter Important Questions and Answers.
RBSE Class 11 Chemistry Chapter 5 Important Questions States of Matter
Multiple Choice Questions:
Question 1.
The ratio of diffusion rate of oxygen and unknown gas is 8 : 10, the molecular weight of unknown gas is
(1) 64
(2) 25
(3) 32
(4) 50
Answer:
(4) 50
Question 2.
Which has minimum number of molecules
(1) 0.1 mol SO2
(2) 11 LSO2
(3) 22 g CO2
(4) 22.4 × 100 mL SO2
Answer:
(1) 0.1 mol SO2

Question 3.
Which gas has maximum diffusion rate?
(1) O2
(2) CO2
(3) NH3
(4) N2
Answer:
(3) NH3
Question 4.
At which condition vander Waal's real gases behave as ideal gas
(1) at high temperature and low pressure
(2) at low temperature and high pressure
(3) at high temperature and high pressure
(4) at low temperature and low pressure
Answer:
(1) at high temperature and low pressure
Question 5.
1 mol of CO2 contains
(1) 6.02 × 1023 carbon atoms
(2) 6.02 × 1023 oxygen atoms
(3) 18.1 × 1023 CO2 molecules
(4) 5 g atoms of CO2
Answer:
(1) 6.02 × 1023 carbon atoms
Question 6.
Gas constant 'R' depends upon
(1) temperature of gas
(2) volume of gas
(3) number of moles of gas
(4) none of the above
Answer:
(4) none of the above
Question 7.
For any gas, rms velocity change with vapour density (d) at fixed pressure
(1) d2
(2) d
(3) \(\sqrt{d}\)
\(\text { (4) } \frac{1}{\sqrt{d}}\)
Answer:
\(\text { (4) } \frac{1}{\sqrt{d}}\)

Question 8.
The relative diffusion rate of a gas (M, wt = 98) with hydrogen will be
(1) 1/5
(2) 1/3
(3) 1/7
(4) 1/9
Answer:
(2) 1/3
Question 9.
There is deviation in behaviour of ideal gas because their molecules
(1) are of negligible volume
(2) have attraction force
(3) are polymolecules
(4) are not attached to each other
Answer:
(1) are of negligible volume
Question 10.
At fixed volume of gas, the increase in pressure with temperature for fixed moles of gas occurs due to
(1) increase in average velocity of molecules
(2) increase in moles of gas
(3) increase in attraction
(4) decrease in average free path
Answer:
(1) increase in average velocity of molecules
Question 11.
Which one is gas equation
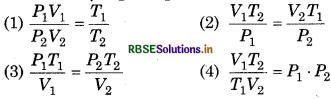
Answer:
\(\text { (1) } \frac{P_1 V_1}{P_2 V_2}=\frac{T_1}{T_2}\)
Question 12.
Kinetic energy for 1 mol of gas is

Answer:
\(\text { (1) } \frac{3}{2} R T\)
Question 13.
The volume of 1 mol molecule of gas at NTP is 22.4 L. This fact was proposed by
(1) Dalton's theory
(2) Avogadro's principle
(3) Berzilius Theory
(4) Law of gaseous volume
Answer:
(2) Avogadro's principle

Question 14.
The gas has equal density to N2 is
(1) C2H6
(2) NH3
(3) CO
(4) CH4
Answer:
(3) CO
Question 15.
The ratio of rms of ozone and oxygen at 27°C is
Answer:
\(\text { (1) } \sqrt{\frac{3}{5}}\)
\(\text { (2) } \sqrt{\frac{4}{5}}\)
\(\text { (3) } \sqrt{\frac{2}{3}}\)
(4) 0.25
Question 16.
P, V, M, T and R are pressure, volume, molar mass, temperature and gas constant respectively, then density of ideal gas will be

Answer:
\(\text { (3) } \frac{P M}{R T}\)
Question 17.
At constant temperature, in fixed mass of ideal gas
(1) product of pressure and volume is always constant
(2) ratio of pressure and volume is always constant
(3) volume is always constant
(4) pressure is always constant
Answer:
(1) product of pressure and volume is always constant
Question 18.
For n mole the vander Waal's equation for real gas is
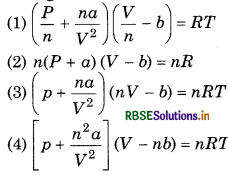
Answer:
\(\text { (1) }\left(\frac{P}{n}+\frac{n a}{V^2}\right)\left(\frac{V}{n}-b\right)=R T\)
Question 19.
The relation between R and K is (if N A Avagadro's number)
(1) R = KN
(2) RK = NA
(3) R + K = NA
(4) K = RNA
Answer:
\((1) R = KN \)
Question 20.
Which one has maximum surface tension
(1) Ethanol
(2) Methanol
(3) H2O
(4) Benzene
Answer:
(3) H2O
Very Short Answer Type Questions:
Question 1.
What is the relation between Kelvin and Celsius scale of temperature?
Answer:
K = t°C + 273.
Question 2.
What is air pressure and write its unit in SI system?
Answer:
The force exerted by molecule of gas at per unit rea is called air pressure and its SI unit is Pascal (Pa).

Question 3.
What is significance of van der Waal's constant 'a' and 'b'?
Answer:
Constant ‘a' tells about strength of intermolecular ttraction and 'b' tells about size of gaseous molecules.
Question 4.
Why size of balloon increases with height (in atmosphere)?
Answer:
As height increases, atmospheric pressure educes, hence the volume expands inside the balloon nd size increases.
Question 5.
Why He and H2 do not liquify at room temperature?
Answer:
Because their critical temperature is less tha room temperature.
Question 6.
What is critical temperature?
Answer:
The maximum temperature at which any gas ca be converted into liquid state and above thi liquification of gas is not possible even at high pressure
Question 7.
What is critical pressure?
Answer:
It is minimum amount of pressure to liquify an gas at its critical temperature.
Question 8.
What is critical volume?
Answer:
It is the volume occupied by 1 mol of any gas a critical temperature and critical pressure.
Question 9.
What is viscosity coefficient?
Answer:
It is the force which is proportional to the area contact of layer and velocity gradient.
Question 10.
What is the effect the effect of temperature viscosity?
Answer:
When we increased the temperature, the viscosit of liquid will be decreased.
Question 11.
Why vapour pressure of water is more tha mercury?
Answer:
Because water contains less intermolecular force of attraction.
Question 12.
What is aqueous tension?
Answer:
It is the pressure exerted by water vapour.
Question 13.
When real gas behaves likes ideal gas?
Answer:
At high temperature and atmospheric pressur real gas behaves like ideal gas.

Question 14.
Although gases have different densities i atmosphere however they don't appear i separate layer, why?
Answer:
Because of diffusion.
Question 15.
The liquification of H2 and He is complicated Why?
Answer:
Because their critical temperature is low.
Question 16.
What is the difference between diffusion an effusion?
Answer:
In diffusion, molecules of gas spread out evenly i available space whereas in effusion, molecules of ga escape out through a tiny hole.
Question 17.
Write the name of two gases which become heat in Joule-Thomson effect?
Answer:
H2 and He.
Question 18.
Arrange the SO2, NH3, H2O and CO2 in decreasing order of liquefcation?
Answer:
SO2 > NH3 > H2O > CO2.
Question 19.
What are isothermal lines?
Answer:
These are lines drawn between P and V at constant temperature.
Question 20.
Why the volume of gases is not zero at absolute temperature ?
Answer:
Because each gas is liquefied prior to its absolute temperature hence for gases PV = nRT, is not applicable at absolute temperature.
Question 21.
Why gases liquefy at low temperature and high pressure?
Answer:
Increasing pressure, the molecules come close to each other while on decreasing temperature, the kinetic energy of molecules is reduced.
Question 22.
Which force acts between molecules of oxygen?
Answer:
Since oxygen molecules are non-polar so the force acts between them is London force.
Question 23.
What is the decreasing order of van der Waal's force between Cl2, Br2 and I2?
Answer:
The molecules with high molecular weight experience more van der Waal's forces. Hence, order will be I2 > Br2 > Cl2.

Question 24.
Illustrate the graph between PV and P for any gas.
Answer:
The graph between PV and P will be parallel to e, axis.
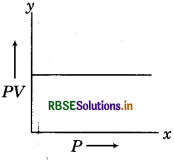
Question 25.
Write the kinetic gas equation?
Answer:
PV = 1/2 m Nu 2
where P = Pressure of gas
V = Volume of gas
m = Mass of molecule of gas
N = Number of molecules of gas
u2 = Square root velocity
Question 26.
Write the relationship between average kinetic energy and Boltzman's constant.
Answer:
Average kinetic energy of a molecule = 3/2 KT
where k = Boltzman constant
T = absolute temperature
Question 27.
What is the relationship between all three speeds of gases?
Answer:
Urms = Square mean root velocity
ū = Average velocity
a = Probability velocity
\(\begin{aligned} & u_{\mathrm{rms}}: \bar{u}: \alpha \\ & \sqrt{\frac{3 R T}{M}}: \sqrt{\frac{8 R T}{M}}: \sqrt{\frac{2 R T}{M}} \\ & 1.7: 1.6: 1.4 \\ & 1: 0.921: 0.816 \end{aligned}\)
Question 28.
What is the significance of critical temperature in liquification of gas?
Answer:
The minimum temperature at which any gas can be converted to liquid state state is called critical 1 temperature. For example, critical temperature of CO2 is 31.1°C.
Question 29.
Why low pressure of air is kept in summer in vehicle tyres?
Answer:
Because on increasing the temperature, pressure of gas also increases.
Question 30.
There is no change in temperature of ideal gases in Joule Thomson effect, why?
Answer:
Because, there is no attraction force between molecules of ideal gas. Thus in Joule Thomson effect, there is no change in temperature of ideal gas.
Question 31.
Out of N2O, O3, NO3, which one has diffusion rate similar to CO2?
Answer:
The diffusion rate of CO2 will similar to that whose molecular weight is similar to CO. So, diffusion rate of N2O will be similar to CO2.
Question 32.
What is isobar curve?
Answer:
The curve plotted at constant pressure is known as isobar curve.

Question 33.
What is real gas?
Answer:
Real gas is that which does not shows the behaviour of ideal gas under all conditions of temperature and pressure.
Question 34.
At STP, if 1 g of CO, H2O, CH4 and NO2 is taken then (i) Which gas will show maximum volume and (ii) Which one shows minimum?
Answer:
(i) At STP, 1 g of CH4 has maximum volume and
(ii) NO will contain minimum volume.
Question 35.
Define Boyle's temperature?
Answer:
It is defined as "the temperature at which a real gas obeys ideal gas law over an appreciable range of pressure.
Question 36.
In which condition, Boyle's law is applicable?
Answer:
Boyle's law is appliciable, when temperature of gas constant.
Question 37.
Is it possible to apply Dalton's law of partial pressure at the mixture of CO and O2?
Answer:
No, this law is not fit for these gases because these gases react with each other. CO and O2 react together to form CO2.
Question 38.
If molecular collision would be elastic then how it affects molecular kinetics?
Answer:
If molecular collision would be elastic then it owers the kinetic energy of molecules. The consistent decrease in kinetic energy of molecules will lead to stop the motion of molecules.
Question 39.
For any gas if compressibility factor Z is less than 1, then what does it mean?
Answer:
If Z <1, then it indicates that gas is more compressible than ideal gas.
Question 40.
In NH3 and N2, which has maximum value of 'a' and which has maximum value of 'b'.
Answer:
1. NH3 contains more value of 'a' because in NH3, nteraction takes place due to polarity and hydrogen bonding.
2. In N2, the value of 'b' will be maximum because size of molecule of Në is greater than that of NH3.
Question 41.
From the following pairs of liquid, separate the liquid with high vapour pressure:
(1) Alcohol and Glycerine.
(2) Petrol and Kerosene.
(3) Mercury and Water.
(4) Water and Honey.
(5) Alcohol and Ether.
Answer:
The vapour pressure of liquid is inversely proportional to intermolecular forces of attraction etween molecules. So, liquid with high vapour pressure
(1) Alcohol
(3) Water
(2) Petrol
(4) Water
(5) Ether.

Question 42.
Explain the effect of temperature 01 following:
(1) Density;
(2) Surface tension;
(3) Viscosity
(4) Vapour density of liquid.
Answer:
(1) On increasing temperature, the density decreases.
(2) On increasing temperature, the surface tension decreases.
(3) On increasing temperature, the viscosity decreases.
(4) On increasing temperature, the vapour density decreases.
Question 43.
Physical properties of ice, water and vapour are different, what will be the chemica composition of water in all three states?
Answer:
In all states, the chemical composition of water wil remain same i.e., it will remain as H2O.
Question 44.
Which factors are responsible to determine the states of matter?
Answer:
These factors are pressure, temperature, mass and volume.
Question 45.
What will be molar volume of Nitrogen and Argon at 273.15 K and 1 atm pressure?
Answer:
22.4 L.
Question 46.
Arrange the following in increasing order of their surface tension:
Water, Alcohol and Hexane.
Answer:
Hexane < Alcohol < Water.
Question 47.
Name that energy, which is produced due to motion of molecules or atoms. What is the effect of temperature on this energy?
Answer:
It is known as thermal energy, it rises with rise in temperature.
Question 48.
Name that intermolecule force which is produced during liquid state of HF.
Answer:
Dipole-dipole interaction.
Question 49.
Critical temperature and critical pressure of CO2 is 30.98°C and 73 atm respectively. Is it possible to liquefy CO2 gas at 32°C and 80 atm?
Answer:
It is not possible to liquefy CO2 gas at 32°C even when pressure is 80 atm because this temperature is more than its critical temperature.
Question 50.
Arrange the following gases in their increasing value of b and explain its reason also? O2, CO2, H2, He
Answer:
H2 < He < O2 < CO2. In this order the size
increases, hence value of 'b' increases.

Question 51.
Arrange the CH4, O2, H2 in their decreasing order of 'a'.
Answer:
CH4 > O2 > H2.
Short Answer Type Questions:
Question 1.
Why non-crystalline solids are considered as super cooled liquid?
Ans.
The constitutent particles of non-crystalline solids are not arranged in regular manner i.e., they are arranged like liquid state. Therefore, non-crystalline solids are considered as super cooled liquid due to high viscosity.
Question 2.
Prove that average kinetic energy of a molecule is equal to 3/2 RT.
Answer:
Suppose mass of gas molecule = m, and square root velocity = u then
Average kinetic energy = 1/2 mv2 ....... (1)
According to kinetic equation of gas
\(\begin{aligned} & P V=\frac{1}{3} m N \bar{u}^2=\frac{2}{3} \times \frac{1}{2} m N \bar{u}^2 \\ & P V=\frac{2}{3} \mathrm{KE} \quad\left[\because \mathrm{KE}=\frac{1}{2} m N \bar{u}^2\right] \end{aligned}\) ........ (2)
Hence KE = 3/2 PV ....... (3)
Ideal gas equation PV = nRT ..... (4)
Hence from equation (4) and eq (3)
KE = 3/2 nRT ...... (5)
In eq (5) divide by Avogrado number N,
kinetic energy of gas molecules KE = 3R/2NT = 3/2KT
where K = Boltzman constant.
Question 3.
Define the critical constants of gas?
Answer:
Critical temperature, critical pressure and critical volumes are collectively known as critical constants of gas. These are denoted by Tc, Pc and Vc respectively. Critical Temperature It is the maximum temperature at which any gas can be converted to liquid state. Above this temperature, liquefication of gas is not possible even at high pressure. It is denoted by Tc. For example, Tc for CO2 gas is 31.1°C.
- Critical Pressure: It is a minimum amount of pressure s to liquefy any gas at its critical temperature. It is b denoted by Pc. For example, the critical pressure of CO2 F gas is 72.9 atm.
- Critical Volume: The volume occupied by 1 mol of any gas at its critical temperature and critical pressure. It is denoted by Vc. For example, the Vc for CO2 is 94 mL.
Question4.
What is the effect of vapour pressure on boiling point?
Answer:
Effect of Vapour Pressure on Boiling Point: The vapour pressure affects the boiling point of any liquid. For example: For the normal boiling point of water, the vapour pressure is equal to 1 atm pressure. If external pressure is less than 1 atm pressure then water will boil at low temperature. This is the reason that on hill station, food boils with difficulty.
Question 5.
Explain why the viscosity of liquid decreases with increase in temperature?
Answer:
The viscosity of liquid decreases with increasing temperature because on increasing temperature the kinetic energy of molecules also increases. Due to more kinetic energy, the flow of molecules increases. Arrhenius and Geezman gave equation on the basis of experiments which shows the effect of temperature on viscosity.
n = Ae -Ea/RT
\(\log \frac{\eta_1}{\eta_2}=\frac{E a}{2.303 R}\left[\frac{T_2-T_1}{T_1 T_2}\right]\)

Question 6.
Which methods are used to determine the vapour pressure. Describe any one of them in detail?
Answer:
Following methods are used to determine vapour pressure:
(A) Static method
- Barometric Method
- Isotonisocpic Method
(B) Dynamic Method
(C) Gas Saturation Method.
Barometric Method: As depicted in figure, two barometric tubes are filled with mercury and placed inverted in a container. Now the liquid, to which vapour pressure is to be determined is placed in container with help of curved pipette. This liquid reached to top through mercury, gets vaporized and reached at equilibrium. The level of mercury in one tube falls down ecause in tube there is vapour pressure of liquid. Reference tube is used for comparison. So by this method, vapour pressure can be determined by lifference in level of mercury in tubes.
f the difference in mercury level in both tube is h cm, the ›ressure exerted by vapour is = hdg
where d density of mercury and g = gravitational acceleration.
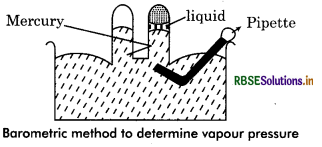
Question 7.
Write applications of liquefied gases?
Answer:
Applications of Liquefied Gases: The liquefied cases have following applications :
- Liquefied gases are used to maintain minimum temperature in laboratory.
- Liquid Freons (CCl2F2) etc., are used as refigerating agent in air conditioner.
- Liquefied air is used in rocket and jet as main source of oxygen.
- Liquefied oxygen is used in welding.
- Liquefied Cl2 is used as insecticide for water treatment.
- Liquefaction of gases is useful to separate same gases mixture. For example, Ne and Ar are separated from air.
Question 8.
Why alcohol and ether are stored in cold place?
Answer:
Both alcohol and ether are volatile in liquid form ecause of this their boiling points are very low. At high emperature, they volatile easily. Hence, to minimize heir evaporation, they are placed at cold place.
Question 9.
Why bottle of liquid ammonia is cooled before opening?
Answer:
The liquid ammonia is filled in bottle at high ressure. If bottle is opened without cooling, then it can e burst because there will be reduction in pressure and ue to this, kinetic energy of molecule will be high. So if ve cool the bottle, then the kinetic energy of molecules ot be increased and gas will come outside slowly and vill not cause any accident.
Question 10.
Why is food cooked fast in pressure cooker?
Answer:
There is more vapour pressure in pressure cooker so boiling point of water is high i.e., water can accep more temperature that's why temperature of pressur cooker becomes high, and results in fast cooking in pressure cooker.
Question 11.
Why soda water bottles are kept refrigerator in summer season?
Answer:
Soda water is the mixture of CO2 and water. T make it, gas is flowed in water at high pressure. Sinc gas is insoluble in water, hence at high pressure, i dissolves easily. In summer season, the solubility of gas in water is less due to high temperature. Hence, summer, gas accumulates at bottom of bottle and pressure increases inside the bottle which can not tolerated by bottle and it may burst. To avoid this bottles are kept in refrigerator.
Question 12.
Which air has high weight? Dry or wet.
Answer:
Dry air mainly contains oxygen, nitrogen and som other gases. It also contains some amount of wate vapour. The vapour density of water vapour is less tha that of N2 and O2. Since molecular mass of N2 is 28, O2 is 32 and H2O is 18. In wet air, the heavy molecules of N and O2 are displaced by water vapour. Hence, dry air heavy than wet air.

Question 13.
Define heat of vaporisation?
Answer:
Heat of Vaporisation: The amount of energ required to evaporate a fixed amount of liquid a constant temperature is called heat of vaporisation of energy required to evaporate 1 mol of liquid is called molar heat or molar energy.
For example : Molar energy for water at 25°C or 298 F is 44.180 kJ/mol.
H2O → H2O(g)
= + 44.180 kJ/mol
ΔΗ. Heat of vaporisation is related to strength of attraction forces between molecules. If force of attraction is high then heat of vaporisation will also be high.
Question 14.
Write the value of universal gas constant in various units?
Answer:
(i). Value of universal gas constant (R) in litr atmosphere per Kelvin per mol.
= 0.0821 L atm K-1mol-1
(ii) Value of R in erg per Kelvin per mol
= 8.314 × 107 erg K-1mol-1
(iii) Value of R in Joule per Kelvin per mol
= 8.314 J K-1mol-1
(iv) Value of R in cubic cm-bar per Kelvin per mol
= 82.1 cm3bar K-1 mol-1
(v) Value of R in litre-bar per Kelvin per mol
= 0.0831 L bar K-1 mol-1
(vi) Value of R in calorie per Kelvin per mol
= 2 cal K-1mol-1
Numerical Examples:
Question 1.
The volume of any gas is 100 cm3. If it is expanded by 30% then calculate the final temperature at constant pressure if initial temperature is 20°C.
Answer:
Increase in volume = 30%
Initial volume (V1) = 100 cm
Increase in initial volume =
Final volume (V2) = 100 + 30 = 130 cm
Now, V1 = 100 cm3, V2 = 130 cm3
T1 = 20°C = 20 + 273 - 293 K,
T2 = ?
According to Charles' law,

= 380.9 K
Question 2.
About 5 L hydrogen gas is filled in a balloon at 22°C. If temperature increases upto 25°C, then what will be the volume of balloon?
Answer:
Given, V1 = 5L, V2 =?
22 + 273 = 295 K;
T2 = 25 + 273 = 298 K.
According to Charles' law,
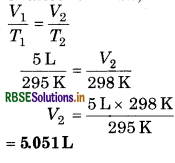
Question 3.
A gas sample occupies 75 dm3 volume at temperature 298 K and pressure 1 bar. If volume is reduced to 10 dm3 at same temperature then how much extra pressure will be required?
Answer:
Given, P1 = 1 bar, V1 = 75 dm3, P2 = ?,
V2 = 10 dm3
From Boyle's law,
1 bar × 75 dm = P2 × 10 dm3
\(P_2=\frac{1 \mathrm{bar} \times 75 \mathrm{dm}^3}{10 \mathrm{dm}^3}\)
P2 = 7.5 bar
Extra pressure
7.5 - 1 = 65 bar

Question 4.
The pressure of a cylinder filled with kitchen gas is 11 bar at temperature 25°C. If tolerence capacity of cylinder is 16 bar then at what temperature it will burst?
Answer:
Given: Maximum pressure (P1) = 16 bar
T1 =?, P2 = 11 bar, T2 = 25 + 273 = 298 K
From Gay Lussac's law,
\(\begin{aligned} \frac{16 \mathrm{bar}}{T_1} & =\frac{11 \mathrm{bar}}{298 \mathrm{~K}} \\ T_1 & =\frac{16 \mathrm{bar} \times 298}{11 \mathrm{bar}} \end{aligned}\)
T1 = 433.45 K
T1 = 433.45 - 273
= 160.45°C
Question 5.
For any given sample of gas how much amount of pressure should be apply that it can compress by 3/4 of its original volume?
Answer:
Given; V1 = V; V2 = 3/4V; P1 = P; P2 = ?
From Boyle's law,
P1V1 = P2V2
P× V = P2 x 3/4
\(P_2=\frac{4}{3} P\)
So, pressure will be 4/3 times.
Question 6.
A gas occupies 0.6 dm3 volume at pressure of 0.92 bar. Calculate that pressure at which the volume of gas will be reduced upto 20% of its original volume,
volume, when temperature constant.
Answer:
Given; P1 = 0.92 bar; P2 =? V1 = 0.6 dm3 ;
V2 = 20% reduction of original volume = \(\frac{0.6 \times 20}{100}\)
V2 = Original volume - 20% volume
= 0.6 - 0.12 = 0.48 dm3
Now, according to Boyle's law,
P1V1 = P2V2
\(P_2=\frac{0.92 \mathrm{bar} \times 0.6 \mathrm{dm}^3}{0.48 \mathrm{dm}^3}\)
= 1.15 bar
Question 7.
The volume of oxygen was found to be 12.0 dm3 at 423 K and 0.987 bar. Calculate the mass of oxygen?
Answer:
Given; P = 0.987 bar; ;V = 12.0 dm3; R = 0.831 bar m3k-1mol-1; T = 423 K; n = ?
From ideal gas equation,
PV = nRT
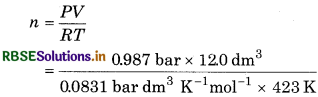
= 0.336 mol
Molar mass of oxygen = 32 g mol -1
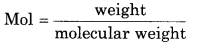
weight = mol x molar volume
= 0.336 × 32
= 10.782 g
So mass of oxygen = 10.782 g.
Question 8.
Using the formula PV = nRT, calculate the value of 1 g hydrogen at STP (R = 0.0821L atm K-1 mol-1).
Answer:
Given, Standard pressure (P) = 1 atm
absolute temperature (T) = 273 K
R = 0.0821 L atm K-1mol-1
No. of moles of hydrogen (n) = 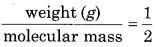
V = ?
Now, from ideal gas equation,
PV = nRT
\(\begin{aligned} V & =\frac{n R T}{P} \\ & =\frac{0.5 \mathrm{~mol} \times 0.0821 \mathrm{~L} \mathrm{~atm} \mathrm{~K}^{-1} \mathrm{~mol}^{-1} \times 273 \mathrm{~K}}{1 \mathrm{~atm}} \end{aligned}\)
= 11.2 L
∴ Volume of 1 g hydrogen at STP = 11.2 L
Question 9.
If 1 L flask at 7.6 × 10-10 mm Hg, oxygen gas is present. Calculate the number of molecules of oxygen at 0°C temperature.
Answer:
From ideal gas equation, PV = nRT
P = 7.6 × 10-10 mm (Hg)
\(=\frac{7.6 \times 10^{-10} \mathrm{~mm}(\mathrm{Hg})}{760 \mathrm{~mm}(\mathrm{Hg})} \times 1 \mathrm{~atm}\)
V = 1L; R = 0.0821 L atm K-1 mol-1
T = 0 + 273 = 273 K
∴ 
According to Avogadro's law, number of molecules in 1 mol
= 6.022 × 1023
∴ Number of molecules of oxygen
= n × 6.022 × 1023
\(=\frac{1 \times 10^{-12} \times 6.022 \times 10^{23}}{0.0821 \times 273}\)
= 2.69 × 1010
∴ Number of molecules of oxygen
= 2.69 × 1010

Question 10.
The volume of a gas is 11 g dm at STP. At constant pressure, how much temperature is required so that volume of gas would be 8 g dm-3 ?
Answer:
As we know that;
d1T1 = d2T2 (at constant pressure)
Given, d1 = 11 g dm3,
d2 = 8 g dm-1
T1 = 273 K, T2 = ?
\(T_2=\frac{d_1 T_1}{d_2}=\frac{11 \mathrm{~g} \mathrm{dm}^{-3} \times 273 \mathrm{~K}}{8 \mathrm{~g} \mathrm{dm}^{-3}}\)
T2 = 375.38 K
T2 = 375.38 - 273 = 102.38°C
Question 11.
In 2 L container, 1023 molecules of NO2 are filled at 27°C. Calculate the pressure in container?
Answer:
Given, T = 27+ 273 = 300 K; V = 2.0 L
R = 0.0821 L atm K-1mol-1
Number of moles of NO2
\(=\frac{10^{23}}{6.022 \times 10^{23}}\)
0.166 mol
From ideal gas equation,
PV = nRT
\(P=\frac{n R T}{V}\)
\(=\frac{0.166 \mathrm{~mol} \times 0.0821 \mathrm{~L} \mathrm{~atm} \mathrm{~K}{ }^{-1} \mathrm{~mol}^{-1} \times 300 \mathrm{~K}}{2.0 \mathrm{~L}}\)
= 0.204 atm
So, pressure in container = 0.205 atm.
Question 12.
Temperature of a gas is t K, at which temperature, the pressure and volume of gas remains half?
Answer:
From gas equation
\(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)
Given; T1 = t K, T2 = ?, P1 = P mm,
P2 = p/2
V1 = V mL, V2 = V/2
Substituting the values
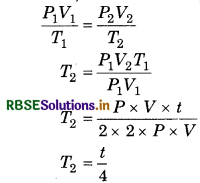
T2 = 0.25t K
Question 13.
A pot, whose volume is 2.461 L contains 0.3 mol N2, 0.5 mol He and 6.2 mol O2 at 27°C. Calculate the partial pressure of gases in mixture?
Answer:
First Method
From ideal gas equation,
\(P=\frac{n R T}{V}\)
T 27 + 273 = 300 K
R = 0.0821 L atm K-1mol-1
V = 2.461 L
Partial pressure of Nitrogen:
\(P_{\mathrm{N}_2}^{\prime}=\frac{0.3 \times 0.0821 \times 300}{2.461}\) = 3.002 atm
Partial pressure of Helium
\(P_{\mathrm{He}}^{\prime}=\frac{0.5 \times 0.0821 \times 300}{2.461}\)
= 5.004 atm
Partial pressure of Oxygen
\(P_{\mathrm{O}_2}^{\prime}=\frac{6.2 \times 0.0821 \times 300}{2.461}\)
= 62.050 atm
Second Method:
Total number of moles in mixture
0.3 + 0.5 + 6.2= 7 moles
Total pressure 'PT' \(=\frac{n \times R \times T}{V}\)
\(=\frac{7 \times 0.0821 \times 300}{2.461}\)
= 70.06 atm
Partial pressure = Total pressure × mole fraction Now, partial pressure of Nitrogen
P'N2 = PT × Mole fraction of N2 gas
= 70.06 × 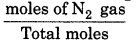
= 3.002 atm
Partial pressure of Helium
\(P_{\mathrm{He}}=\frac{70.06 \times 0.5}{7}\)
= 5.004 atm
Partial pressure of Oxygen
\(P_{\mathrm{O}_2}^{\prime}=\frac{70.06 \times 6.2}{7}\)
= 62.050 atm

Question 14.
The density of mixture of N2 and O2 is 1.3 g/L at NTP. Calculate the partial pressure of O2 and N2?
Answer:
Suppose n1 moles of O2 and no moles of N2 are mixed together.
So, average molecular weight of gaseous mixture
\((M)=\frac{32 \times n_1+28 \times n_2}{n_1+n_2}\)
Similarly for mixture,
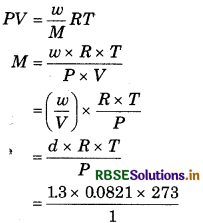
M = 29.137 g mol-1
From eq. (1) and (2),
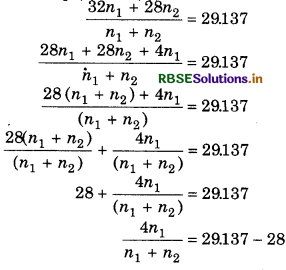
Mole fraction of O2 = 0.284
Mole fraction of N2 = 1 - 0.284 = 0.716
According to Dalton's partial pressure law,
PO2 = PT × nO2
= 1 x 0.284
= 0.284 atm
PN2 = PT × nN2
= 1 × 0.716
= 0.716 atm
So,
PO2 = 0.284 atm
PN2 = 0.716 atm
Question 15.
The pressure of mixture of H2 and N2 filled in a pot is 750 bar. If partial pressure of N2 in mixture is 150 bar then calculate the ratio of molecules of H2 and N2 in mixture?
Answer:
Total pressure of mixture = 750 bar
Partial pressure of N2 = 150 bar
partial pressure of H2 = 750 - 150 = 600 bar
According to ideal gas equation
\(\begin{aligned} & P_{\mathrm{N}_2}=\frac{n_{\mathrm{N}_2} R T}{V}=150 \mathrm{bar} \\ & P_{\mathrm{H}_2}=\frac{n_{\mathrm{H}_2} R T}{V}=600 \mathrm{bar} \end{aligned}\)
Since, volume V and temperature T are equal
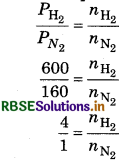
So,
nN2 : nH2 = 4 : 1
Question 16.
In a mixture, 2.0 g of gas A and 1.4 g of gas B are mixed. If molar mass of A and B are 120 and 90 respectively, and total pressure of mixture is 0.921 bar. Then calculate partial pressure of A and B?
Answer:
Moles of A = 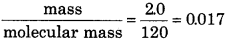
moles of B = 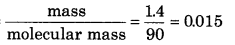
Total moles = 0.017 + 0.015 = 0.032
Total pressure of mixture = 0.921 bar
\(\begin{aligned} P_A & =\frac{0.017}{0.032} \times 0.921 \\ & =0.489 \mathrm{bar} \\ P_B & =\frac{0.015 \times 0.921}{0.032} \end{aligned}\)
= 0.432 bar
Question 17.
80 mL NHg gas is diffused from a pot in 9 mi at 20°C and 550 mm Hg pressure. At the sam temperature and pressure, how much tim will be consumed by unknown gas whos molar mass is 70 and volume is 50 mL.
Answer:
Volume of NH3 = 80 mL
Time taken in diffusion
\(r_{\mathrm{NH}_3}=\frac{80}{9}\)
Volume of unknown gas = 50 mL
time for diffusion = t min
\(r_{\text {gas }}=\frac{50}{t}\)
According to Graham's law of diffusion
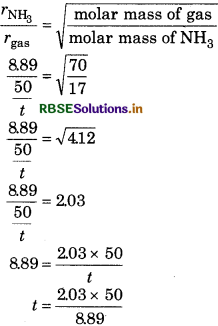
= 11.42 min
Question 18.
Determine the ratio of diffusion rates of 235 UF and 238 UFC?
Answer:
Molecular weight of 235UF6
Molecular weight of 238 UF6 = 352
From Graham's law of diffusion,
\(\begin{aligned} & \frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1}} \\ & \frac{r_1}{r_2}=\sqrt{\frac{352}{349}} \end{aligned}\)
10043: 1

Question 19.
Determine the amount of KClO3, which is heated to obtain 2.5 L oxygen at 28°C temperature and 750 mm Hg pressure?
Answer:
Calculation of volume of free oxygen at NTP 5. V1 = 2.5 L, V2 =?, P1 = 750 mm Hg, P2 = 1 atm = 760 mm n Hg, T1 = 28 + 273 = 301 K, T2 = 273 K.
From gas equation,
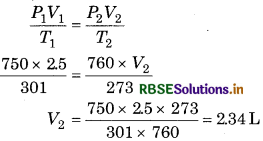
Amount of KClO3 :
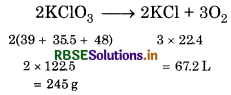
Amount of KCIO3 required to release 67.2 L of
O2 = 245 g
for 1 L Oxygen = \(\frac{245}{67.2 \mathrm{~g}}\)
= 8.53 g
Question 20.
When zinc powder (ZnO + Zn) is dissolved in dilute H2SO4, then 150 mL H2 gas is released at NTP. Calculate the percentage of Zn in zinc powder? (Atomic mass of Zn = 65 g mol-1).
Answer:
Both Zn and ZnO react with dil. H2SO4 but only Zn releases H2 gas.
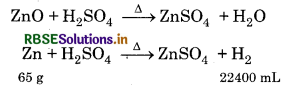
∵ Amount of Zn, required to release 22400 mL H2 gas = 65 g
∴ for 150 ml H2 = \(\frac{65 \times 150}{22400}\) = 0.435 g
% of Zn in zinc powder =
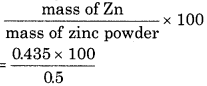
= 87%
Question 21.
How much volume of CO2 will be obtained if A 10.0 g pure marble is treated with HCl at 30°C and 800 mm pressure?
(The aqueous tension at 30°C is 26.7 mm)
Answer:
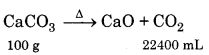
∵ 100 g CaCO3 gives = 22400 mL CO2
∴ 10 g will give = imm = 2240 mL
Calculation of volume of CO2 :
V1 = 2240 mL, V2 =?, P1 = 760 mm, P2 = 800 - 26.7
= 773.3 mm, T1 = 273 K, T2 = 30 + 273 = 303 K.
From gas equation

= 2443.39 mL
Question 22.
Calculate the value of critical temperature (Tc) and critical pressure (Pc) for oxygen if van der Waal's constant a and b are 1.32 and 0.312 dm3 mol-1
respectively.
Answer:
α = 1.32 dm3 mol-1,
b = 0.0312 dm3 mol-1,
R = 0.0821 dm3 bar mol-1K-1
Critical temperature of oxygen
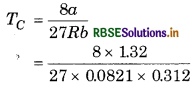
= 152.69 K
Critical pressure of Oxygen
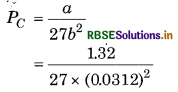
= 50.22 bar
Question 23.
Determine the square root velocity (urms), average velocity (ū) and probable velocity (a) of SO2 at STP.
Answer:
Given, T = 0 + 273 = 273 K, M = 64 g mol
\(u_{\mathrm{rms}}=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 273}{64}}\)
= 3.26 × 104 cm/s
verage velocity (ū) = 0.9213 × Urms
= 0.9213 x 3.26 × 104
= 3.00 × 104 cm/s
probable velocity (a) = 0.816 × Urms
0.816 × 3.26 × 104
= 266 x 104 cm/s

Question 24.
The compressibility factor for 1 mol van der Waal's gas at 0°C and 100 atm pressure is 0.5. Calculate the van der Waal's constant ‘a' considering the volume of gas molecules negligible.
Answer:
Compressibility factor (Z) = \(\frac{P V}{R T}\)
V = 0.112 L
According to vander Waal's equation
\(\left(P+\frac{a}{V^2}\right)(V-b)=R T\)
If b is negligible then,
\(\left[100+\frac{a}{(0.112)^2}\right]\) = 0.0821 × 273
a = 1253 L2 mol-2 atm
Question 25.
Calculate the compressibility factor of 0.4 L CO2 at 300 K and 40 atm pressure?
Answer:
Compressibility factor (Z)
\(=\frac{40 \mathrm{~atm} \times 0.4 \mathrm{~L}}{1 \mathrm{~mol} \times 0.0821 \mathrm{~L} \mathrm{~atm} \mathrm{~K} \mathrm{~K}^{-1} \mathrm{~mol}^{-1} \times 300 \mathrm{~K}}\)
Z = 0.65
Question 26.
Calculate, the average speed of molecules of 15 g gas whose volume is 12.5 L at 3 atm pressure.
Answer:
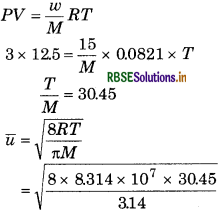
= 8.03 × 104 cm/s
Question 27.
What will be the total pressure of mixture formed by mixing 8 g O2 and 4 g H2 at 27°C in a flask whose volume is 1 dm3?
Answer:
Number of moles of oxygen
\(\begin{aligned} \left(n_1\right) & =\frac{\text { mass of oxygen }}{\text { molar mass }} \\ & =\frac{8 \mathrm{~g}}{32 \mathrm{~g} \mathrm{~mol}^{-1}}=0.25 \mathrm{~mol} \end{aligned}\)
Number of moles of H2
\(\begin{aligned} \left(n_2\right) & =\frac{\text { mass of Hydrogen }}{\text { molar mass }} \\ & =\frac{4 \mathrm{~g}}{2 \mathrm{~g} \mathrm{~mol}^{-1}}=2 \mathrm{~mol} \end{aligned}\)
Partial pressure of O2
\(\begin{aligned} \left(P_{\mathrm{O}_2}\right) & =\frac{n_1 R T}{V} \\ & =\frac{0.25 \times 0.83 \times 300}{1 \mathrm{dm}^3} \end{aligned}\)
= 6.225 bar
Partial pressure of H2
\(\begin{aligned} \left(P_{\mathrm{H}_2}\right) & =\frac{n_2 R T}{V} \\ & =\frac{2 \times 0.083 \times 300}{1 \mathrm{dm}^3} \end{aligned}\)
= 49.8 bar
Total pressure PTotal = PO2 + PH2
= 6.225 + 49.8
= 56.025 bar
Question 28.
If 1010 wheat grains are spread out in second then how much time is required t spread wheat grains equivalent Avogadro's number?
Answer:
If 1010 wheat grains spread in 1 second.
Then time required for 6.022 × 1023 grains

= 1.91 × 106 years
Question 29.
Payload is obtained when mass of displace air is subtracted from mass of balloon. If 10 kg helium is filled in a balloon whose radiu is 10 m, at temperature 27°C and pressure 1.6 bar. Then calculate payload. (density of air, d = 12k gm and R = 0.08 bar dm3 K-1mol-1)
Answer:
Calculation of displaced air
radius of balloon r = 10 cm
volume of balloon (V) = \(\frac{4}{3} \pi r^3\)
\(=\frac{4}{3} \times \frac{22}{7} \times(10)^3\)
= 4190.5 m3
Mass of displaced air = volume of balloon
= 4190.5 × 12
= 5028.6 kg
Calculation of mass of balloon
Moles of He (n) \(=\frac{P V}{R T}\)
\(=\frac{1.66 \times 4190.5 \times 10^3}{0.083 \times 300}\)
= 279.37 × 103 mol
Mass of He = Mol of He × Mass of He
= 279.37 × 103 × 4
= 1117.48 × 103
= 1117.48 kg
So mass of filled balloon
= 100 + 1117.48
= 1217.48 kg
Calculation of Payload
Payload = Mass of displaced air - Mass of filled balloon
= 5028.6 - 1217.48
= 3811.12 kg
Competitive Exam Questions:
Question 1.
A gas with molecular weight 39 g mol-1 ha critical density 0.1 g cm3. Its critical volum in L mol-1 will be
(a) 0.390
(b) 0.90
(c) 0.039
(d) 390
Answer:
(a) 0.390

Question 2.
Which of the following relation is correct fo liquid vapour in liquid-vapour equilibriu state
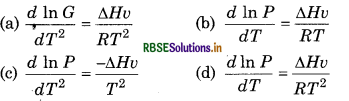
Answer:
\(\text { (d) } \frac{d \ln P}{d T}=\frac{\Delta H v}{R T^2}\)
Question 3.
Equal moles of O2 and H2 are kept in a p which can escape through a tiny hole. Ho much part of oxygen will escape whe hydrogen escape to half
(a) 1/8
(b) 1/4
(c) 3/8
(d) 1/2
Answer:
(a) 1/8
Question 4.
In a mixture, of gases the ratio of H2 and gas is 1 : 4 (w/W). In mixture, what will b molar ratio of these gases
(a) 4 : 1
(b) 16 : 1
(c) 2 : 1
(d) 1 : 4
Answer:
(a) 4 : 1
Question 5.
At what condition, CO will follow ideal gas law
(a) at high temperature and low pressure
(b) at low temperature and high pressure
(c) at high temperature and high pressure
(d) at low temperature and low pressure
Answer:
(a) at high temperature and low pressure
Question 6.
The square root velocity of molecules of CO is 1000 m/s at 27°C. What will be square root velocity of molecule of N2 at 600 K ?
(a) 2000 m/s
(b) 1414 m/s
(c) 1000 m/s
(d) 1500 m/s
Answer:
(b) 1414 m/s
Question 7.
A gas can be liquefied at temperature T and pressure P
(a) T = Tc and P < Pc
(b) T = Tc and P > Pc
(c) T > Tc and P > Pc
(d) T > Tc and P < Pc
Answer:
(b) T = Tc and P > Pc
Question 8.
If Z is compressibility coefficient then at low pressure, van der Waal's equation can be written as
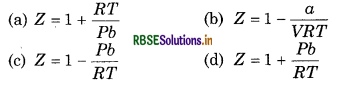
Answer:
\(\text { (b) } Z=1-\frac{a}{V R T}\)
Question 9.
Equal mass of H2, O2 and CH pot of volume V at 27°C under same condition, the ratio of volumes of H2 : O2 : CH4 will be
(a) 16 : 1 : 2
(b) 8 : 1 : 2
(c) 8 : 16 : 1
(d) 16 : 8 : 1
Answer:
(a) 16 : 1 : 2

Question 10.
Maximum deviation from ideal gas is expected from.
(a) NH3 gas
(b) H2 gas
(c) N2 gas
(d) CH4 gas
Answer:
(a) NH3 gas
Question 11.
vander Waal's equation changes into ideal gas equation at.
(a) high temperature and low pressure
(b) low temperature and low pressure
(c) low temperature and high pressure
(d) high temperature and high pressure
Answer:
(a) high temperature and low pressure
Question 12.
For gaseous state, if maximum possible speed is C*, average speed is C and mean square speed is C then what will be their ratio for large number of molecules.
(a) C* C : C= 1.225 : 1.128 : 1
(b) C : C* : C = 1.128 : 1.125 : 1
(c) C* : C : C = 1 : 1.128 : 1.125
(d) C C* : C=1 : 1.125 : 1.128
Answer:
(c) C* : C : C = 1 : 1.128 : 1.125
Question 13.
The density of N2 (28) gas at 227°C and 5.00 atm will be (R = 0.082 L atm K-1mol-1)
(a) 1.40 g/mL
(b) 0.81 g/mL
(c) 3.41 g/mL
(d) 0.29 g/mL
Answer:
(c) 3.41 g/mL
Question 14.
In same condition, 50 mL of each of gas A and B take 150 second and 200 second respectively to diffuse through small pore. If molecular mass of B is 36, then what will be molecular mass of A.
(a) 96
(b) 128
(c) 32
(d) 64
Answer:
(c) 32
Question 15.
At high pressure, the compressibily factor of real gas will be.

Answer:
\(\text { (c) } 1+\frac{P b}{R T}\)
Question 16.
For gases, a and bare van der Waal's constants. Chlorine is liquefied easily as compared to ethane because.
(a) value of a and b of Cl2 > value of a and b of C2H6
(b) value of a and b of Cl2 < value of a and bof C2H6
(c) ‘a' of Cl2 < 'a' of C2H6 but 'b' of Cl2 > 'b of Cl2 H
(d) ‘a' of Cl2 >‘a' of C2H6 but 'b' of Cl2 < 'b' of C2H6
Answer:
(d) ‘a' of Cl2 >‘a' of C2H6 but 'b' of Cl2 < 'b' of C2H6
Question 17.
If temperature (K) is doubled then average speed of gaseous molecule will be increased by factor.
(a) 1.4
(b) 2.0
(c) 2.8
(d) 4.0
Answer:
(a) 1.4
Question 18.
A gaseous mixture was prepared by taking equal moles of CO and N2. If total pressure of mixture is 1 atm, then what will be partial pressure of N2 in mixture.
(a) 1 atm
(b) 0.5 atm
(c) 0.8 atm
(d) 0.9 atm
Answer:
(b) 0.5 atm
