RBSE Class 11 Chemistry Important Questions Chapter 1 Some Basic Concepts of Chemistry
Rajasthan Board RBSE Class 11 Chemistry Important Questions Chapter 1 Some Basic Concepts of Chemistry Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 11 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 11 Chemistry Chapter 1 Important Questions Some Basic Concepts of Chemistry
Multiple Choice Questions:
Question 1.
Number of moles of oxygen in 1 mol oxygen will be:
(1) 1
(2) 100
(3) 6.022 × 1023
(4) 0.623 × 1023
Answer:
(3) 6.022 × 1023
Question 2.
Unit of equivalent weight is:
(1) g
(2) kg
(3) g/L
(4) None of these
Answer:
(4) None of these
Question 3.
Volume of 1 g molecular weight of each gas at N.T.P.
(1) 224 L
(2) 224 cc
(3) 2.24 L
(4) 22.4 L
Answer:
(4) 22.4 L

Question 4.
Number of molecules present in 44 g CO2 are:
(1) 12 × 1023
(2) 3 × 1010
(3) 6 × 1023
(4) 1 × 1023
Answer:
(3) 6 × 1023
Question 5.
Volume of 0.25 mol NH3 gas at S.T.P:
(1) 224 L
(2) 5.6 L
(3) 112 L
(4) 22.4L
Answer:
(2) 5.6 L
Question 6.
For a metal, the formula of its chloride is MC1 and equivalent weight of metal is 9, what wi be atomic weight of element :
(1) 9
(2) 18
(3) 27
(4) 3
Answer:
(3) 27
Question 7.
Atomicity of any element represents:
(1) Atomic number of element
(2) Atomic weight of element
(3) Avogadro number of element
(4) Number of atoms in molecule of element
Answer:
(4) Number of atoms in molecule of element
Question 8.
Equivalent weight of H2SO4 will be:
(1) 98
(2) 49
(3) 196
(4) 448
Answer:
(2) 49
Question 9.
Total number of ions in 111g CaCO3:
(1) 1 mol
(2) 2 mol
(3) 3 mol
(4) 4 mol
Answer:
(3) 3 mol
Question 10.
Air is :
(1) Compound
(2) Mixture
(3) Element
(4) None of these
Answer:
(2) Mixture
Question 11.
1 mol H2O contains:
(1) 6.022 × 1023 oxygen atoms
(2) 6.022 × 1023 hydrogen atoms
(3) 18.1 × 1023 H2O atoms
(4) 3g molecules of H2O
Answer:
(1) 6.022 × 1023 oxygen atoms

Question 12.
What is equivalent weight of oxalic acid (H2C2O42H2O) if its molecular weight 126?
(1) 98
(2) 63
(3) 196
(4) 126
Answer:
(2) 63
Question 13.
Which pair follow the law of multiple proportions?
(1) GO and CO2
(2) H2O and D2O
(3) NaCl and NaBr
(4) MgO and Mg (OH)2
Answer:
(1) GO and CO2
Question 14.
Equivalent weight of MnSO4 remains half molecular weight, when this conversion appears:
(1) in Mn2O3
(2) in MnO2
(3) in MnO4
(4) in MnO2-
Answer:
(2) in MnO2
Question 15.
5 mol X and 9 mol Y 'formed Z according t given reaction X + 2Y → Z, then how muc mol of Z will be formed?
(1) 5 mol Z
(2) 8 mol Z
(3) 14 mol Z
(4) 4 mol Z
Answer:
(4) 4 mol Z
Question 16.
How much g of oxygen will react with 27 g aluminium?
(1) 8 g.
(2) 16 g
(3) 32 g
(4) 24 g
Answer:
(4) 24 g
Question 17.
How much amount of CaCO3 will give 56 kg of CaO?
(1) 1000 kg
(2) 56 kg
(3) 2 kg
(4) 100 kg
Answer:
(4) 100 kg
Question 18.
5.3 g of Na2CO3 is dissolved in 500 mL of water. Molarity of solution will be
(1) 0.1 M
(2) 0.2 M
(3) 0.3 M
(4) 10 M
Answer:
(1) 0.1 M
Question 19.
Which has the minimum number of molecules?
(1) 0.1 mol CO2
(2) 11 ml CO2
(3) 22 g CO2
(4) 22.4 × 103 ml CO2
Answer:
(4) 22.4 × 103 ml CO2

Question 20.
Percentage of oxygen in NaOH is:
(1) 40
(2) 16
(3) 8
(4) 1
Answer:
(1) 40
Very Short Answer Type Questions:
Question 1.
Sometimes air is considered as heterogeneous mixture, why?
Solution:
When dust particles present in air then it is considered as heterogeneous mixture.
Question 2.
Which state of matter has indefinite shape and volume?
Solution:
Gaseous state.
Question 3.
In which state of matter, the intermolecular space is larger and smaller?
Solution:
In gaseous state, intermolecular space is larger whereas in solid, it is smaller.
Question 4.
By which force, molecules attract each other?
Solution:
Intermolecular force.
Question 5.
What is International System of units?
Solution:
International system of unit is known as S.I. It was accepted in 1960 by Conference Generale des Poids et Measures, CGPM.
Question 6.
What is S.I. unit of length?
Solution:
The S.I. unit of length is metre.
Question 7.
How much metre present in one micron?
Solution:
10-6 metre.
Question 8.
How much seconds are present in one solar day?
Solution:
86400 s.
Question 9.
Light year is the unit of which physical quantity?
Solution:
Distance.

Question 10.
What is difference between base units and derived units?
Solution:
Base units are those which are obtained from S other physical quantities and derived units are obtained from base units,
Question 11.
What is Indian standard of time?
Solution:
It is time obtained from caesium watch kept M in National Physical Laboratory (N.P.L).
Question 12.
What is Angstrom (Å) ?
Solution:
It is used for measurement of size of nucleus and wavelength of light. 1Å = 10-10 m
Question 13.
The radius of an atom is 10-10 m. Calculate it is in micrometre.
Solution :
\(10^{-10} \mathrm{~m}=\frac{10^{-10}}{10^{-6}}=10^{-4} \mu \mathrm{m}\)
∵ 1 μm = 10-6
Question 14.
When vanadium is mixed with iron, it forms alloy. The density of vanadium is 5.96 g/cm3. (i What will be its S.I. unit?
Solution:
The density of vanadium will be 5.96 kg/m3 in S.I. or M.K.S. system.
Question 15.
Convert 40 calorie in joule.
Solution:
1 cal = 42 J
∵ 40 cal = 42 × 40 J
= 168.0 J
Question 16.
How much joules are present in 1 erg?
Solution:
1 erg = 10-4 joule.
Question 17.
Write the 0.000213 cm in scientific notation.
Solution:
2.13 × 10-4 cm
= 2.13 × 10-6 m
Question 18.
Convert the 20°C in Fahrenheit.
Solution:
°F = \(\frac{9}{5}\left({ }^{\circ} \mathrm{C}\right)\) (°C) + 32
\(\circ \mathrm{F}=\frac{9}{5} \times 20+32\)
= 36 + 32 = 68
20°C = 68°F
Question 19.
Convert 10°F in °C.
Solution:
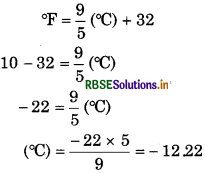
10°F = - 12 22 °C
Question 20.
What is scientific notation of measurement ?
Solution:
In scientific notation, the measurement is ritten in exponential form. It is represented as N × 10n, where N is the number (digit) and n is ponent.

Question 21.
What do you mean by precision and accuracy?
Solution:
Precision refers to closeness of various measurements for the same quantity where as accuracy the agreement of a particular value to the true value of e result.
Question 22.
Express the following in scientific notations?
(i) 0.0012;
(ii) 2130000;
(iii) 2002;
(iv) 600.0;
(v) 5000;
(vi) 7216.3
Solution:
(i) 0.0012 = 12 × 10-3
(ii) 2130000 = 2.13 × 106
(iii) 2002 = 2.002 × 10-3
(iv) 6000 = 6.00 × 102
(v) 5000 = 5.0 × 103
(vi) 7216.3 = 7.2163 × 103
Question 23.
What is the weight of C-12 atom in gram?
Solution:
1.992648 × 10-23 g≈ 1.99 × 10-23 g
Question 24.
When zero is not significant figure for any number?
Solution:
If zero is at left side of non-zero digit then it is ot significant figure. For example; in 0.014, both zero e not significant figures.
Question 25.
May all digit significant in any numbers?
Solution:
Yes, if all digits are non-zero or if zero exists etween two non-zero digits.
Question 26.
Which is more accurate measurement in 5.0 or 5.00 g ?
Solution:
5.00 is more accurate because in this measurement is taken upto two points after decima point.
Question 27.
In which ratio, element combines with mass.
Solution:
According to law of constant proportion.
Question 28.
Who gave law of conservation of mass?
Solution:
Antoine Lavoisier in 1785.
Question 29.
Who gave principle of relativity?
Solution:
Albert Einstein.
Question 30.
What is the energy conservation law Einstein?
Solution:
E = mc2
Question 31.
What do you mean by a.m.u.?
Solution:
It is the Atomic Mass Unit.
Question 32.
Which isotope of carbon is used to expres the relative mass of element?
Solution:
C-12 isotope.

Question 33.
Why atomic masses of an element fractional?
Solution:
Due to presence of their isotopes.
Question 34.
How much atoms are present in 1g atom any element?
Solution:
6.022 × 1022 atoms.
Question 35.
What is atomic mass unit?
Solution:
Atomic mass unit is the 1/12th part of C-12 atom.
So,
1 a.m.u. = \(\frac{1.9926 \times 10^{-23}}{12}\) = 1.66 × 10-24 g
Question 36.
What is the average atomic mass?
Solution:
The average atomic mass of an element refer to the atomic masses of isotopes of the elements takin into account the different abundance of the element' isotopes.
Question 37.
What is molecular mass?
Solution:
Molecular mass shows how many times molecule of substance is heavier than that 1th of 12 th mass of an atom of C-12.
Question 38.
What is molar mass?
Solution:
When mass of any substance is expressed in terms of moles then it is called molar mass for instance molar mass of H2O = 18.02 g.
Question 39.
Calculate the molecular mass of hydrogen if its atomicity is 2.
Solution:
Molecular mass Atomic mass × Atomicity
= 1.008 × 2 = 2.016 u.
∴ Molecular mass of hydrogen will be 2.016 u.
Question 40.
Calculate the number of oxygen atoms in 48u of ozone?
Solution:
Number of atoms of oxygen will be \(\frac{48 u}{16 u}\) = 3
So, there will be 3 atoms of oxygen.
Question 41.
Does molar volume of H2S and CO2 differ ?
Solution:
No, these will be same because at S.T.P., molar volume of all gases is 22.4 L.
Question 42.
Is law of mass conservation valid for nuclear reactions?
Solution:
No, this law is not valid for nuclear reactions.

Question 43.
What will be volume of 1 mol CO2 at S.T.P. ?
Solution:
The volume of 1 mol CO2 at S.T.P. will be 22.4 L.
Question 44.
If a person spends ₹ 1000000 per second, can he spend 1 mol in his life span?
Solution:
∵₹1000000 spends = in 1 second
∴ 6.022 × 1023 rupees will spend
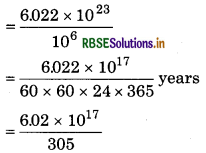
= 2 × 1010 years.
So, it is not possible to spend in life time.
Question 45.
Who expounded the following laws?
(i) Law of constant proportions
(ii) Law of conservation of mass
(iii) Law of multiple proportions
(iv) Law of reciprocal proportions
(v) Law of gaseous volume
Solution:
(i) Joseph Proust,
(ii) Lavoisier,
(iii) Dalton,
(iv) Ritcher,
(v) Gay Lussac.
Question 46.
When Gay Lussac's law of gaseous volume is disobeyed?
Solution:
It is disobeyed when there is solid or liquid state of one of reactant or product.
Question 47.
What is Dulong Patit's law?
Solution:
According to this law, for any metal, the product of atomic mass and specific heat is 6.4.
So, Atomic mass × Specific heat = 6.4
Question 48.
Write the name of two other states of matter?
Solution:
(i) Plasma,
(ii) Bose Einstein Condensate (BEC).
Question 49.
Substract 0.0016 from 3.82 and give answer in significant figures.
Solution:
\(\begin{array}{r} 3.82 \\ -0.0016 \\ \hline 3.8184=3.82 \\ \hline \end{array}\)
Short Answer Type Questions:
Question 1.
Define the relative atomic mass of an element.
Solution:
Atomic mass of any element is that number which represents that how much an atom of element is heavier than 1/12th part of C-12 by mass or 1.008 part by mass of hydrogen. Average relative mass of an atom of S an element as compared to the mass of an atom of C-12.
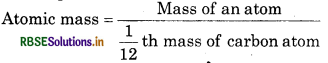
Question 2.
What is meant by formula of compound?
Solution:
The brief representation of molecules in compound is symbol of their elements is known as formula of compound. It is classified as :
- Empirical formula and
- Molecular formula.
1. Empirical Formula: It gives the simplest whole number ratio of the atoms of various elements present in one molecule of compound.
2. Molecular Formula: It gives the actual number of atoms of various elements present in one molecule of the compound:

Question 3.
What is the relation between empirical formula and molecular formula ?
Solution:
Molecular formula = n × Empirical formula.
(Where n = 1, 2, 3, ...)
Question 4.
Differentiate molarity and molality.
tablee
Numerical Questions:
Question 1.
If 2.12 g glucose and 2.925 g salt are mixed in 30.2 g of water. Calculate the mass of solution.
Solution:
Mass of glucose = 2.12 g
Mass of salt = 2.925 g
Mass of water = 302 g
Total mass of solution = 2.12 + 2.925 + 302
= 35.245 g
So, by rounding off the number 35.245 ≈ 35.2 g
Question 2.
Mass of silver metal is 6.342g and its density is 1.28 g/cm3. Calculate its volume.
Solution:
Mass of silver = 6.342 g
Density of silver = 128 g/cm
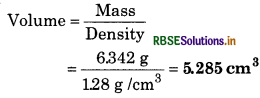
By rounding off it will be 5.3 cm3, which has 2 significant figures.
Question 3.
Mass of 1 atom of hydrogen is 1.008 u. What will be mass of 10 atoms of hydrogen?
Solution:
Mass of 1 atom of hydrogen = 1.008 u
Mass of 10 atoms of hydrogen = 10 × 1.008 u
= 10.08 u
Since 10 is accurate number so its significant number will be infinite. Hence the number of significant figures o resultant number should be equal to significant figure of other numbers. Thus total mass is 10.08 u.
Question 4.
Express the following into International
System of Units (S.I.) :
(i) 90 million miles (Distance between Earth and Sun)
(ii) 90 miles/hour (Speed of Rajdhani Express Train)
(iii) 5 Feet 6 inches (Average length of Indian males)
Solution:
(i) 90 millon miles - S.I. unit of distance is metre.
So,
1 mile = 1.60 km
= 1.60 × 103 metre
= 1.60 × 103 m
Conversion coefficient
= \(\frac{1.60 \times 10^3 \mathrm{~m}}{1 \mathrm{mile}}\)
For 90 million miles = 90 x 106 miles
= 90 x 106 miles × conversion coefficient
\(=\frac{90 \times 10^6 \text { miles } \times 1.6 \times 10^3 \mathrm{~m}}{1 \mathrm{mile}}\)
= 90 × 1.6 × 106 m
= 1.44 × 1011 metre
(ii) 90 miles per hour
1 mile = 1.6 km = 1.6 × 103
Conversion coefficient = \(\frac{1.6 \times 10^3 \mathrm{~m}}{1 \mathrm{mile}}\)
1 Hour = 60 × 60 s = 3.6 × 103s
Conversion coefficient =
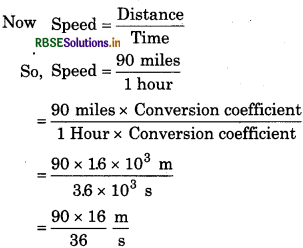
= 40 m/s
(iii) 5 Feet 6 inches = 5 × 12 + 6 = 66 inches
1 inch = 2.54 × 10-2 m
Conversion coefficient = 2.54 × \(\frac{10^{-2} \mathrm{~m}}{\text { inches }}\)
66 inches = 66 inches × conversion coefficient
= 66 × 2.54 × 10-2 m
= 167.64 × 10-2 m
= 1.68 m

Question 5.
Convert the volume of 10L H2O in metre.
Solution:
1 L = 1000 cm3
1m = 100 cm
1L = 10-3 m
Conversion coefficient = \(\frac{10^{-3} \mathrm{~m}^3}{1 \mathrm{~L}}\)
So 10L = 10L × conversion cofficient
\(=\frac{10 . \mathrm{L} \times 10^{-3} \mathrm{~m}^3}{\mathrm{~L}}\)
= 10-2m3
Question 6.
How much mass of sodium chloride will be separated by 4.9 g H2SO4 if 6 g sodium hydrogen sulphate (NaHSO4 ) and 1.825 g HCl formed during the reaction?
Solution:
The chemical equation will be
NaCl + H2SO4 → NaHSO4 + HCl
Mass of reactants = Mass of NaCl + Mass of H2SO4
= x + 4.9 g
Mass of products = Mass of NaHSO4 + Mass of HCl
6.0 g + 1.825g = 7.825 g
According to law of mass conservation
Mass of reactants = Mass of products x + 4.9 g = 7.825 g
x = 7.825 - 4.9 g
Hence, mass of NaCl = 2.925 g
Question 7.
When 10 g CaCO3 is heated, then 5.6 g CaO and 2.24 litre CO2 gas (at S.T.P.) are formed. Prove that data follow the law of mass conservation.
Solution:
Chemical reaction: 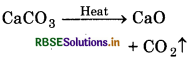
Mass of CaCO3 = 10 g
Mass of CaO = 5.6 g
∵ Mass of 22.4L CO2 at STP = 44 g
Mass of 1L CO2 at STP = \(\frac{44}{22.4} \mathrm{~g}\)
∴ Mass of 2.24L CO2 at STP = \(\frac{44 \times 2.24}{22.4}\) = 4.4 g
Total mass of products = 5.6 + 44 = 10 g
Since mass of products is equal to the mass of reactants. So, data follow law of conservation of mass.

Question 8.
When 2.16 g metallic copper is heated with HNO3, 2.7 g copper oxide is obtained. In other experiment, 1.15 g copper oxide is reduced to give 0.92 g copper. Show that data follow law of constant proportions.
Solution:
From first experiment:
Weight of metallic copper = 2.16 g
Weight of copper oxide = 2.7 g
Weight of oxygen = 2.7 - 2.16 = 0.54 g
Percentage of copper = 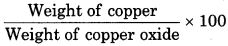
\(=\frac{2.16 \times 100}{2.7}=80 \%\)
Percentage of oxygen = 100 - 80 = 20%
From second experiment :
Weight of metallic copper = 0.92 g
Weight of copper oxide = 1.15 g
Weight of oxygen = 1.15 - 0.92 = 023 g
Percentage of copper = \(\frac{0.92}{1.15}\) × 100 = 80%
Percentage of oxygen = 100 - 80 = 20%
In both experiments, ratio of mass of copper and oxygen are same. So, data obey the law of constant proportions.
Question 9.
Prove that following data obey law of constant proportions. Mass of carbon in the different samples of carbon monoxide are following:
(i) 1.26 g carbon in 1.42 g of carbon monoxide.
(ii) 1.008 g carbon in 1.136 g of carbon monoxide.
Solution:
In first sample:
Mass of CO = 142 g
Mass of C = 126 g
Mass of O = 1.42 - 1.26 = 0.16 g
% of carbon = \(\frac{126}{1.42}\) × 100 = 88.73%
% of oxygen = 100 - 88.73 = 11.27%
In second sample:
Mass of CO = 1.136 g
Mass of C = 1.008 g
Mass of O = 1.136 - 1.008
= 0.128 g
Mass of carbon
% of carbon = 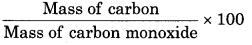
\(=\frac{1.008 \times 100}{1.136}\)
= 88.73%
% of oxygen = 100 - 88.73 = 1127%
Since percentage of carbon and oxygen is same in given data, so it obeys law of constant composition proportions.
Question 10.
In a copper sulphate crystal, 25.45% copper and 36.07% water are present. If law of constant proportion is valid then calculate the weight of copper from 40 g copper sulphate crystal.
Solution:
From given data
% of copper = 25.45% ·
% of water = 36.07%
% of sulphate = 100 - (25.45 + 36.07)
= 100 - 61.52 = 38.48%
From the law of constant composition proportion, the % of copper sulphate in second crystal should be same as first one, then
Amount of Cu in 100 g CuSO4 = 25.45 g
Amount of Cu in 40 g CuSO4 = \(\frac{25.45 \times 40}{100}\)
= 10.18 g
Question 11.
In two sulphides of an element, the mass of sulphur are 53.33% and 36.36% respectively. Prove that data obey the law of multiple proportions.
Solution:
In first sulphide :
Amount of sulphur = 53.33%
Amount of element = 100 - 53.33
= 46.47%
The ratio of percentage of element and sulphur is
46.67 : 53.33 = 1 : 1.14
In second sulphide :
Amount of sulphur = 36.36%
Amount of element = 100 - 36.36
= 63.64
The ratio of percentage of element and sulphur is
= 63.64 : 36.36 = 1 : 0.57
Since amount of element in both sulphides is fixed. Then ratio in both amount is a multiple 0.57 : 1.14 or 1 : 2, which is multiple proportions. So data follow the law of multiple proportions.
Question 12.
Different amounts of A and B mutually react and form three compounds as X, Y and Z.
0.3 g A + 0.4 g B→ 0.7 g X
18.0 g A + 43.0 g B → 66.0 g Y
40.0 g A + 159.99 g B → 199.99 g Z
On the basis of above data, prove the law of multiple proportions.
Solution:
In compound X, Y and Z, the amounts of B
react with A, are
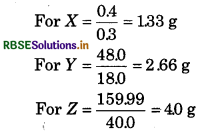
Hence, amounts of B, reacted with A are in following ratio :
1.33 : 2.66: 4.0
or
1: 2 : 3
So law of multiple proportion is obeyed.

Question 13.
Reaction of H2 and Cl2 in presence o sunlight gives HCl gas. Calculate the volum of HCl gas obtained from 20 mL of hydrogen
Solution:
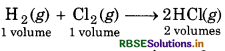
∵1 volume hydrogen gives = 2 volumes HCl
∴ 20 volumes hydrogen will produce = 2 × 20 ml HCl
= 40 ml HCI
Question 14.
Two oxides are formed from a metal. From these oxides, 1g of each is reduced and yiel 0.798 g and 0.888 g metal respectively. Thes results obey which law of chemica combination?
Solution:
In first oxide, amount of oxide = 1.000 g
Amount of metal = 0.798 g
Amount of oxygen = 1.000 - 0.798
=0.202 g
∴ 0.202 g oxygen is combined with = 0.798 g of metal
∴ 1g oxygen will be combined with = \(\frac{0.798}{0.202}\) g of metal
= 3.95 g
In second oxide,
Amount of oxide = 1.00 g
Amount of metal = 0.888 g
Amount of oxygen = 1.00 - 0.888
= 0.112 g
0.112 g oxygen is combined with = 0.888 g of metal
∵ 1g oxygen will be combined with = \(\frac{0.888}{0.112}\) = 7.92 g
So, amount of metal combined to oxygen are 3.95 an 7.92 respectively which are in simple ratio of 1 : 2. S given data follow the law of multiple proportions.
Question 15.
If nitric oxide is formed by followin equation :
N2(g) + O2(g) → 2NO (g)
then calculate the volume of nitroge required to produce 30 L of NO.
Solution:
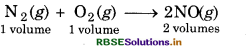
∵ 2 L of NO is obtained by = 1 L of nitrogen
∴ 30 L of NO will be obtained by = 30/2 = 15 L of Nitrogen
Question 16.
6.488 g of lead reacts with 1.002 g of oxygen form PbO2. This PbO2 is also obtained whe lead nitrate is heated, in which percentage o oxygen is 13.38%. Does above information prove the law of constant composition proportions?
Solution:
In first oxide,
Mass of lead peroxide = 6.488 g + 1.002 g = 7.490 g
∵ 7.49 g PbO2 contains = 1.002 g oxygen
∴ 100 g PbO2 will contain = \(\frac{1.002 \times 100}{7.49} \mathrm{~g}\)
= 13.38%
Since in second experiment, percentage of oxygen is 13.38% which is equal to first. So, it proves law of constant composition proportions.
Question 17.
Calculate the molar mass of following:
(i) CH3COOH,
(ii) P4,
(iii) S8,
(iv) PCl5,
(v) SO2Cl2
Solution:
(i) Molar mass of CH3COOH
= 2 × 12 + 4 × 1 + 2 × 16
24 + 4 + 32 = 60 g/mol
(ii) Molar mass of P4
= 31 × 4 = 124 g/mol
(iii) Molar mass of Sg
= 32 × 8 = 256 g/mol
(iv) Molar mass of PCl5 = 31 + 35.5 × 5
= 31+ 177.5 = 208.5 g/mol
(v) Molar mass of SO2Cl2 = 32 + 16 × 2 + 2 × 35.5
= 32 + 32 + 71.0
= 135 g/mol.

Question 18.
Calculate the number of atoms and gram atoms in 120 g carbon.
Solution:
Gram atoms = 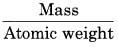
Number of atoms = No. of gram atoms × 6.022 × 1023
= 10 × 6.022 × 1023
= 6.022 × 1024 atoms
Question 19.
Calculate the mass of 1 carbon atom.
Solution:
Atomic mass of carbon = 12 g
Atomic mass of 1 mol of carbon = 12 g
or Atomic mass of 6.022 × 1023 atoms of carbon = 12 g
So, atomic mass of 1 carbon atom = \(\frac{12}{6.022 \times 10^{23}} \mathrm{~g}\)
= 1.99 × 10-23g
Question 20.
Calculate the number of moles in 5.75 g of sodium. (Atomic mass of sodium 23 u)
Solution:
1 mol = Atomic mass
∵ 23 g sodium = 1 mol sodium
= 0.25 mol sodium
Question 21.
Calculate the number of sodium atoms in 2.3 g of sodium. (Atomic weight of Na = 23)
Solution:
∵ No. of sodium atoms in 23 g sodium = 6.022 × 1023
∴ No. of sodium atoms in 2.3 g sodium = \(\frac{6.022 \times 10^{23}}{23} \times 2.3\)
= 6.022 × 1022 sodium atoms
Question 22.
Calculate the number of Cl ̄ ions in 0.585 g of NaCl.
Solution:
Since 58.5 g NaCl contains = 6.022 × 1023
NaCl molecules.
Thus, no. of NaCl molecules in 0.585 g will be
= \(\frac{6.022 \times 10^{23} \times 0.585}{58.5}\) NaCl molecules
= 6.022 × 1021 NaCl molecules
1 molecule of NaCl contains = Cl-
6.022 × 1021 molecule of NaCl will contain
= 6.022 × 1021 CI ions.
Question 23.
Find the molecular formula of ammonia formed by combination of hydrogen and nitrogen.
Solution:
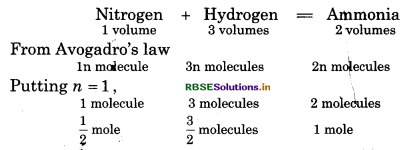
So, molecular formula of ammonia will be NH3
or mass of n × (NH3) = 17 n
But molecular mass of NH3 = 17
So \(n=\frac{17}{17}=1\)
So, molecular formula of ammonia will be NH3.
Question 24.
Calculate the mass of 3.01 × 1022 molecules of NH3.
Solution:
Molar mass of NH3 = 14+ 3 = 17 g/mol
∵ Mass of 6.022 × 1023 molecule of NH3 = 17 g/mol
∴ Mass of 3.01 x 1022 molecules of NH3
\(=\frac{17 \times 3.01 \times 10^{22}}{6.022 \times 10^{23}}\)
= 8.5 × 10-1
= 0.85 g/mol
Question 25.
Find out the number of atoms in 2.31 × 10-6 copper. (Atomic mass of Cu 63.5 a.m.u.).
Solution:
63.5 g Cu = 6.022 × 1023 Cu-atoms.
2.31 × 10-6 Cu = \(\frac{6.022 \times 10^{23} \times 2.31 \times 10^{-6}}{63.5}\)
= 0.219 × 1017
= 2.19 × 1016 Cu-atoms

Question 26.
The density of liquid mercury is 13.6 g/cm3 Calculate the number of moles in 1 L mercury metal.
Solution:
Mass of Hg = Density > Volume
= 13.6 × 1000 = 13600 g
No. of moles of Hg = 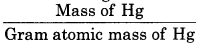
\(=\frac{13600}{200}\)
= 68 mol
Question 27.
Find out the gram molecules in 4.9 g of H2SO4.
Solution:
Molecular mass of H2SO4
= 2 × 1 + 32 + 16 × 4
= 2 + 32 + 64 = 98 g
∵ 98 g H2SO4 = 1 g/mol
∴ 4.9 g H2SO4 = 1/98 × 4.9 g/mol
= 0.05 g mol
Question 28.
Sodium carbonate decahydrate (Na2CO3 10H2O) is an important industrial chemical. Calculate its formula mass.
Solution:
Na2CO3.10H2O
=[2 × 23 + 12 + 3 × 16 + 10(2 × 1 + 16)] g
= [46 +12 + 48 + 180]
= 286 g
Question 29.
Calculate the mass of following:
(i) 1 mol atom of N
(ii) 5 moles atom of Al
(iii) 5.0 moles of Na+ ion
(iv) 10 moles of NaOH
Solution:
(i) Atomic mass of N = 14 g
So mass of 1 mol N = 14 g
(ii) Mass of 5 moles atom of Al
Atomic mass of Al = 27 g
∵ Mass of 1 mol of Al = 27 g
∴ Mass of 5 moles of Al = 5 x 27 = 135 g
(iii) Mass of 5.0 moles of Na+
Atomic mass of Nation = 23 g
∴ Mass of 1 mol of Na+ = 23 g
∴ Mass of 5.0 moles of Na+ = 5 x 23 = 115 g
(iv) Mass of 10 moles of NaOH
Mass of NaOH = 40 g
∴ Mass of 1 mol NaOH = 40 g
∵Mass of 10 moles of NaOH = 10 × 40 = 400 g
Question 30.
Convert the following into mol:
(i) 12 g SO2 gas,
(ii) 25 g H2O,
(iii) 22 g CO2 gas
Solution:
(i) Atomic weight of SO2 gas = 32 + 32 = 64g
64 g SO2 = 1 mol SO2
12 g SO2 = \(\frac{12}{64}\) mol SO2
= 0.1875 mol
(ii) 25 g H2O
Molecular weight of H2O = 2 + 16 = 18 g
18 g H2O = 1 mol H2O
25 g H2O = \(\frac{25}{18}\) mol H2O
= 1.39 mol H2O
(iii) 22g CO2
CO2 = 12 + 32 = 44 g
44 g CO2 = 1 mol CO2
22 g CO2 = \(\frac{22}{44}\)
= 1/2 mol of CO2
= 0.5 mol CO2
Question 31.
Calculate the total number of electrons in 1.4 nitrogen gas.
Solution:
Molar mass of N2 = 14 x 2 = 28 g
No. of electrons present in 28 g N2 = 14 mol electrons
No. of electrons present in 1.4 g nitrogen
= \(\frac{14 \times 1.4}{28}\) mol electrons
= 0.7 mol electrons
= 0.7 × 6.022 × 1023 electrons
= 4. 215 × 1023 electrons
Question 32.
Find out the number of each atom present in 3.42g sucrose (C12H22O11).
Solution:
Molecular weight of sucrose C12H22O11
= 12 × 12 + 22 × 1 + 11 × 16
= 144 + 22 + 176 = 342 g
∵ 342 g sucrose contains = 6.022 × 1023
∴ 3.42 g sucrose will contain
\(=\frac{6.022 \times 10^{23} \times 3.42}{342}\)
= 6.022 × 1021 molecules
∵1 molecule of sucrose = 12 carbon atoms
∴ 6.022 × 1021 molecules of sucrose will contain
= 12 × 6.022 × 1021 carbon atoms
= 72.24 × 1021 carbon atoms
= 7.224 × 1022 C atoms
∴ 1 molecule of sucrose = 22 hydrogen atoms
∵ 6.022 × 1021 molecules of sucrose will contain
= 22 × 6.022 × 1021
= 132.44 × 1021 H atoms
= 1.32 × 1023 H atoms
∵ 1 molecule of sucrose = 11 oxygen atoms
6.022 × 1021 molecules of sucrose will contain
= 11 × 6.022 × 1021 oxygen atoms
= 6.622 × 1021 O-atoms
= 6.622 × 1022 O-atoms
Question 33.
Calculate the number of aluminium ions present in 1.21 g of aluminium oxide (Al2O3).
Solution:
Molecular weight of Al2O3 = 2 × 27 + 3 × 16
= 54 + 48 = 102 g/mol
No. of aluminium ions present in 102 g Al2O3
\(=\frac{2 \times 1.21}{102}\) mol aluminium ions
= 0.024 mol aluminium ion
= 0.024 × 6.022 × 1023 aluminium ions
= 0.144 × 1023 Al ions
= 1.44 × 1022 Al ions
Question 34.
Calculate the number of atoms present in 3.2 g of sulphur.
Solution:
Atomic weight of S = 32
No. of atoms in 32 g S = 6.022 × 1023
No. of atoms in 3.2 g S = \(\frac{6.022 \times 10^{23} \times 32}{32}\)
= 6.022 × 1022

Question 35.
What will be the number of molecules in SO2 in which 8.0 g oxygen is present?
Solution:
Molecular weight of SO2 = 32 + 2 × 16 = 64 g
n 32 g oxygen will present in = 64 g SO2
8 g oxygen will present in = \(\frac{64 \times 8.0}{32}\)
= 16 g SO2
64g SO2 contains = 6.022 × 1023 molecules
16 g SO2 contains = \(\frac{6.022 \times 10^{23} \times 16}{64}\)
= 1.505 × 1023 molecules
Question 36.
If 1021 molecules are removed from 200 mg of H2S. Calculate the number of moles present in H2S.
Solution:
Molecular weight of H2S = 2 + 32 = 34 g
Number of molecules in 34 g H2S = 6.022 × 1023
Number of molecules in 200 × 10-3 g H2S
\(=\frac{6.022 \times 10^{23} \times 200 \times 10^{-3}}{34}\)
= 35.41 × 1020
= 3.54 × 1021 molecules.
Number of molecules removed = 1021
So, no. of remaining molecules
molecules
= 3.54 × 1021 - 1.00 × 1021
= 2.54 × 1021 molecules.
∵6.022 × 1023 H2S molecules are present = 1 mol H2S
∵ For 2.54 × 1021 H2S molecules = imm mol H2S
= 4.2 × 10-3 mol H2S
Question 37.
What will the number of molecules (density of water = 1g/mL) in 1L of water?
Solution:
Mass of 1L water 1 x 1 = 1g
So,
no. of molecules = \(\frac{1}{18}\) × 6.022 × 1023
= 3.34 × 1022 molecules
Question 38.
The molecular weight of haemoglobin is 89600. It has 0.25% (by mass) iron. Calculate the number of iron atoms present in one molecule of haemoglobin.
Solution:
According to question,
100 g haemoglobin contains Fe = 0.25 g
So, 89600 g haemoglobin will contain Fe
= \(\frac{0.25}{100}\) × 89600 = 224 g Fe
So, 1 mol haemoglobin contains Fe
or
= \(\frac{224}{56}\)
4 g Fe atoms
Hence, the number of Fe atoms in 1 mol haemoglobin will be 4 g.
Question 39.
If 1 g of any gas at N.T.P. occupies 1400 mL volume, what is molecular weight of gas? How much molecules are present in gas at N.T.P.?
Solution:
Weight of 1400 mL gas = 1 g
At N.T.P., weight of 22400 mL gas = \(\frac{22400}{1400}\)
Molecular weight of gas = 16 g
Number of molecules, at N.T.P. in 22400 mL gas
= 6.022 × 1023 molecules
Hence, number of molecules in 1 mL gas
\(=\frac{6.022 \times 10^{23}}{22400}\)
= 2.688 × 1019 molecules
Question 40.
What will be volume of 61 g oxygen at N.T.P.?
Solution:
Molecular weight of O2 = 16 x 2 = 32
Volume of 32 g O2 at N.T.P. = 22.4 L
Volume of 16 g O2 at N.T.P. = \(\frac{22.4 \times 16}{32}\)
= 11.2 L
Question 41.
What will be the number of molecules in 10 mL water vapour at S.T.P. ?
Solution:
At S.T.P., volume of 6.022 × 1023 molecules = 22.4 L
At S.T.P., 22400 mL water vapour contains = 6.022 × 1023 molecules
So, at S.T.P., 10 mL of water vapour will contain
\(=\frac{6.022 \times 10^{23} \times 10}{22400}\)
= 2.69 × 1020 molecules
Question 42.
Boron occurs in nature in its two isotopes whose atomic masses are 10 and respectively. What will be the % abundance of that sample which has average atomic mass as 10.8?
Solution:
Suppose, percentage of B10 = x
B11 = 100 - x
Average atomic mass of boron
\(\begin{aligned} & =\left(\frac{10 x}{100}\right)+\left[\frac{11(100-x)}{100}\right] \\ 10.8 & =\left(\frac{10 x}{100}\right)+\left[\frac{1100-11 x}{100}\right] \end{aligned}\)
10.8 × 100 = 1100 - x
1080 = 1100x
So, percentage of B10 and percentage of B11 = 100 - 20 = 80%
Question 43.
Find out the percentage of K, Cl and oxyge in KClO3.
Solution:
Molar mass of KClO3
= 39 + 35.5 + 3 × 16 = 122.5
% of K = \(\frac{39 \times 100}{122.5}\) = 31.83%
% of Cl = \(\frac{35.5}{122.5} \times 100\) = 28.97%
% of O = \(\frac{48}{122.5} \times 100\) = 39.20%
Question 44.
Find out the percentage of crystallized wate in pure sample of washing soda (Na2CO3·10H2O).
Solution:
Molecular weight of Na2CO3·10H2O
= 2 × atomic weight of Na + atomic weight of C + 3 atomic weight O + 10 × atomic weight of H2O.
= 2 × 23 + 12 + 3 × 16 + 10 × 18
= 46 + 12 + 48 + 180
= 286
Amount of H2O in 286 g of Na2CO3·10H2O = 180 g
100 g Na2CO3·10H 2O = \(\frac{180 \times 100}{286}\)
= 62.94%

Question 45.
In a compound, Na, S, H and O are presen The percentage of Na, S and H are 14.28% 9.92%, 6.20% respectively. Determine th molecular formula of compound. If a H-atoms are present in H2O form compound then give its crystallized formula when its molar mass is 322 g.
Solution:
% of O = 100 - (% of Na + % of S + % of H)
= 100 - (14.28 + 9.92 + 6.20) = 100 - 30.40 = 69.60
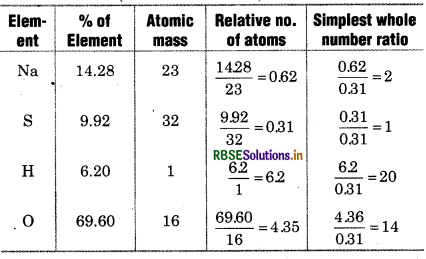
So, empirical formula of compound = Na2H2OO14
Empirical formula mass = 23 × 2 + 32 + 20 + 14 × 16
46 + 32 + 20 + 224
= 322 g
Molar mass of compound = 322 g

Molecular formula of compound is Na2SH2OO14. The formula of crystallized compound will be Na2SO410H2O.
Question 46.
When a compound is analysed, following data were obtained. C = 54.5%, H= 9.9%, O = 36.41%, if vapour density of compound is 44.1 then determine its molecular formula.
Solution:
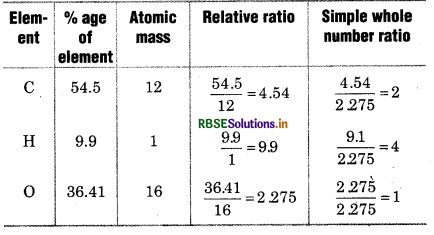
Empirical formula = C2H4O
Vapour density = 44.1
Molecular Weight = 2 × Vapour density
= 2 × 44.1882
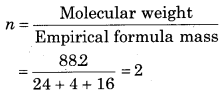
So, molecular formula = 2 × C2H4O = C4H8O2
Question 47.
5.325 g carbon compound was analysed and it was found that it contains 3.758 g carbon, 0.316 g hydrogen and 1.251 g oxygen. Determine its empirical formula and molecular formula if its vapour density is 68.
Solution:
Mass of compound = 5.325 g
Mass of carbon = 3.758 g
Mass of hydrogen = 0.316 g
Mass of oxygen = 1251 g
% of carbon = \(\frac{3.758}{5.325} \) × 100 = 70.57%
% of hydrogen \(=\frac{0.316}{5.325}\) × 100 = 5.93%
% of oxygen = \(\frac{1.251}{5.325}\) × 100 = 23.50%
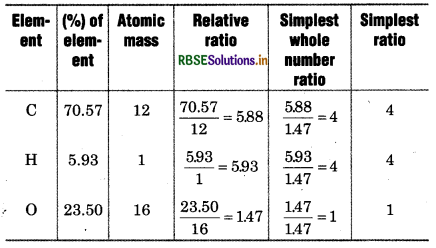
Empirical formula of compound = C4H4O
Empirical formula mass = (4 × 12) + (4 × 1) + 16 = 68
Molecular mass of compound = 2 × Vapour density
= 2 × 68 = 136
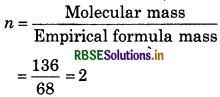
So, molecular formula = 2 × C4H4O
= C8H8O2
Question 48.
50 g nitrogen gas and 8 g hydrogen gas are combined to produce NH3 gas. Which is the limiting reagent of reaction? Calculate the amount of NH3 gas produced.
Solution:
Balanced chemical equation is
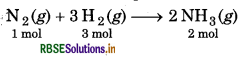
Number of moles of nitrogen = 50 g x \(\frac{1 \mathrm{~mol}}{28 \mathrm{~g}}\) = 1.786 mol
Number of moles of hydrogen = 8 g × \(\frac{1 \mathrm{~mol}}{2 \mathrm{~g}}\) = 4 mol
According to equation,
Number of moles of H2 reacts with 1.786 mol N2
= 3/1 × 1.786 = 5.358 moles
But moles present in H2 = 4
So complete H2(g) will take part in reaction. So, H2 iş limiting reagent.
Amount of NH3 (g) formed by 3 mol of H2 = 2 mol
Ammonia formed by 4 mol of H2 = \(\frac{2}{3} \times 4 \mathrm{~mol}\)
= 2.66 mol
2.66 mol = 2.66 × 17 = 45.22 g
Question 49.
3.0 g H2 reacts with 30.0 g O2 to form H2O. What is the limiting reagent of reaction? Calculate the amount of H2O formed. Also calculate the amount of excess reactant.
Solution:
Following will be chemical reaction:
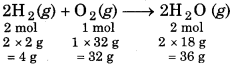
For limiting reagent,
4 g H, reacts with = 32 g O2
3 g H2 will react with = \(\frac{32 \times 3}{4}\) = 24 g O2
But according to problem, we have 30 g 02. viz O2 is in excess, So, the limiting reagent for this reaction is ydrogen.
4 g H2 form = 36 g H2O
3 g H2 will produce = \(\frac{36 \times 3}{4}\)
= 27 g H2O
Question 50.
Combustion of 1g Mg is furnished in a closed vessel which contains 0.5 g O2. What is limiting reagent for reaction? Calculate the amount of MgO produced in reaction.
Solution:
Balanced equation is:
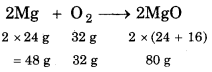
∵ 48 g Mg react with = 32 g O2
∴ 1g Mg will react with = \(\frac{32}{48}\)
But, as per question, amount of O, is 0.5 g which is less
O2 is limiting reagent.
Determination of amount of MgO
32 g O2 react with Mg and gives 80 g MgO.
0.5 g O2 will react with Mg to give = \(\frac{80 \times 0.5}{32}\)
= 1.25 g MgO
Question 51.
The molecular weight of a salt is 40 g mol-1, if 4 g of salt is dissolved in 250 g H2O. Calculate the molality of solution.
Solution:
Weight of solute 'W' = 4g
Weight of solvent 'W' 250 g
Now , molality
\(\begin{aligned} & =\frac{W_B \times 1000}{M_B \times W_A} \\ & =\frac{4 \times 1000}{40 \times 250} \end{aligned}\)
= 0.4 mol/Kg
Question 52.
If 10 g oxalic acid is dissolved in 500 n solution (density = 1.1g mL-1) then calculate the percentage mass of oxalic acid.
solution.
Solution: Weight of solute 'WR' = 10 g
Density of solvent 'd' = 1.1 g/mL
Mass of solution = Volume x Density
= 500 × 1.1
= 550 g
Mass percentage

= 1.82%

Question 53.
Density of 49% (weight percent) solution H2SO4 is 1.109 g/mL. Calculate the molality a molarity?
Solution:
49% (weight percent) means 49 g solu present in 100 g solution.
Weight of solute 'WB' = 49 g
Weight of Solvent 'WA' =100 - 49 = 51 g
Molecular weight of solute 'MB' = 98 g mol-1 (H2SO4)
Density of solution d = 1.109 g/mL
So molality (m)
\(\begin{aligned} & =\frac{W_B \times 100}{M_B \times W_A} \\ & =\frac{49 \times 1000}{98 \times 51} \end{aligned}\)
= 9.8 mol/kg
Molarity (M)
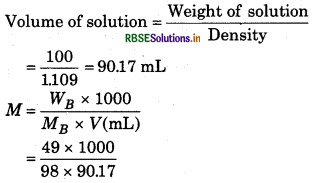
= 5.54 mol/L
Question 54.
In the mixture of 80 g water and 90 g methyl alcohol, calculate the mole fraction of bo compounds.
Solution:
Weight of H2O 'WA' = 80 g
Molecular weight of H2O 'MA'= 18 g/mol
Weight of CH3OH 'WB' = 90 ġ
Molecular Weight of CH3OH 'MB' = 32 g/mol
Mole fraction of CH3OH =
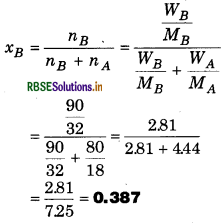
= 0.387
Mole fraction of H2O, xA = 1 - XB
= 1 - 0.387
= 0.613
Question 55.
If 5.85 g NaCl is dissolved in 500 g H2O. Calulate the molality of solution.
Solution:
Weight of solute 'WB' = 5.85 g
Molecular weight of solute 'M' = 23 + 35.5
= 58.5 g/mol
Weight of solvent 'WA' = 500 g
Molality (m)
\(\begin{aligned} & =\frac{W_B \times 1000}{M_B \times W_A} \\ & =\frac{5.85 \times 1000}{58.5 \times 500} \end{aligned}\)
= 0.2 mol/kg
Question 56.
Density of aqueous solution of 2 M oxalic acid is 1.02 g/mL, if molecular weight of oxalic acid is 126g/mol then calculate the molality of solution.
Solution:
Molarity 'M' = 2 M
Density 'd' = 1.02 g/mL
Molecular weight of solute 'MB' = 126 g/mol
Molality (m) = ?
2 M' means that 2 mol of solute is dissolved in 1 L solution.
Volume of solution = 1000 mL
Density of solution = 1.02 g/mL
Weight of solution = ?
So Weight of solution = Density × Volume
= 1.02 × 1000 = 1020 g
Moles of solute = 
'WB' = Moles of solute × Molecular weight
= 2 × 126 = 252 g
Weight of solvent 'WA' = Weight of solution - Weight of solute
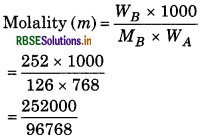
= 2.604 mol/kg.
Question 57.
Calculate the mole fraction of ethylene glycol (C2H6O2) if solution contains 20% mass of C2H6O2.
Solution:
20% C2H6O2 means that 20 g C2H6O2 is So present in 100 g solution.
So,
Weight of solute WB = 20 g
Weight of solute WA = 100 - 20 = 80 g
Weight of solvent W1 Molar mass of solute MB
= 2 × 12 + 6 × 1 + 2 × 16 = 62 g/mol
Molar mass of solvent MA = 18 g
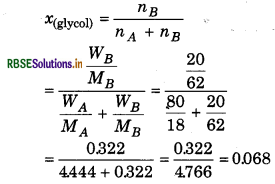
= 0.068
Mole fraction of H2O = 1 - X(glycol)
= 1 - 0.068
= 0.932
So, mole fraction of glycol = 0.068
mole fraction of H2O = 0.932

Question 58.
Write down the chemical equation for a reaction' and balance it by using fit and lapse method.
Magnesium nitrite + Water → Magnesium hydroxide + Ammonia
Solution:
Chemical reaction will be:
Mg3N2 + H2O → Mg(OH)2 + NH3
Balancing of Equation
(i) To balance the atoms of Mg
Mg3N2 + H2O → 3Mg(OH)2 + NH3
(ii) To balance atoms of O
Mg3N2 + 6H2O3 → Mg(OH)2 + NH3
(iii) To balance atoms of N
Mg3N2 + 6 H2O → 3Mg(OH)2 + 2NH3
(iv) H atoms are already balanced.
So
complete balanced equation will be
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3↑
Question 59.
Find out the mass of CaO obtained when 100 kg of 90% pure CaCO3 is heated.
Solution :
CaCO3 → CaO+ CO2
Molar mass of CaCO3 = 100 g
90% pure CaCO3 means 90 kg CaCO3 is present 100 kg CaCO3.
So amount of pure CaCO3 in 100 kg = 90 kg
According to reaction,
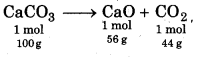
amount of CaO formed from 100g CaCO3 = 56 g
or from 100 kg CaCO3, amount of CaO formed = 56 kg
Therefore, CaO formed from 90 kg CaCO3
= \(\frac{56}{100}\) = 50.4 kg
Question 60.
On complete combustion of gaseous mixture of propane and butane, 10L CO2 is formed. Calculate the composition of gaseous mixture.
Solution:
Suppose, volume of propane in mixture = xL
Volume of butane in mixture = (3 - x) L
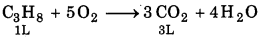
1L C3Hg gives CO2 = 3 L.
x L C3H8 gives CO2 = 3x L
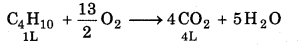
1L C4H10 give CO2 = 4L
(3 - x) LC4H10 gives CO2 = 4 × (3 - x) L
Volume of free CO2 = 10 L
3x + 4(3 - x) = 10
3x + 12 4x = 40
- x = 10 -12
x = 2
Volume of propane = 2 L
Volume of butane = (3 - 2) = 1L
Competitive Exam Questions:
Question 1.
The number of oxygen atoms in 4.4 g CO2 :
(a) 1.2 × 1023
(b) 6 × 1023
(c) 6 × 1023
(d) 12 × 1023
Answer:
(a) 1.2 × 1023
Question 2.
When 2.46 g hydrated salt (MgSO4.x H2O) completely dehydrated, it gives 1.2 g dehydrated salt. If molecular mass of this salt is 120 g mol-1, then value of x will be :
(a) 2
(b) 4
(c) 5
(d) 6
(e) 7
Answer:
(e) 7

Question 3.
In a compound, the weight of sulphur is 8%. Least melecular weight is :
(a) 200
(b) 400
(c) 155
(d) 355
Answer:
(b) 400
Question 4.
In which amount (g), of dioxygen there will be 1.8 × 1022 molecules?
(a) 9.60
(b) 0.096
(c) 96.0
(d) 0.960
Answer:
(d) 0.960
Question 5.
When 50 mL of 16.9% AgNO3 is mixed with 50 mL of 5.8% NaCl. The weight of precipitate will be :
(a) 28 g
(b) 3.5 g
(c) 7 g
(d) 14 g
Answer:
(c) 7 g
Question 6.
What is the volume of H2O used in decompostion of 1.368 kg sucrose?
(a) 0.072 dm
(b) 0.720 dm3
(c) 0.18 dm3
(d) 0.018 dm
Answer:
(a) 0.072 dm
Question 7.
The maximum number of water molecules is present in:
(a) 18 molecules of H2O
(b) 1.8 g H2O
(c) 18 g H2O
(d) 18 mol H2O
Answer:
(d) 18 mol H2O
Question 8.
If Avogadro number NA, 6.022 × 1023 mol-1 converts into 6.022 × 1020 for carbon, then change in mass of 1 mol of carbon will be:
(a) 1.2 × 10-3 g
(b) 12 × 10-3 g
(c) 12 x 10-3 g
(d) 12 × 10-3 g
Answer:
(b) 12 × 10-3 g
Question 9.
When 20.0 g magnesium carbonte decomposes to give CO2 and 8.0 g MgO, then what will be purity of Mg in sample?
(a) 75
(b) 96
(c) 60
(d) 84
Answer:
(d) 84

Question 10.
When Mohr's salt is dissolved in excess of water, then how much ions molecules formed?
(a) 6
(b) 4
(c) 10
(d) 5
Answer:
(d) 5
Question 11.
Which contains highest number of atoms?
(a) 24 g of 12 C
(b) 56 g of 56 Fe
(c) 27 g of 27 Al
(d) 108 g of Ag
Answer:
(a) 24 g of 12 C
Question 12.
1.0 g Mg is burnt with 0.56 g O2 in closed vessel, which reectant is left in excess and how much?
(a) Mg, 0.44g
(b) 02, 0.28 g
(c) Mg, 0.16 g
(d) 02 0.16 g
Answer:
(c) Mg, 0.16 g
Question 13.
When 22.4L H2 (g) is mixed with 11.2L Cl2 (g) each at S.T.P., then how many moles of HCl(g) will be formed?
(a) 0.5 mol HCl (g)
(b) 1.5 mol HCl (g)
(c) 1 mol HCl (g)
(d) 2 mol HCl (g)
Answer:
(c) 1 mol HCl (g)
Question 14.
How much silver (At. wt. 108) will be displaced by such amount of electricity which is used to displace 5600 mL O2 at S.T.P?
(a) 54.0 g
(b) 108.0 g
(c) 5.4 g
(d) 10.8 g
Answer:
(b) 108.0 g
Question 15.
What will be number of H+ ions present in 10 million parts of pure water in 1.33 cm' 3 at 25°C?
(a) 6.023 million
(b) 60 million
(c) 8.01 million
(d) 80.23 million
Answer:
(c) 8.01 million
Question 16.
What will be volume of 0.1 M oxalic acid which is completely oxidised by 20 mL of 0.025 M solution of KMnO4?
(a) 12.5 mL
(b) 25 mL
(c) 125 mL
(d) 37.5 mL
Answer:
(a) 12.5 mL
Question 17.
At S.T.P, 2 g of 100% pure divalent carbonate produces 448 cc CO2 on its complete thermal decomposition. What will be equivalent weight of metal?
(a) 40
(b) 20
(c) 28
(d) 12
(e) 56
Answer:
(b) 20
Question 18.
Which one of the following is the lightest?
(a) 0.2 mol of H2 gas
(b) 6.022 × 1022 molecules of N2
(c) 0.1 g of silver
(d) 0.1 mol of O 2(g)
(e) 1 g H2O
Answer:
(c) 0.1 g of silver
Question 19.
The total number of electrons in 18 mL of H2O will be :
(a) 6.02 × 1023
(b) 6.02 × 1025
(c) 6.02 x 1024
(d) 6.02 × 18 × 1023
Answer:
(c) 6.02 x 1024
Question 20.
If the molecular weight of H3PO, is M, its equivalent weight will be:
(a) M
(b) M/2
(c) M/3
(d) 2M
Answer:
(b) M/2

Question 21.
Which has maximum number of molecules among the following?
(a) 8g H2
(b) 64g SO2
(c) 44g CO2
(d) 48g O3
Answer:
(a) 8g H2
Question 22.
A hydrocarbon contains 80% carbon, empirical formula of this will be :
(a) CH2
(b) CH3
(c) CH4
(d) C2H3
Answer:
(b) CH3
Question 23.
Arrange the following masses in increasing order:
(I) 1 atom of oxygen
(II) 1 atom of nitrogen
(III) 1 x 10-10 mol of oxygen
(IV) 1 x 10-10 mol of copper
(a) II < I < III < IV
(b) 1 < II < III < IV
(c) III < II < IV < I
(d) IV < II < III < I
(e) II < IV < I< III
Answer:
(a) II < I < III < IV

- RBSE Class 11 Chemistry Important Questions Chapter 2 Structure of Atom
- RBSE Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
- RBSE Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- RBSE Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry - Some Basic Principles and Techniques
- RBSE Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- RBSE Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- RBSE Solutions for Class 11 Chemistry Chapter 8 Redox Reactions
- RBSE Solutions for Class 11 Chemistry Chapter 7 Equilibrium
- RBSE Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- RBSE Solutions for Class 11 Chemistry Chapter 5 States of Matter