RBSE Class 8 Science Important Questions Chapter 6 Combustion and Flame
Rajasthan Board RBSE Class 8 Science Important Questions Chapter 6 Combustion and Flame Questions and Answers.
Rajasthan Board RBSE Solutions for Class 8 Science in Hindi Medium & English Medium are part of RBSE Solutions for Class 8. Students can also read RBSE Class 8 Science Important Questions for exam preparation. Students can also go through RBSE Class 8 Science Notes to understand and remember the concepts easily. Browsing through class 8 science chapter 14 extra questions that includes all questions presented in the textbook.
RBSE Class 8 Science Chapter 6 Important Questions Combustion and Flame
Objective Questions
Question 1.
Which of the following is not combustible substance.
(a) Magnisium
(b) Wood coal
(c) Iron
(d) Wood
Answer:
(c) Iron

Question 2.
Which of the following is useful as extinguisher for the fire of inflammable substance like petrol?
(a) Water
(b) CO2
(c) Oxygen
(d) None of the above
Answer:
(b) CO2
Question 3.
The colour of the outermost part of candle flame is
(a) Black
(b) Yellow
(c) Red
(d) Blue
Answer:
(d) Blue
Question 4.
The unit of calorific value of fuel is
(a) kJ/kg.
(b) kg./kJ
(c) Joule
(d) Watt
Answer:
(a) kJ/kg.

Question 5.
The gas produced by the burning of candle is
(a) Nitrogen
(b) Carbon dioxide
(c) Hydrogen
(d) Oxygen
Answer:
(b) Carbon dioxide
Fill in the blanks
1. The substance that undergoes combustion is said to be .........................
Answer:
combustible
2. Ignition temperature of inflammable substances is very .........................
Answer:
low

3. Poisonous ......................... gas is produced by incomplete combustion of fuel.
Answer:
carbon mono - oxide
4. ......................... rain is harmful for crops, buildings and soil.
Answer:
Acid.
True/False
Mark T if the statement is true and 'F' if it is false.
1. Carbon dioxide is essential in air for combustion.
Answer:
False
2. The ignition temperature of inflammable substances is very high.
Answer:
False

3. Water is not used to control the fire of oil.
Answer:
True
4. Light and heat are produced in combustion process.
Answer:
True
5. Poisonous carbon mono oxide gas is produced by the complete combustion of fuel.
Answer:
False
6. CNG is a cleaner fuel.
Answer:
True

Match the words in 'Column-A' with 'Column-B'
Question 1.
|
Column-A (Fuel) |
Column-B (Calorific value kJ/kg) |
|
(i) Wood |
(a) 55,000 |
|
(ii) Coal |
(b) 45,000 |
|
(iii) Petrol |
(c) 33,000 |
|
(iv) LPG |
(d) 22,000 |
Answer:
|
Column-A (Fuel) |
Column-B (Calorific value kJ/kg) |
|
(i) Wood |
(d) 22,000 |
|
(ii) Coal |
(c) 33,000 |
|
(iii) Petrol |
(b) 45,000 |
|
(iv) LPG |
(a) 55,000 |

Question 2.
|
Column-A |
Column-B |
|
(i) Global warming |
(a) Carbon monoxide |
|
(ii) Flammable |
(b) Carbondioxide |
|
(iii) Fire extinguisher |
(c) Petrol |
|
(iv) Poisonous |
(d) Potassium bicarbonate |
Answer:
|
Column-A |
Column-B |
|
(i) Global warming |
(b) Carbondioxide |
|
(ii) Flammable |
(c) Petrol |
|
(iii) Fire extinguisher |
(d) Potassium bicarbonate |
|
(iv) Poisonous |
(a) Carbon monoxide |
Very Short Answer Type Questions
Question 1.
What is combustion?
Answer:
A chemical process in which a substance reacts with oxygen to give off heat is called combustion.
Question 2.
What do you mean by this statement that calorific value of charcoal is 33 kJ/g.
Answer:
This statement means 33 kJ heat is produced by the complete combustion of one gram charcoal.
Question 3.
Define the ignition temperature of a substance.
Answer:
The lowest temperature at which a substance catches fire is called its ignition temperature.

Question 4.
Write the names of two substances which are applied on the head of safety match.
Answer:
- Antimony trisulphide
- Potassium chlorate.
Question 5.
What is meant by inflammable substance?
Answer:
The substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances like petrol.
Question 6.
What is the meaning of spontaneous combustion?
Answer:
The type of combustion in which a material suddenly bursts into flames, without the application of any apparent cause is called spontaneous combustion.

Question 7.
Never sleep in a closed room with burning coal, why?
Answer:
Carbon mono oxide gas is produced by the burning of coal which is poisonous so it may cause death.
Question 8.
Write the names of two gases which are responsible for acid rain.
Answer:
- Sulphur dioxide
- Nitrogen dioxide.
Question 9.
What is the reason of global warming?
Answer:
The reason of global warming is excess of carbondioxide gas in air.

Question 10.
Why does a matchstick not catch fire on its own at room temperature?
Answer:
The ignition temperature of matchstick is more than room temperature so it does not catch fire on its own at room temperature.
Short Answer Type Questions
Question 1.
Why is a special fire extinguisher used to control the fires involving electrical equipments. Write the name of it.
Answer:
For fires involving electrical equipment carbon dioxide (CO2) is the best extinguisher. CO2, being heavier than oxygen, covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled. The added advantage of CO2 is that in most cases it does not harm the electrical equipment. The instrument used to control the fire is called fire extinguisher. The liquid CO2 is filled in it at high pressure.
Question 2.
Water can be boiled in a paper cup without burning it. What is the reason?
Answer:
When we heat a paper cup, the heat supplied to the paper cup is transferred to water continuously by conduction so the temperature of water increases and ultimately water starts boiling but in presence of water the ignition temperature of paper does not reach so it does not bum.

Question 3.
What is ignition temperature and its importance?
Answer:
Ignition temperature:
The lowest temperautre at which a substance catches fire is called its ignition temperature. Every substance has different ignition temperature.
Importance:
For the burning of a substance, it is heated up to ignition temperature. Due to it kerosene oil does not catch fire on its own at room temperature but if it is heated a little it will catch fire. So substances should be preserved on the basis of their ignition temperature.
Question 4.
Why is water used generally to control the fire?
Answer:
When house or other places catch fire fireman generally controls the fire by pouring water on it. Water cools the combustible material so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapours also surround the combustible material, helping in cutting off the supply of air. So, the fire is extinguished.
As we know that there are three essential requirements for producing fire are fuel, air and heat. Fire can be controlled by removing one or more of these requirements. The job of fire extinguisher is to cut off the supply of air, or to bring down the temperature of the fuel, but the fuel, in most cases cannot be eliminated.
Question 5.
Why can the fire of oil or petrol not be controlled by water?
Answerw:
Water is heavier than oil, so, it sinks below the oil and oil keeps burning on top. So for this carbondioxide is used in place of water, which is heavier than oxygen. It covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off so the fire is controlled.

Question 6.
Explain the different types of combustion.
Answer:
Combustion is of three types:
1. Rapid combustion:
Some substances burns even with a small spark like gas in gas stove. It bums with a small spark of lighter. Such type of combustion is rapid combustion.
2. Spontaneous combustion:
This type of combustion in which a material suddenly bursts into flames, without the application of any apparent cause is called spontaneous combustion. Like phosphorous which bum in air at room temperature.
3. Explosion:
Such type of combustion occurs in crackers. When a cracker is ignited a sudden reaction takes place with the evolution of heat, light and sound. Such a reaction is called explosion.
Question 7.
What do you understand by the calorific value of fuel? What will be the calorific value of that fuel, when 20,000 kJ heat is liberated from complete burning of 5 kg fuel? Calculate it.
Or
What is the meaning of calorific value? Explain.
Answer:
Calorific value:
The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its calorific value. The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg).
∵ Heat from liberated complete combustion of 5 kg fuel = 20,000 kJ
∴ Heat liberated from complete combustion of
1 kg fuel = \(\frac{20,000}{5}\) = 4,000 kJ
∴ Calorific value of fuel = 4,000 kJ/kg.

Question 8.
Write the short notes on the following
(1) Global warming
(2) Acid rain.
Answewr:
1. Global warming:
The rise in temperature of the atmosphere due to increased concentration of carbon dioxide and some other gases is called global warming. Global warming may be destructive for all animals if it is not planned.
2. Acid rain:
Oxides of sulphur and nitrogen dissolve in rain water and form acids. Such rain is called acid rain. It is very harmful for crops, buildings and soil.
Question 9.
When the clothes of a person catch fire, the person is covered with a blanket to extinguish fire, why?
Answer:
When the clothes of a person with fire are tightly covered with blanket, the supply of air containing oxygen, which is required for burning is stopped. So due to shortage of air the fire of clothes is extinguished.
Question 10.
Which substances produce flame in the process of combustion?
Answewr:
All the substances do not produce flame in the process of combustion. The substances which vapourise during burning, give flames. For example, kerosene oil and molten wax form flames because these are vapourised. Charcoal, does not vapourise and so it does not produce a flame.

Question 11.
In some conditions the fuel burns with blue flame but in other conditions it burns with yellow flame. What is the reason for it?
Answer:
The burning of a fuel with blue or yellow flame depends on available oxygen for combustion.
1. When the availability of oxygen (or air) is sufficient, combustion of fuel is complete which gives blue flame.
2. When the availability of oxygen (or air) is insufficient, combustion of fuel is incomplete which gives yellow flame, in this condition, the particles of carbon formed by incomplete combustion of fuel reach in flame. When these particles shine by heating, gives yellow light which gives yellow colour to the flame also.
Question 12.
Give any one reason why a piece of paper burns with yellow flame.
Answer:
The piece of paper bums with yellow flame due to incomplete combustion because the availability of oxygen is incomplete. The incomplete combustion of paper produces the unbumt carbon particles. These particles reach upto flame and shine by heating, gives yellow light and yellow colour to the flame also.
Essay Type Questions
Question 1.
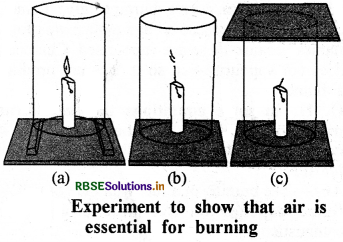
Prove that air is essential for combustion. Give the required diagram.
Answer:
Air is essential for combustion:
Fix a lighted candle on a table. Put a glass chimney over the candle and rest it on a few wooden blocks in such a way that air can enter in the chimney [Fig. (a)]. Now remove the blocks and let the chimney rest on the table [Fig. (b)]. Finally', put a glass plate over the chimney [Fig. (c)J. We see that in condition (a) candle bums unaffected; In condition (b) the air does not enter in the chimney from lower side, so flame flickers and gives smoke. In condition (c) flame flicker is off, because air is not available to it. It proves that air is essential for combustion.

Question 2.
How is the modern safety match prepared? Explain.
Answer:
Modern safety match:
Safety match means that it bums on ignition automatically. This was developed about two hundred years ago. A mixture of antimony trisulphide, potassium chlorate and white phosphorus with some glue and starch was applied on the head of a match made of suitable wood. When struck against a rough surface, white phosphorus got ignited due to the heat of friction. This started the combustion of the match. However, white phosphorus proved to be dangerous.
So now a days the head of the safety match contains only antimony trisulphide and potassium chlorate. The rubbing surface has powdered glass and a little red phosphorus. When the match is struck against the rough surface, some red phosphoms gets converted into white phosphoms. This immediately reacts with potassium chlorate in the matchstick head to produce enough heat to ignite antimony trisulphide and start the combustion.
Question 3.
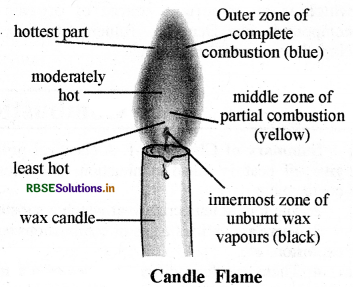
Give the description of candle flame with diagram.
Answer:
Candle flame has three zones:
1. Innermost zone:
The inner most or lowermost zone of candle flame (which is near the wick) is black. This black colour is due to unburnt carbon particles of fuel. The temperature of this zone is minimum.
2. Middle zone of partial combustion or Luminous zone:
The middle zone of candle flame is the zone of incomplete combustion of wax which is due to insufficient availability of oxygen. When unbumt particles of carbon go to upper side from the middle part of the candle flame they shine by heating and give yellow light, which gives yellow colour to the flame also. The temperature of this zone is moderate.
3. Outer zone of complete combustion or Non - luminous zone:
The outermost zone of candle flame is blue because the complete combustion of fuel (wax) occurs due to sufficient availability of oxygen (or air) blue part of candle flame is very small. The temperature of this zone of candle flame is highest.

Question 4.
Write the five special features of ideal fuel.
Answer:
Special features of ideal fuels are as follows:
- The calorific value of fuel must be high so that it can give more heat according to per unit weight.
- The ignition temperature of fuel must be optimum so that it can bum easily. The ignition temperature of fuel should not be very high or very low.
- In the fuel the amount of non - flammable substances should be less so that it does not leave behind more ash.
- No poisonous and harmful gas should be produced by the burning of fuel which can pollute the air.
- The fuel should be cheap and easily available.
Question 5.
What are the effect of harmful products produced by the combustion of fuel. Write in detail.
Answer:
The increasing fuel consumption has harmful effects on the environment. Out of these some are as follows:
1. Carbon fuels like wood, coal, petroleum release unbumt carbon particles. These fine particles are dangerous pollutants causing respiratory diseases, such as asthma. Wood is used as fuel for which cutting of trees is required which leads to deforestation. Due to decrease in forest environmental pollution is increasing.
2. Incomplete combustion of wood, coal etc. gives carbon monoxide gas, which is very poisonous. If the burning coal is left in a closed room, it may cause death of persons sleeping in that room.
3. Combustion of most fuels releases carbon dioxide in the environment. Increased concentration of carbon dioxide in the air is believed to cause global warming, due to which the temperature of earth is increasing. This results in the melting of glaciers, which leads to a rise in the sea level, causing the danger of floods in the coastal areas.
4. Burning of coal and diesel release sulphur dioxide gas. It is an extremely suffocating and corrosive gas. Moreover, petrol engines give off gaseous oxides of nitrogen. Oxides of sulphur and nitrogen dissolve in rain water and form acids. Such rain is called acid rain which is very harmful for crops, buildings and soil.

- RBSE Class 8 Science Notes in Hindi & English Medium Pdf Download
- RBSE Class 8 Science Important Questions in Hindi Medium & English Medium
- RBSE Solutions for Class 8 Science in Hindi Medium & English Medium
- RBSE Class 8 Science Important Questions Chapter 14 Chemical Effects of Electric Current
- RBSE Class 8 Science Notes Chapter 16 Light
- RBSE Class 8 Science Notes Chapter 1 Crop Production and Management
- RBSE Class 8 Science Notes Chapter 2 Microorganisms: Friend and Foe
- RBSE Class 8 Science Notes Chapter 3 Synthetic Fibres and Plastics
- RBSE Class 8 Science Notes Chapter 4 Materials: Metals and Non-Metals
- RBSE Class 8 Science Notes Chapter 5 Coal and Petroleum
- RBSE Class 8 Science Notes Chapter 6 Combustion and Flame