RBSE Class 8 Science Important Questions Chapter 14 Chemical Effects of Electric Current
Rajasthan Board RBSE Class 8 Science Important Questions Chapter 14 Chemical Effects of Electric Current Questions and Answers.
Rajasthan Board RBSE Solutions for Class 8 Science in Hindi Medium & English Medium are part of RBSE Solutions for Class 8. Students can also read RBSE Class 8 Science Important Questions for exam preparation. Students can also go through RBSE Class 8 Science Notes to understand and remember the concepts easily. Browsing through class 8 science chapter 14 extra questions that includes all questions presented in the textbook.
RBSE Class 8 Science Chapter 14 Important Questions Chemical Effects of Electric Current
Objective Questions
Question 1.
The long wire of Light Emitting Diode (LED) is joined to which terminal of battery
(a) Positive side
(b) Negative side
(c) Either positive or negative
(d) Neither positive nor negative
Answer:
(a) Positive side

Question 2.
On electrolysis of water, hydrogen gas collects at
(a) anode (positive terminal)
(b) cathode (negative terminal)
(c) neither cathode nor anode
(d) anode as well as cathode
Answer:
(b) cathode (negative terminal)
Question 3.
On electrolysis of water, gas that collects at positive terminal is
(a) oxygen
(b) hydrogen
(c) oxygen and hydrogen both
(d) nitrogen
Answer:
(a) oxygen
Question 4.
Cycle handle bar is coated with
(a) Copper
(b) Silver
(c) Chromium
(d) Iron
Answer:
(c) Chromium
Question 5.
Which of the following is bad conductor of electricity?
(a) Juice of lemon
(b) Distilled water
(c) Vinegar
(d) Seawater
Answer:
(b) Distilled water
Fill in the blanks
1. Substances are most acceptably divided into good conductors or bad conductors instead of ...................... and ......................
Answer:
Conductors, Insulators
2. Tin is less reactive than ......................
Answer:
Iron.
True/False
In the following statement write 'T' for true and 'F' for false.
1. Salt solution is a good conductor of electricity.
Answer:
True
2. Distilled water is bad conductor of electricity.
Answer:
True

3. Electroplating is a useless process.
Answer:
False
4. Iron has a nature of being corroded and rusted.
Answer:
True
Match the words given in 'Column-A' with 'Column-B'
Question 1.
|
Column-A |
Column-B |
|
(i) Good conductor |
(a) Rubber |
|
(ii) Poor conductor |
(b) Tin |
|
(iii) Bad conductor |
(c) Air |
|
(iv) Electroplating |
(d) Copper |
Answer:
|
Column-A |
Column-B |
|
(i) Good conductor |
(d) Copper |
|
(ii) Poor conductor |
(c) Air |
|
(iii) Bad conductor |
(a) Rubber |
|
(iv) Electroplating |
(b) Tin |
Very Short Answer Type Questions
Question 1.
What are electrodes?
Answer:
Eelectrodes are conducting rods, plates, etc. through which electric current enters or exits with minimum loss, in the electrolyte.
Question 2.
How electric current conducts in electrolyte solution?
Answer:
Electrolyte gives ion in their solution. Positive charges move towards cathode and negative ion move towards anode and due to their movement electric current flows.
Question 3.
Which electrolyte is used in silver electroplating?
Answer:
Solution of silver nitrate is used.
Question 4.
In coating of copper, pure copper is accumulated at which terminal of battery?
Answer:
During copper coating, pure copper is accumulated on the electrode which is connected to negative terminal of the battery (i.e. cathode).
Question 5.
What is the chemical effect of electric current?
Answer:
The process by which some liquid carries current through them and dissociates into its ions, is called Chemical Effects of Electric Current.
Question 6.
Write the names of those liquids which do not conduct electricity.
Answer:
Distilled water, oil.

Question 7.
Write the name of such liquid which is a good conductor electricity but on passing electric current does not get electrolysed.
Answer:
Mercury.
Question 8.
Name four liquids that conduct electricity.
Answer:
- Lemon juice
- Vinegar
- Lime water
- Sodium hydroxide (NaOH) solution.
Short Answer Type Questions
Question 1.
What is the full form of LED? Write its one use.
Answer:
The full form of LED is "Light Emitting Diode".
Use: LED is used when weak electric current is flowing because it even glows in it.
Question 2.
What is electrolysis process? Write its two uses.
Answer:
The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Uses:
- To protect the base metal from corrosion.
- To make any metal appear attractive and expensive.
Question 3.
What do you understand by good conductors and bad conductors (i.e. insulator of electricity)?
Answer:
Substances that allow electricity to pass through them are called good conductors. In contrast, substances which do not allow electricity to pass through them are bad conductors of electricity. Copper (Cu) and Aluminium (Al) conduct electricity whereas rubber, plastic and wood do not conduct electricity.
Question 4.
Draw the diagram of tester.
Answer:

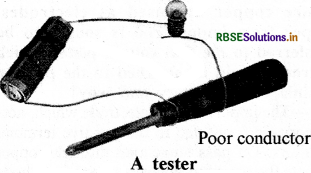
Question 5.
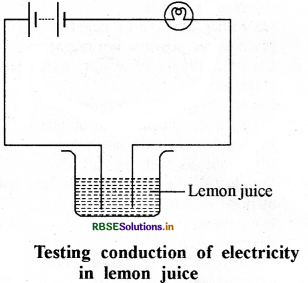
How will we test conduction of electricity through lemon juice?
Answer:
In a plastic beaker, take a little amount of lemon juice. Bring the tester near it and dip the ends in juice. Take care that both ends are not more than 1 cm apart, but do not touch each other. If bulb brightens, it shows that lemon juice conducts electricity.
Write the name of a device which will glow even when a weak current flow's through it.

Question 6.
Write the name of electrical appliance which can be elluminated even in the weak electrical current. Draw a diagram of electrical circuit.
Answer:
Light Emitting Diode (LED) is lighted even in weak electric current.
Connecting LED in circuit:
LED has two wires connected to it called leads. One lead is slightly longer than the other. While joining LED to a circuit, the longer lead is connected to positive terminal while shorter lead to the negative terminal of battery.
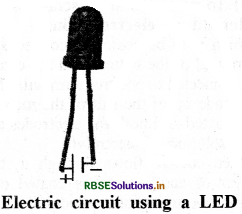
Question 7.
How is a tester produced by magnetic effect of electricity?
Answer:
Take an empty tray of match - box. On the tray, wrap some electricity wires as shown in figure. Put a magnetic needle in the tray. Now connect one free end of wire to one terminal of battery, and leave the other end open. Take another piece of wire and connect it with the other terminal of battery. Join both free ends of wire together for just a moment. Magnetic needle will deflect. This way a tester is ready with two open / free ends of wire.
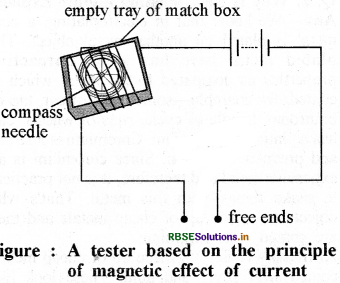
Question 8.
Show how distilled water can be made conducting.
Answer:
Take a clean and dry plastic beaker, and fill it with distilled water. By using a tester, it will be acknowledged that distilled water does not conduct electricity. Take a pinch of salt and dissolve it in distilled water. Test it with tester. It will be found that it conducts electricity.
Question 9.
Write few chemical effects of electric current.
Answer:
- Chemical activities take place when current passes through a conducting solution.
- Due to chemical effect, gas bubbles can be found on electrodes.
- Deposition of a metal can take place on electrodes. Colour of electrolytes can also change.
Question 10.
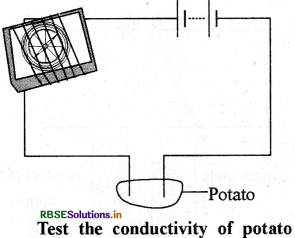
How will we test conductance of potato?
Answer:
Cut a potato in two equal parts and put the copper wires of tester in it. Wires connected to +ve terminal have a blue spot produced on it. This shows that potato is a good conductor.
Question 11.
Anode of Cu voltameter is made up of copper. Why?
Answer:
By flow of electricity in copper voltameter, electrolyte of copper sulphate dissociates into copper ion (Cu++) and sulphate ion (SO4--). Cu2+ ions are attracted to negative -terminal of battery and is deposited on it, but due to this, Cu in the solution becomes less. To restore it, anode is made of copper, so same amount of copper dissolves in solution. This way, amount of copper that was reduced in solution is replenished. This is the reason why copper voltameter is made of copper.

Question 12.
Why does molten NaCl conducts electricity?
Answer:
Solid NaCl is also ionic in nature. But these ions are very close to each other and are bounded. When NaCl is in molten state, attractive forces between Na+ and Cl decreases and so ions can move freely. These ions conduct electricity.
Question 13.
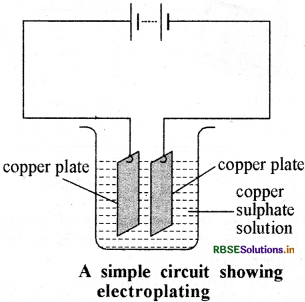
By the help of a diagram, explain the process of coating copper on any metallic object.
Answer:
Mix 2 spoons of copper sulphate in a 250 mL distilled water in a dry beaker. To make it more conducting, add few drops of dilute sulphuric acid. Clean two copper plates and dip them in the solution and connect these to the terminals of battery. Flow the electric current through circuit for 15 minutes. By this, copper accumulates on the negative plate. After stopping the electric current, on analysis of the plates, we find that on the negative side, a coat of copper is seen. On the basis of this we can say that by the process of electroplating, the metallic object is coated with copper.
Essay Type Questions
Question 1.
What is electroplating? Why is it done? Explain its working with the help of a diagram.
Or
What is electroplating process? Write its two uses.
Or
Name the process of gold plating on silver ornaments and also draw a diagram of the process.
Answer:
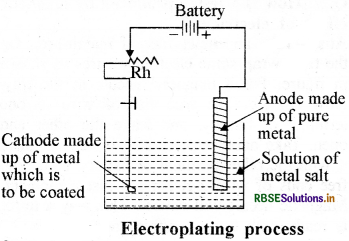
Electroplating:
Coating of unreactive metal on any other metal with the help of electricity is called electroplating, e g. Gold plating on the silver jewellery is done by this method.
The reasons for electroplating are:
- Main element is to be protected against corrosion.
- To make it attractive.
- To protect it from scratches.
Method of electroplating:
The substance which is to be coated in connected to negative terminal of the battery and the coating material is connected to positive terminal. The electrolyte is made up of the salt of the metal which is to be coated and both the electrodes are dipped in the solution of electrolyte. When current flows through it, then the thin layer of anode metal is coated on the object which is acting as cathode.

Question 2.
Why is electroplating useful? Explain.
Answer:
We know that in electroplating, a coat metal is plated on another metal object. The plated metal here has some unreactive properties as compared to the metal which is coated, for example - some parts of car, tap of bathroom, handle of cycle, rims of wheels, etc. has a plating of chromium. Chromium is lustrous and prevents corrosion. Since chromium is an expensive metal and therefore, it is not practical to make things with this metal. That's why objects are made up of cheap metals and then are coated with chromium.
Artificial jewellery is made up of cheap metals coated with silver and gold. These look like silver and gold but are very cheap. Cans storing food are made up of iron but are electroplated with tin as it is less reactive than iron so food material does not get spoiled in cans coated with tin. Bridges and self - driven vehicles are made strong by using iron. But iron gets easily corroded and rusted so to protect it, it is coated with zinc by electroplating. In this way electroplating is widely used.

- RBSE Class 8 Science Notes in Hindi & English Medium Pdf Download
- RBSE Class 8 Science Important Questions in Hindi Medium & English Medium
- RBSE Solutions for Class 8 Science in Hindi Medium & English Medium
- RBSE Class 8 Science Notes Chapter 16 Light
- RBSE Class 8 Science Notes Chapter 1 Crop Production and Management
- RBSE Class 8 Science Notes Chapter 2 Microorganisms: Friend and Foe
- RBSE Class 8 Science Notes Chapter 3 Synthetic Fibres and Plastics
- RBSE Class 8 Science Notes Chapter 4 Materials: Metals and Non-Metals
- RBSE Class 8 Science Notes Chapter 5 Coal and Petroleum
- RBSE Class 8 Science Notes Chapter 6 Combustion and Flame
- RBSE Class 8 Science Notes Chapter 7 Conservation of Plants and Animals