RBSE Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 9 Coordination Compounds
RBSE Class 12 Chemistry The Coordination Compounds InText Questions and Answers
Question 9.1.
Write the formulae for the following coordination compounds:
(i) tetraammine diaquacobalt (III) chloride
(ii) potassium tetracyanonickelate (II)
(iii) tris (enthane-1, 2-diamine) chromium (III) chloride
(iv) amminebormidochloridonitrito-N-platinate (II)
(v) dichloridobis (ethane-1, 2-diamine) platinum (IV) nitrate
(vi) iron (III) hexacyanoferrate (II)
Answer:
(i) [CO(NH3)4(H2O)2]Cl3
(ii) K2[Ni(CN)4]
(iii) [(Cr(en)3]Cl2
(iv) (Pt(NH3)BrCl(NO2)]
(v) [PtCl(en)2] (NO3)2
(vi) Fe [Fe(CN)6]3
Question 9. 2.
Write the IUPAC names of the following coordination compounds:
(i) [(CO(NH3)6]
(ii) [(CO(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl (NH2CH3)]Cl
Answer:
(i) hexaamminecobalt (III) chloride
(ii) pentaamminechloridocobalt (III) chloride
(iii) potassium hexacyanoferrate (III)
(iv) potassium trioxalatoferrate (III)
(v) potassium tetrachloridopalladate (II)
(vi) diamminechlorido (methylamine) platinum (II) chloride

Question 9.3.
Indicate the types of isomerism exhibited by the following complexes and draw the structures of these isomers:
(i) K[Cr(H2O)2 (C2O4)2]
(ii) [CO(en)3]Cl3
(iii) (CO(NH3)5 (NO2)] (NO3)2
(iv) [Pt(NH3)(H2O)Cl2]
Answer:
(i) The complex K[Cr(H2O)2 (C2O4)2] shows geometrical isomerism structure of its cis and trans isomers are as follows:
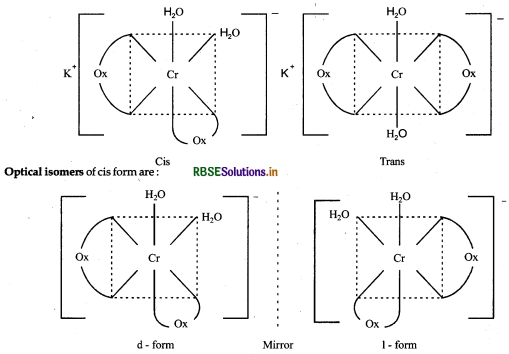
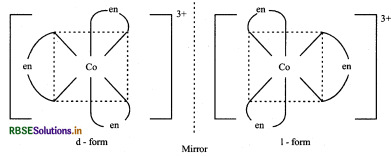
(ii) The complex [CO(en)3]Cl3 shows optical isomerism and it has two optical isomers.
(iii) The complex [CO(NH3)5 (NO3)] (NO3)2 shows ionisation isomerism and linkage isomerism.
(a) Ionisation isomerism: [CO(NH3)5 (NO2)] (NO3)2 and CO(NH3)5 (NO3)] (NO2) (NO3)
(b) Linkage isomerism: [CO(NH3)5 (NO2) (NO3)2 and [CO(NH3)5 (ONO) (NO3)2
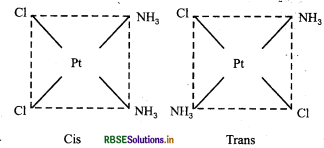
(iv) The complex [Pt(NH3)(H2O)Cl2] shows geometrical isomerism.
Question 9.4.
Give evidence that [CO(NH3),Cl]SO4 and [CO(NH3)5SO4]Cl are ionisation isomers.
Answer:
Ionisation isomers give different ions in aqueous solution. Therefore, they react differentially with different reagents.
[CO(NH3)5Cl]SO4 gives sulphate ions in aqueous solution which gives the precipitate of BaSO4 on adding of BaCl2
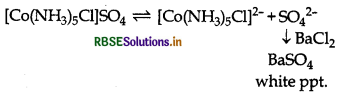
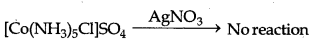
[CO(NH3)5SO4]Cl gives chloride ions in aqueous solution which gives the precipate of Agcl on addition of AgNO3.
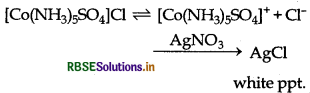
But it does not react with BaCl2 solution.
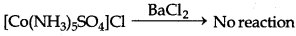
Question 9.5.
Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square planar structure is diamagnetic and the [NiCl4]2- ion with tetrahedral geometry is paramagnetic.
Answer:
In both the complexes, Ni is in +2 oxidation state and has 3d8 configuration in the presence of CN- which is a strong field ligand, the 3d electrons of Ni2+ ion pair up leaving no unpaired electron. Therefore it involves dsp2 hybridisation and is diamagnetic in nature.
In the presence of Cl- which is a weak field ligand, the 3d electrons do not pair up. Hence the complex [NiCl4]2- contains two unpaired electrons and is paramagnetic.
It can again explained as follow:
Magnetic behaviour of [Ni(CN)4]2-: It has CN- a strong field ligand.
The complex [Ni(CN)4]2- has no unpaired e-, so, it is diamagnetic in nature.
Magnetic behaviour of [NiC4]2- It has Cl-, a weak field ligand.
However, it does not react with AgNO3.
Ni (28) ground state
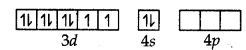
Ni (II) ground state
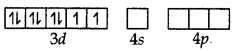
Ni (II) excited state (when ligands approach central metal ion to form coordinate bonds)
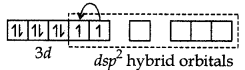
dsp3 hybrid orbitals of Ni2+ ion
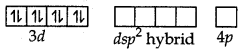
[CO(NH3)5SO4]Cl gives chloride ions in aqueous solution which gives the precipitate of AgCl on addition of AgNO3.
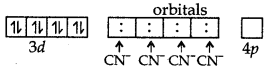
The complex [Ni(CN)4]2- has no unpaired e-, so, it is diamagnetic in nature.

Question 9.6.
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Explain.
Answer:
In the complex [NiCl4]2- Ni is in +2 oxidation state and has 3d configuration. In the presence of Cl- (a weak field ligand), 3d electrons do not get paired up. Hence the complex Ni contains two unpaired electrons and is paramgnetic.
In the complex [Ni(CO)4] Ni is in zero oxidation state. In the presence of CO, (a strong field ligand), the unpaired d-electrons of Ni gets paired up leaving no unpaired electron Hence the complex [Ni(CO)4] is diamagnetic.
Question .9.7.
[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3- is weakly paramagnetic. Explain?
Answer:
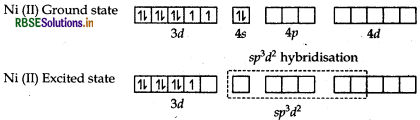
In both the complexes, Fe is in + 3 oxidation state and has 3d5 configuration. In the presence of H2O (a week field ligand), 3d electrons do not pair up. The hybridisation is sp3d2 forming an outer orbital complex containing five unpaired electrons. Hence it is strongly paramagnetic In the presence of CN, (a strong field ligands) the 3delectrons pair up leaving only one unpaired electron. The hybridisation in d2sp3 forming inner orbital complex. Hence the complex [Fe(CN)6]3- is weakly paramagnetic.
Question 9.8.
Explain [CO(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
Answer:
In the complex (CO(NH3)6]3+, Co is in + 3 oxidation state and has d6 configuration. In the presence of NH3 (a strong field ligand), 3d-electrons pair up leaving two 3d-orbitals vacant. Hence it involves d2sp3 -hybridisation and forms inner orbital complex. In the complex [Ni(NH3)6]2+, Ni is in + 2 oxidation state and has 3d8 configuration. In case of Ni2+ ion, two empty 3d orbitals are not available even after pairing. Hence Nilion undergoes spd hybridisation using its 4d-orbitals forming outer orbital complex.
Question 9.9.
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2- ion.
Answer:
The atomic number of platinum is 78 and its electronic configuration is 5d9 6s1. In this complex, the oxidation state of Pt is +2 and has 5d8 configuration. For square planer geometry, the hybridisation is dsp2. Hence the unpaired electrons in 5d-orbitals pair up to make one d-orbital empty for dsp2-hybridisation. Thus there is no unpaired electron.
Pt (78) ground state
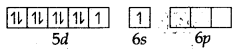
pt (II) ground state
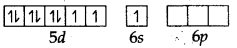
Pt (II) excited state (when ligands approach central metal ion to form covrdinate bonds)
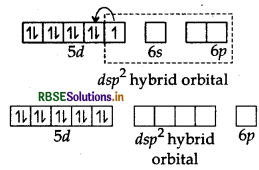
[Pt (CN)4]2-
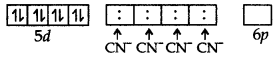
Hence, number of unpaired electrons in [Pt (CN)4]2- is zero.
Question 9.10.
The hexaaquomanganese (II) ion contains five unpaired electron, while the hexacyano ion contains only one unpaired electron. Explain using crystal field
theory.
Answer:
Mn2+ ion has 3d5-configuration. In the complex [Mn(H2O)6]2+, water in a weak field ligand. Therefore ∆0 < P. Hence the configuration of Mn2+ will be (t2g)3 (eg)2 i.e., it has five unpaired electrons on the other hand in the complex [Mn(CN)6]4-, CN- is a strong field ligand. Therefore ∆0 > P. Hence the configuration of Mn2+ will be (t2g)5 (eg)0. It indicates that it has one unpaired electron.

Question 9.11.
Calculate the overall complex dissociation equilibrium constant for the [Cu(NH3)4]2+ ion given that B4 for this complex is 2.1 x 1013.
Answer:
The overall dissociation constant is the reciprocal of the overall stability constant. Thus, overall
dissociation constant \(\frac{1}{\beta_4}=\frac{1}{2 \cdot 1 \times 10^{13}}\) = 4.7 x 10-14.
RBSE Class 12 Chemistry Coordination Compounds Textbook Questions and Answers
Question 9.1.
Explain the bonding in coordination compounds in terms of Werner's postulates.
Answer:
- In coordination compounds metals show two types of linkages (valencies) - primary and secondary.
- The primary valencies are normally ionisable and are satisfied with negative ions.
- The secondary valencies are non-ionisable. These are satisfied by neutral molecules or negative ions. The secondary valency is equal to the coordination number and is fixed for a metal.
- The ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements corresponding to different coordination numbers.
e.g., Structure of [COCl2(NH3)4]+ Cl- is as follows: CO has 3 as Primary valency and 4 as Secondary valency.
Question 9.2
FeSO4 solution mixed with (NH4)2SO4 solution in 1 : 1 molar ratio gives the test of Fe2+ ion but CusO4. solution mixed with aqueous ammonia in 1 : 4 molar rati does not give the test of Cu2+ ion. Explain why.
Answer:
FeSO4 solution mixed with (NH4)2SO4 in 1 : 1 molar ratio gives a double salt. This double salt is FeSO4 (NH4)2SO4. 6H2O (Mohr's salt) which dissociates in aqueous solution to give Fetion. Therefore, it gives test for Fe2+ ion.
FeSO4 (NH4)2SO4 → Fe2+ + 2NH4+ + 2SO42-
CuSO4, solution mixed with aqueous ammonia in 1 : 4 molar ratio gives a coordination compound of formula [Cu(NH3)4]SO4. The complex ion [Cu(NH3)4]2+ does not dissociate in aqueous solution to give Cu2+ ion. Hence it does not give test for Cu2+ ion.

Question 9.3.
Explain with two examples each of the following:
Coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Answer:
(i) Coordination entity: A coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. Example: [CO(NH3)3Cl3] is a coordination entity in which cobalt ion is surrounded by three ammonia molecules and three chloride ions. Other examples are:
(i) [Pt(NH3)2Cl2]
(ii) [Fe(CN)6]4-
(iii) [CO(NH3)6]3+ etc.
(ii) Ligand: The ions or molecules bound to the central metal atom/ion in the coordination entity are called ligands. These may be simple ions as Cl- and may be large molecule as NH2CH2CH2NH2 or even macromolecules like proteins.
(iii) Coordination number: It is the number of ligand donor atoms to which the metal is directly bonded. For example, [PtCl6]2- the coordination number of Pt is 6 while in [Ni(NH3)4]2+, the C.N. of Ni is 4.
(iv) Coordination polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. e.g., octahedral, square planar and tetrahedral polyhedron.
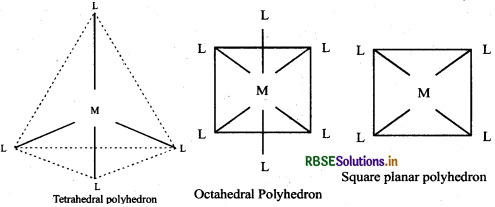
(v) Homoleptic complexes: Complexes in which a metal is bounded to only one kind of donor groups e.g, [CO(NH3)6]3+ [Cr(H2O)6]3+ etc.
(vi) Heteroleptic complexes : Complexes in which a metal is bound to more than one kind of donor groups.e.g. [CO(NH3)4Cl2]+ [Cr(H2O)4Br2]+ etc.
Question 9.4.
What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Answer:
(a) Monodentate ligands: The ligands which can coordinate to central metal ion through single donor atom are called monodentate ligands.
Examples: Cl-, H2O, NH3, CO, NO3- NO2- etc.
(b) Bidentate ligands : The ligands which can coordinate to central metal ion through two donor atoms are called bidentate ligands.
Examples: NH2CH2CH2NH2 (ethane-1, 2-diamine) C2O42- (oxalate ion) etc.
(c) Ambidentate ligands: The unidentate or monodentate ligands which can coordinate to central metal ion through two different atoms are called ambidentate ligands.
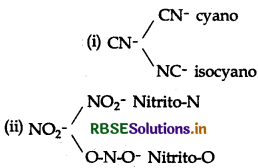

Question 9.5.
Specify the oxidation number of the metals in the following coordination entities:
(i) [CO(H2O)(CN)(en)2]2+
(ii) [PtCl4]2-
(iii) [Cr(NH3)3Cl3]
(iv) [COBr2(en)2]+
(v) K3[Fe(CN)6]
Answer:
(i) [CO(H2O)(CN)(en)2]2+
x + 0 + (-1) + 2 x 0 = + 2
x - 1 = +2
x = +3
(ii) [PtCl4]2-
x + 4 x (-1) = -2
x - 4 = -2
x = +2
(iii) [Cr(NH3)3Cl3]
x + 0 + 3 x (-1) = 0
x + 0 - 3 = 0
x = +3
(iv) [COBr2(en)2]+
x + 2 x (-1) + 2 x 0 = +1
x - 2 + 0 = +1
x = +3
(v) K3[Fe(CN)6]
3 x (+1) + x + 6 x (-1) = 0
3 + x - 6 = 0
x = +3
Question 9.6.
Using IUPAC norms write the formulae for the following:
(i) tetrahydroxozincate (II)
(ii) potassium tetrachloridopalladate(IT)
(iii) diamminedichloridoplatinum (II)
(iv) potassium tetracyanonickilate (IT)
(v) pentaamminenitrito-O-cobalt(III)
(vi) hexammine cobalt(III) sulphate
(vii) potassium tri (oxalato) chromate (IIT)
(viii) hexaammine platinum (IV)
(ix) tetrabromidocuprate (II)
(x) pentaammine nitrito-N-cobalt(IIT)
Answer:
(i) [Zn(OH)4]2-
(ii) k2 [PdCl4]
(iii) [Pt(NH3)2Cl2]
(iv) K2[Ni(CN)4]
(v) [CO(NH3)5(ONO)]2+
(vi) [CO(NH3)6]2 (SO4)3
(vii) K3[Cr(C2O4)]
(viii) [Pt(NH3)6]4+
(ix) [CUBr4]2-
(x) [CO(NH3)5(NO2)]2+
Question 9. 7.
Using IUPAC norms write the systematic names of the following:
(i) [CO(NH3)6]Cl3
(ii) [Pt(NH3)2 Cl (NH2CH3)]Cl
(iii) [Ti(H2O)6]3+
(iv) [CO(NH3)4 Cl(NO2)]Cl
(v) [Mn(H2O)6]2+
(vi) [NiCl4]2-
(vii) [Ni(NH3)6]Cl2
(viii) [CO(en)3]3+
(ix) [Ni(CO)4]
Answer:
(i) hexaammine cobalt(III) chloride
(ii) diammine chlorido (methyl amine) platinum (II) chloride
(iii) hexaaquatitanium (III)ion
(iv) tetraamminechlorido nitrito-N-cobalt(III) chlordie
(v) hexaquamanganese (II) ion
(vi) hexaamminenickel (II) chloride
(vii) trans (ethane-1, 2-diamine) cobalt(III) ion
(ix) tetracarbonylnickel (0)

Question 9.8.
List various types of isomerism possible for coordination compounds, giving an example of each.
Answer:
Coordination compounds show two types of isomerism:

(1) Structural isomerism: It can be further classified as follows:
(i) Ionisation isomerism
Examples:
- [CO(NH3)5CI]Br
- [CO(NH3)NO2]NO3 and [CO(NH3)5NO3]NO2
(II) Linkage isomerism Examples:
- [CO(NH3)5(NO2)]Cl2 and [CO(NH3)5 (ONO)]Cl2
- [CO(NH3)5 (CN)Cl2 and [CO(NH3)(NC)]Cl2
(iii) Coordination isomerism
Examples:
- [CO(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [CO(CN)6]
- [Pt(NH3)4] [PtCl4] and [Pt(NH3)3Cl] [PtCl3(NH3)]
(iv) Solvate isomerism
Example:
- [Cr(H2O)6]Cl and [Cr(H2O)6Cl] Cl2.H2O
- [CO(H2O)6]Br3 and [CO(H2O)5Br] Br2.H2O
(2) Stereo isomerism:
(i) Geometrical isomerism
Example:
- [Pt(NH3)2 (Cl)2]
- [CO(en)2Cl2]
(ii) Optical isomerism
Examples: cis [CO(en)2Cl2]
Question 9.9.
How many geometrical isomers are possible in the following coodination entities:
(a) [Cr(C2O4)3]3
(b) [CO(NH3)3Cl3]
Answer:
(a) [Cr(C2O4)3]3 does not show geometrical inomerism.
(b) [CO(NH3)3Cl3]
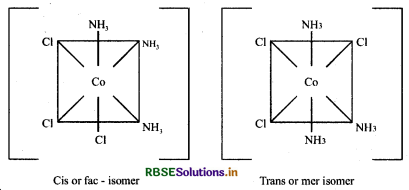
Question 9.10.
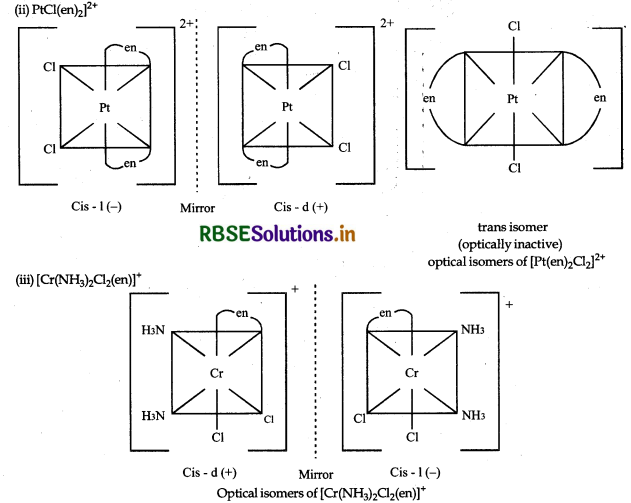
Draw the structures of optical isomers of:
(i) [Cr(C2O4)3]3-
(ii) pt[Cl(en)2]2+
(iii) [Cr(NH3)2Cl2(en)]+
Answer:
(i) [Cr(C2O4)3]3-
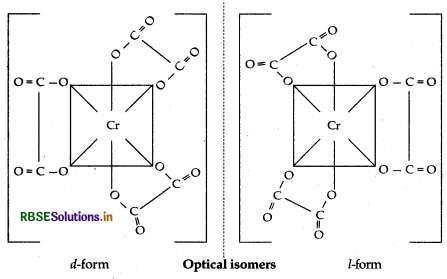

Question 9. 11.
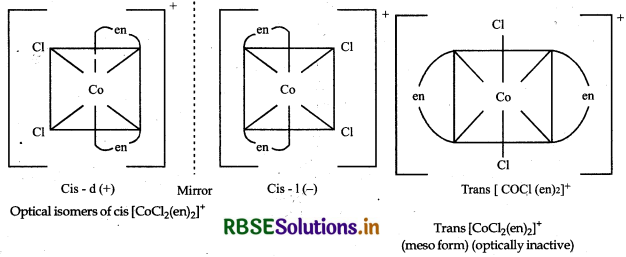
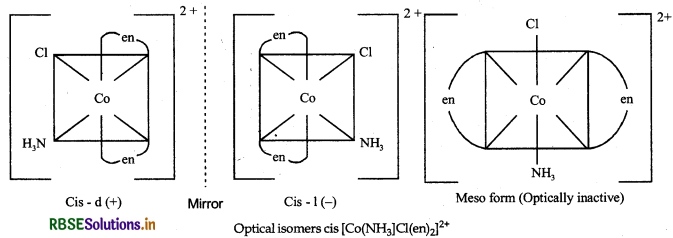
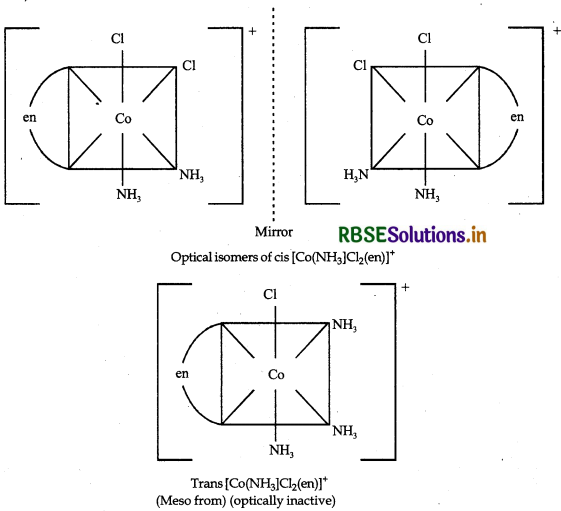
Draw all the isomers (geometrical and optical) of:
(i) [COCl2(en)2]+
(ii) [CO(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl(en)]+
Answer:
(i) [COCl2(en)2]+
(ii) [CO(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl(en)]+
Question 9.12.
Write all the geometrical isomers of [Pt(NH3)(Br)(Cl) (Py)] and how many of these will exhibit optical isomers?
Answer:
This complex has three possible geometrical isomers:
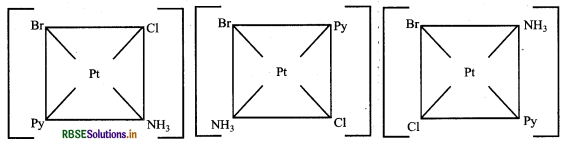
These types of isomers do not show optical isomerism because square planar complexes rarely show optical isomerism
Question 9.13.
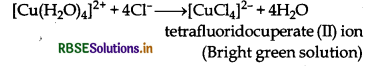
Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride and
(ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Answer:
Aqueous copper sulphate solution exists as [Cu(H2O)4]2+ H2O. This solution has blue colour due to the presence of [Cu(H2O)4]2+ ions.
(i) When potassium fluoride is added, then F- ligands replaces weak H2O ligands and form [CuF4]2- ions which is a green coloured complex.
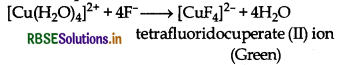
(ii) When potassium chloride is added, then Cl- ligands replace weak H2O ligands and form (Cuculions which have bright green colour.
Question 9.14.
What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
Answer:
When aqueous solution of KCN is added to an aqueous solution of copper sulphate then first of all cupric cyanide is formed which dissociates to give cuprous cyanide and cyanogen. Cuprous cyanide disselves in excess of KCN to give K3[Cu(CN)4]
CUSO4 + 2KCN → CU(CN)2 + K2SO4 x 2
2CU(CN)2 → CU2(CN)2 + (CN)2
CU2(CN)2 + 6KCN → 2K3[CU(CN)4] + 2K2SO4 + (CN)2↑
In this way, the coordination entitity formed is [Cu(CN)4]3- Since CN- is a strong field ligand, it dissociates to give Cu2+ ion. Hence no precipitate is formed on passing H2S.
Question 9.15.
Discuss the nature of bonding in the following coordination entites on the basis of valence bond theory:
(a) [Fe(CN)6]4-
(b) [FeF6]3-
(c) [CO(C2O2)3]3-
(d) [COF6]3-
Answer:
(a) [Fe(CN)6]4-:
The atomic number of Fe is 26 and its electronic configuraton is 3d64s2. The oxidation state of Fe in this complex is +2.
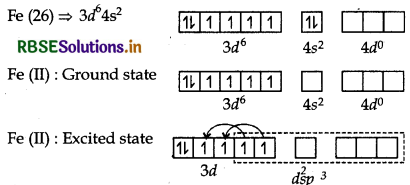
Since CN- is a strong field ligand, hence 3d electrons are paired up to make twod-orbitals vacant for ds2sp3 hybridisation d2sp3 hybrid orbitals of Fe (II)
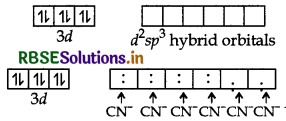
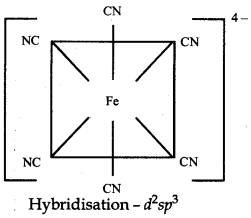
Geometry → Octahedral
Colour → colourless because there is no unpaired electrons
Magnetic Character → Diamagnetic because there is no unpaired electrons
Nature of the complex → Inner orbital complex
(b) [FeF6]3-: The atomic number of Fe is 26 and it has 3d64s2 configuration. The oxidation state of Fe in this complex is +3. Here, F- is a weak field ligand. Therefore, 3d electrons do not pair up. Fe(26) 3d 4s.
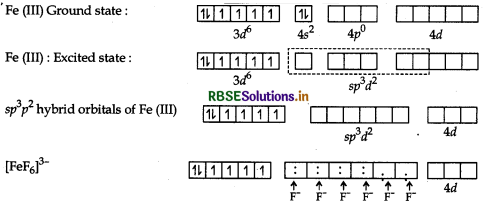
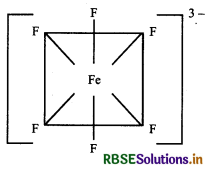
Hybridisation → sp3 d2
Geometry → Octahedral
Colour → Colourless because of unpaired electrons
Magnetic character → Paramagnetic due to the presence of unpaired electrons
\(\mu=\sqrt{n(n+2)} \text { B.M. } \mu=\sqrt{5(5+2)} \text { B.M. } \mu=\sqrt{35} \text { B.M. }\)
(c) [CO(C2O4)3]3-: The atomic number of cobalt is 27 and has electronic configuration 3d74s2. The oxidation state of CO in this complex is +3. Here, C2O42- is a strong field ligand. Therefore, 3d electrons are paired up and inner orbital (low spin) complex is formed.
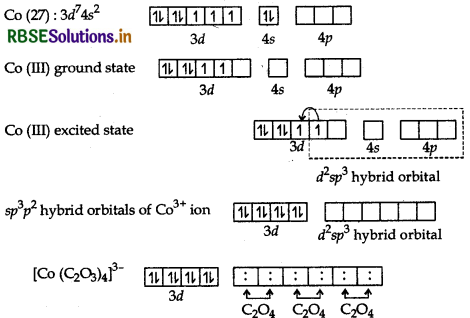
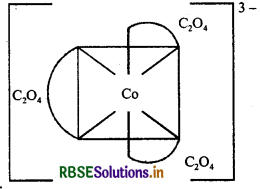
Hybridisation: d2sp3
Geometry: Octrahedral
Colour: Colourless because there is no unpaired electrons
Magnetic character: Dimagnetic because there is no unpaired electrons
Nature of the complex: Inner orbital (low spin) complex
(d) [COF6]3-: The atomic number of cobalt is 27 and has configuration 3d74s2. The oxidation state of cobalt in this complex is +3.
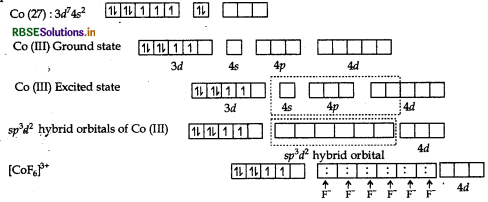
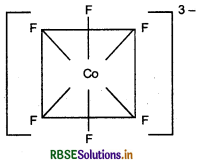
Hybridisation: sp3d2
Geometry: Octahedral
Colour: Coloured due to the presence of unpaired electrons
Magnetic character: Paramagnetic due to the presence of unpaired electrons
μ = μ = μ Nature of the complex: Outer orbital (high spin) complex

Question 9.16.
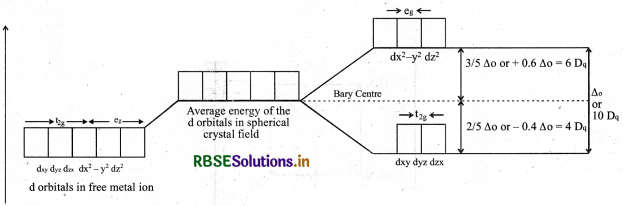
Draw figure to show the splitting of d-orbitals in an octahedral crystal field.
Answer:
When six ligands approach to central metal ion, they give degenerate d-orbitals of central metal ion split into two sets of degenrate orbitals having degenerate energies.
These two sets are tag and e orbitals. The difference in energies between these two sets are known as crystal field splitting energy (CSFE, ∆0)
Question 9.17.
What is spectochemical series? Explain the differences bn a weak field ligand and a strong field ligand.
Answer:
The ligands can be arranged in a series in the order of increasing field strength. This series is called spectochemical series and is given below:
I- < Br- < SCN- < CI- < S2- < F < OH- < C2O42- < H2O < NCS- < EDTA4- < NH3 < en < CN- < CO.
Question 9.18.
What is crystal field splitting energy? How does the magnitude of A, decide the actual configuration of d-orbitals in a coordination entity?
Answer:
In an octahedral field, when ligands approach to the central metal ion to form coordination bonds, the five degenerated d-obitals of central metal ion splits in two sets of orbitals having different energies. This splitting of degenerated d-orbitals is called central field splitting and the energy difference between two sets of orbitals is called crystal field splitting energy.
The configuration of d-orbitals in a coordination complex depends on the value of ∆o < p, the fourth electron enters one of the orbitals hence configuration becomes (t2g)z (eg)1 and high spin complex is formed. The ligands if ∆o > p, fourth electron get paired up in one of the 12 orbitals hence configuration becomes (t2g)4 (eg)0 and low spin complex is formed. The ligands for which pare called strong field ligands.
Question 9.19.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2- is diamagnetic. Explain why?
Answer:
[Cr(NH3)6]3+:
Atomic number of cobalt is 24 and electronic configuration is 3d5ns1 and the oxidation state is +3.
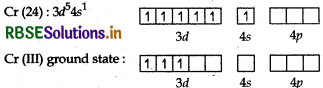
NH3 is a strong field ligand but two d-orbitals are already vacant hence pairing of electron is not required. Cr(III) Excited state
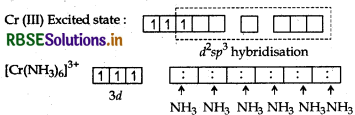
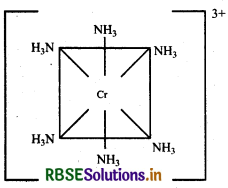
Hybridisation → d2sp3
Geometry → Octahedral
Colour → Coloured due to the presence of unpaired electrons.
Magnetic character → Paramagnetic due to presence of unparied electrons.
Magnetic Moment
= \(\sqrt{n(n+2)} B M\)
= \(\sqrt{3(3+2)} B M\)
= \(\sqrt{15}\)
(b) [Ni(CN)4]2-:
Atomic number of Ni is 28, electronic configuration is 3d84s2 and oxidation state is +2.
Ni (28): 3d8 4s2
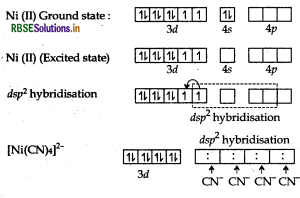
Hybridisation - dsp2
Geometry - Square planar
Colour - Colourless because there is no unpaired electrons.
Magnetic character - Diamagnetic because there is no unpaired electron.

Question 9.20.
A solution of [Ni(H2O)4]2+ is green but a solution of [Ni(CN)4]2- is colourless, explain.
Answer:
[Ni(H2O)4]2+: Atomic number of nickel is 28, electronic configurationis 3d84s2 and oxidation state is +2.
H2O is a weak field ligand. Hence it cannot paired up the electrons in 3d orbitals so 4d-orbitals take part in hybridisation So it is green.

Since there are two unpaired electrons, hence d-d transitions would occur so colour of solution become green.
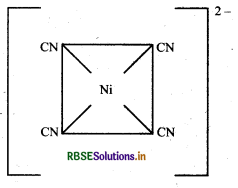
[Ni(CN)4]2-: Atomic number of Ni is 28, electronic configuration in 3d84s2 and oxidation state is +2.
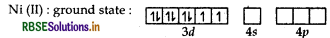
Since there is no unpaired electron, no d-d transitions would occur and hence the complex is colourless.
Question 9.21.
[Fe(CN)6]4- and [Fe(H2O)6]2+ are of different colours in dilute solutions, Why?
Answer:
In both the complexes, the oxidations state of Fe is +2 and electronic configuration of Fe2+ is 3d64so. In the presence of CN- (a strong field ligand), 3d electrons get paired up leaving one unpaired electron in the complex [Fe(CN)4]4- but in the presence of H2O (a weak field ligand) 3d electrons do not get paired up and therefore there are five unpaired electrons in the complex [Fe(H2O)]2+ Since both the complexes have different number of unpaired electrons so different region of visible light will be absorbed and therefore transmitted colour would be different i.e., they will give different colours in their solutions.
Question 9.22.
Discuss the nature of bonding in metal carbonyls.
Answer:
The metal carbon bond in metal carbonyl possesses both s-and p-character. The M-C σ - bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a empty orbital of the metal. The M-C- π - bond is formed by the donation of a pair of electrons from a filled d-orbitals of metal into vacent antibonding π* orbital of carbon mono-oxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal.
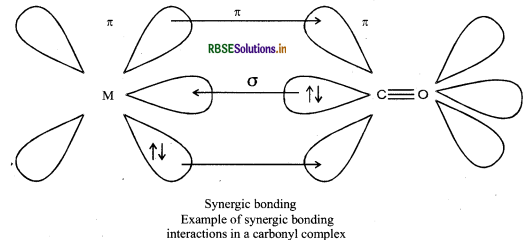
Question 9.23.
Give the oxidation state, d-orbital configuration and coordination number of the central metal ion in the following complexes:
(i) K3[CO(C2O4)3]
(ii) Cis-[Cr(Cn)2Cl2]Cl
(iii) (NH4)2 [COF4]
(iv) [Mn(H2O)6]SO4
Answer:
(i) K3[CO(C2O4)3]
= x + 3 (-2) = -3
x = +3
(ii) Cis-[Cr(Cn)2Cl2]Cl
= x + 0 + 2(-1)
= +1
x = +3
(iii) (NH4)2 [COF4]
= x + 4(-1) = -2
x = +2
(iv) [Mn(H2O)6]SO4
x + 0 = +2
x = +2

Question 9.24.
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex.
(i) K[Cr(H2O)2 (C2O4)2].3H2O
(ii) [CrCl3(Py)3]
(iii) [CO(NH3)5Cl]Cl2
(iv) Cs(FeCl4)
(v) K4(Mn(CN)6]
Answer:
(i) K[Cr(H2O)2 (C2O4)2].3H2O
IUPAC Name - Potassium diaquadioxalatochromate (III) trihydrate
Oxidation State -
K[Cr(H2O)2 (C2O4)2]
+ 1 + x + 2 x 0 + 2 x (-2)' = 0
1 + x - 4 = 0
x = + 3
Coordination Number - 6
Stereochemistry - Since the coordination number is 6, geometry is octahedral.
Magnetic moment and electronic configuration Cr(24); 4s1 3d5
Cr3+ : 4s0 3d9; [(t2g)3 (eg)o]
Unpaired e- = 3
Three unpaired electrons hence paramagnetic. Magnetic moment
\(\begin{aligned} (\mu) &=\sqrt{n(n+2)} B M \\ &=\sqrt{3(3+2)} B M \\ &=\sqrt{3 \times 5} B M \\ &=\sqrt{15} \end{aligned}\)
= 3.18 BM
(ii) [CrCl3(Py)3]
IUPAC Name:Trichloridotripyridinechromium (III)
Oxidation number:
[CrCl3(Py)3]
x + 3 x (-1) + 3 x 0 = 0
X - 3 = 0
Y = +3
Coordination Number - 6
Stereochemistry - Since the coordination number is 6, geometry is octahedral
Magnetic moment and electronic configuration:
Cr(24) : 4s1 3d5
Cr3+ (21) : 4s° 3d3 [(t2g)3 (eg)0]
d2sp3 hybridization
Three unpaired electrons hence paramagnetic
Magnetic moment
= \(\sqrt{n(n+2)} B M\)
\(\begin{aligned} &=\sqrt{3(3+2)} B M \\ &=\sqrt{3 \times 5} B M \\ &=\sqrt{15} B M \end{aligned}\)
= 3.87 BM
(iii) [CO(NH3)5Cl]Cl2
IUPAC Name - Pentaamminechloridocobalt(III) chloride
Oxidation State - [CO(NH3)5Cl]Cl2
x + 5 x (0) + (-1) + 2 x (-1) = 0
x - 1 - 2 = 0
x = +3
Coodination number - 6
Stereochemistry - Since the cordination number is 6, geometry of the complex is octahedral.
Magnetic moment and electronic configuration
CO(27): 4s2 3d7
CO3+ (24): 4S0 3d6 [(t2g)6 (eg)0]
d2sp3 hybridization
No unpaired electrons hence diamagnetic
Magnetic moment (μ) = 0
(iv) Cs[FeCl4]
IUPAC name-Caesium tetrachloridoferrate (III)
Oxidanitn state: Cs[FeCl4]
+ 1 + x + 4 x (-1) = 0
X - 3 = 0
x = +3
Cordiantion number - 4
Stereochemistry - Tetrahedral
Magnetic moment and electronic configuration
Fe (26): 4s2 3d6
Fe3+ (23): 4so 3d [(eg)2 (t2g)3]
sp3 hybridisation
Five unpaired electrons hence paramagnetic
Magnetic moment (u) = \(\sqrt{n(n+2)}\)
\(\begin{aligned} &=\sqrt{5(5+2)} \\ &=\sqrt{5 \times 7} \\ &=\sqrt{35} \mathrm{BM} \end{aligned}\)
= 5.92 B M
(v) K4[Mn(CN)6]:
IUPAC Name-Potassium hexacyanomanganate (II)
Oxidation State: K4[Mn(CN)6]
4 x (+1) + x + 6 x (-1) = 0
4 = N -6 = 0
N = +2
Coordination Number - 6
Stereochemistry - Octahedral
Magnetic moment and electronic configuration
Mn (25): 3d5 4s2
Mn2+ (23): 3d6 4s0 [(t2g)5 (eg)0]
d2sp3 hybridisation
One unpaired electron hence paramagnetic.
Magnetic moment (u) = \(\sqrt{n(n+2)}\)
\(\begin{aligned} &=\sqrt{5(5+2)} \\ &=\sqrt{5 \times 7} \\ &=\sqrt{35} \mathrm{BM} \end{aligned}\)
= 1.732 B M

Question 9.25
What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Answer:
Mostly the complex ions are highly stable, the reaction between a metal ions and ligands is considered as a Lewis acid-base reaction in which the ligands act as Lewis bases and the central metal ion acts as a Lewis acid. If the interaction between central atom as ion and ligands is strong, a stable complexion is formed. The stability of the complex in solution refers to the degree of association between metal ion and ligands in Volved in the state of equibrum Ine magnitude of the stability as formation) equilibrium constant for the association quantitatively express the stability. If we consider the fol lowing reaction
\(\mathrm{M}+4 \mathrm{~L} \rightleftharpoons \mathrm{ML}_4\)
Then stability constant is given by
\(k=\frac{\left[\mathrm{ML}_4\right]}{[\mathrm{M}][\mathrm{L}]^4}\)
Higher the value of stability contant, greater the amount of ML4 and lesser the amount of free metal ions in solution hence complex will be stable.
Factors affecting the stability of complexes:
(1) In Analytical Chemistry: Coordiantion compounds are used in many qualitative and quantitative chemical analysis. Some metal ions coordinate with a number of ligands (especially chelating ligand), to form columned complexes which form the basis for their detection and estimation by classicial and instrumental methods of analysis. For example,
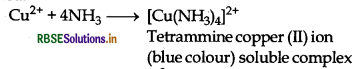
(i) The presence of CU2+ ions are tested by adding ammonia to the solution salt when a blue coloured complex is formed.
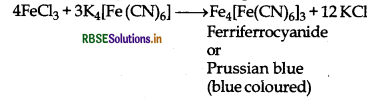
(ii) The presence of Fe3+ ions are tested by adding potassium ferrocyanide solution to the salt solution when ferriferrocyanide or prussian blue complex is formed.
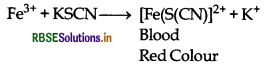
(iii) Fe3+ ions are also detected by adding potassium thioyanate solution to the salt solution when a red coloured complex is formed.
Question 9.26.
What is mornt by chelate effect? Give an example.
Answer:
When a di - or polydentate ligand coordinates to the central metal atom or ion using its to as more donor atoms, then a ring like structure is formed which increase the stability of the complex. This enhanced stability of the complexes containing bidentate of polydentate ligands (chelating ligands) in called chelate effect :
Example: [Cu(CN)2]2+
Question 9.27.
Discuss briefly giving an example in each can the role of coordinatioin compounds in (i) biological systems (ii) analytical chemistry (iii) medicinal chemistry (iv) extraction/metallurgy of metals.
Answer:
Factors affecting the stability of complexes:
(1) In Analytical Chemistry: Coordiantion compounds are used in many qualitative and quantitative chemical analysis. Some metal ions coordinate with a number of ligands (especially chelating ligand), to form columned complexes which form the basis for their detection and estimation by classicial and instrumental methods of analysis. For example,
(i) The presence of CU2+ ions are tested by adding ammonia to the solution salt when a blue coloured complex is formed.

(ii) The presence of Fe3+ ions are tested by adding potassium ferrocyanide solution to the salt solution when ferriferrocyanide or prussian blue complex is formed.

(iii) Fe3+ ions are also detected by adding potassium thioyanate solution to the salt solution when a red coloured complex is formed.

Question 9.28.
How many ions are produced from the complex CO(NH3)6Cl2 in solution
(i) 6
(ii) 4
(iii) 3
(iv) 2
Answer:
Oxidation number of cobalt = 6
The complex [CO(NH3)6] Cl2 ionises as
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_2 \stackrel{e q}{\longrightarrow}\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{2+}+2 \mathrm{Cl}^{-}\)
Hence three ions are produced.
Therefore option (ii) is correct.
Question 9.29.
Amongst the following ions which one has highest magnetic moment?
(i) [Cr(H2O)6]3+
(ii) [Fe(H2O)6]2+
(iii) [Zn(H2O)6]2+
Here oxidaiton states are - Cr(III), Fe(II), Zn(II)
Electronic configuration of Cr3+ = 3d [(t2g)3 (eg)0]
Number of unpaired electrons = 3
Electronic configuration of Fe
Electronic configuration of Zn2+ = 3d10
Number of unpaired electron = 0
Magnetic moment μ = \(\sqrt{n(n+2)}\)
Since n = 4 is highest, the magnetic moment of option (iii) will be highest.

Question 9.30.
The oxidation number of cobalt in K[CO(CO)4]
(i) +1
(ii) +3
(iii) -1
(iv) -3
Answer:
K[CO(CO)4]
+ 1 + x + 4 x (0) = 0
1 + x = 0
x = -1
Hence option (iii) is correct.
Question 9.31.
Among the following the most stable complex is:
(i) [Fe(H2O)6]2+
(ii) [Fe(NH3)6]3+
(iii) [Fe(C2O4)3]3-
(iv) [FeCl6]3-
Answer:
In each of the above complexes, the oxidation state of Fe is +3, Since C2O42- is a chelating ligand and it forms chelate ring, therefore option (iii) is most stable complex.
Question 9.32.
What will be the correct order of the wavelengths of absorption in the visible region for the following:
[Ni(NO2)6]4-, [Ni(NH3)6]2+, [Ni(H2O)6]2+
Answer:
Since the metal ion is fixed, the increasing order of field strength of the ligands from the spectrochemical series is H2O < NH3 < NO. Thus, the order of energies absorded for excitation is as follows:
[Ni(NO2)6]4-, [Ni(NH3)6]2+, [Ni(H2O)6]2+
As E = \(\frac{h c}{\lambda}\) the wavelengths absorbed will be in the opposite order.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम