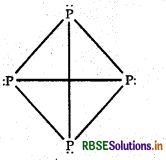
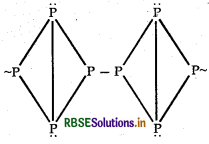
RBSE Solutions for Class 12 Chemistry Chapter 7 The p-Block Elements
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 7 The p-Block Elements Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 7 The p-Block Elements
RBSE Class 12 Chemistry The p-Block Elements InText Questions and Answers
Question 7.1.
Why are pent halides more covalent than trihalides?
Answer:
According to Fajan's rule, higher the positive oxidation state, higher will be the polarising power hence more will be the covalent character. In penta halide, the oxidation state is more (+5) than in trihalide (+3). Hence penta halides are more covalent than trihalides.
Question 7.2.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group-15 elements?
Answer:
Among the hydrides of group-15 BiH3 is the least stable hydride due to its largest size, Hence it can readily lose H-atom. So BiH3 behave as the strongest reducing agent.

Question 7.3.
Why is N less reactive at room temperature?
Answer:
N2 is less reactive at room temperature because. It has strong pa-pa overlaping triple bond (N = N) hence have very high bond dissociation enthalpy.
Question 7.4.
Mention the conditions required for the maximum yield of ammonia.
Answer:
In Haber's process, ammonia can be manufactured as:
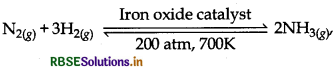
According to Le-chatelier's principle, the yield can be maximise as follow:
(a) High Pressure: To get the high yield of ammonia, the pressure should be high i.e. 200 atm.
(b) Low Temperature: As the reaction is exothermic so the Temperature should be Low i.e. 700 K temperature is sufficient.
(c) Catalyst: To get the high yield of ammonia, catalyst iron oxide is used.
(d) Promoter: Along with catalyst promotors like K2O, Al2O3 or Mo are used to increase the efficiency of the catalyst.
Question 7.5.
How does ammonia react with a solution of Cu2+?
Answer:
Ammonia reacts with the solution of Cuation and a deep blue coloured complex is formed.
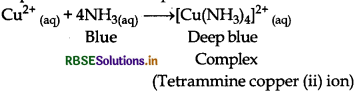
Question 7.6.
What is the covalence of nitrogen in N2O5?
Answer:
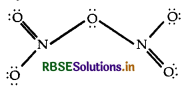
The covalency depends upon the number of shared pair of electrons. The structure of N2O5 is as follow:
Since the nitrogen atom has four shared pair of electron hence its covalency is 4.
Question 7.7.
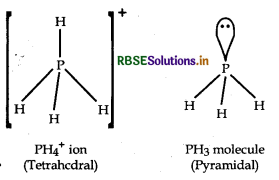
Bond angle in PH4+ is higher than that is PH3 Why?
Answer:
In both PH3 and PH4+ ion, phosphorus atom is sp3-hybridised but the bond angle in PH+ is 109°28' but in PH3 it is 93.6°. It is due to the presence of lone pair of electron on phosphorus atom of PH3 which creates lone pair-bond pair repulsion which decreases the bond angle.
Question 7.8.
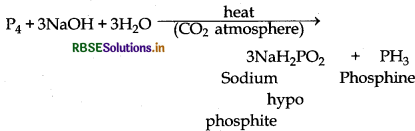
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2
Answer:
When white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2 then sodium hypophosphite and phosphine gas is formed.
Question 7.9.
What happens when PCl5 is heated?
Answer:
On heating PCl5, it dissociates into PCl3, and Cl2 because is PCl5, two P-Cl bonds are longer than three P-Cl(e) bonds attached to central phosphorus atom.
PCl5 → PCI3 + Cl2
Question 7.10.
Write a balanced equation for the hydrolytic reaction of PCl5, in heavy water.
Answer:
PCl5, reacts with heavy water to form phosphorus oxychloride (POCl3) and deuterium chloride (DCI)
PCl5 + D2O → POCl3 + 2DCI
Question 7.11.
What is the basicity of H3PO4?
Answer:
Basicity is decided by P-OH bond.
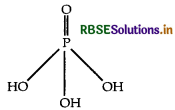
As three P-OH bonds are present in H3PO4. Therefore its basicity is three.
Question 7.12.
What happens when H3PO4 is heated?
Answer:
On heating H3PO3 disproportionates to give orthophonsphric acid and phosphine.

Question 7.13.
List the important sources of sulphur?
Answer:
In earth's curst sulphur exists in combined state in the form of sulphides and sulphates. Some examples are:
Sulphides: Galena (Pb), Zinc blende (ZnS), Copper phrites (CuFeS).
Sulphates: Gypsum (CaSO4. 2H2O), epsom salt (MgSO4.7H2O).
Traces of sulphur also occur as H2S in volcanoes. Some organic materials like eggs, protens, garlic, onion, mustard, hair and wool also contain sulphur.
Question 7.14.
Write the order of thermal stability of the hydrides of group-16 elements?
Answer:
As the size of central atom increases, bond length increases and dissociation energy decreases. Hence on moving down the group thermal stability decreases. H2O > H2S > H2Se > HeTe > H2PO(Thermal stability)

Question 7.15.
Why is H2O a liquid and H2S a gas?
Answer:
Due to small size and high electronegativity of oxygen atom, water molecules are associated by hydrogen bonding hence exist in liquid state while H2S molecules are not associated by hydrogen bonding so it is a gas.
Question 7.16.
Which of the following does not react with oxygen directly?
Zn, Ti, Pt, Fe
Answer:
Pt. because it is a noble metal and have very high value of ionisation energy, on the other hand, Zn, Ti and Fe are active metal and can directly combine with oxygen to form respective oxids.
Question 7.17.
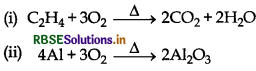
Complete the following reactions:
(i) C2H4 + O2 →
(ii) 4Al + 3O2
Answer:
Question 7.18.
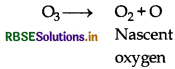
Why does O3 act as a powerful oxidising agent?
Answer:
Ozone can easily be liberated because of atoms of nascent oxygen hence act as a powerful oxidising agent.
Question 7.19.
How is ozone estimated quantitatively?
Answer:
When ozone reacts with an excess of potassium iodide solution buffered with a borate buffer (pH 9.2), iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method for the estimation of ozone gas.

Question 7.20.
What happens when sulphur dioxide is passed through an aqueous solution of Fe (III) salt?
Answer:
Sulphur dioxide behave as reducing agent hence it reduces an aqueous solution of Fe (iii) salt into Fe (ii) salt.
SO2 + 2H2O → SO42- + 4H+ + 2e-
2Fe3+ + → 2e- + 2Fe2+
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42- + 4H+
Question 7.21.
Comment on the nature of two S-O bonds formed in SO2 molecule. Are the two S-O bonds in this molecule equal ?
Answer:
Both the S-O bond in sulphur dioxide molecule are covalent in nature. They have equal bond length and equal strength due to the presence of resonance.
Question 7.22.
How is the presence of sodetected?
Answer:
SO2 has pungent smell. It can be detected by the following two tests:
(a) SO2 turns orange colour of acidified K2Cr2O7 into green colour
SO2 + 2H2O → SO42- + 4H+ + 2e-
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
Cr2O72- + 3SO2 + 2H+ → 2Cr3+ + 3SO42- + H2O
(b) SO2 turns pink colour of KMnO4 colourless.
SO2 + 2H2O → SO42- + 4H+ + 2e-
MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
2MnO4- + 5SO2 + 2H2O → 2Mn2+ + 5SO42- + 4H+
Question 7.23.
Mention three areas in which H2SO4 plays an important role.
Answer:
Main three areas in which H2SO4 plays an important role are:
- It is used in batteries.
- In manufacturing of pigments, paints and dyestuff intermediates.
- As an important laboratory reagent.
Question 7.24.
Write the conditions to maximise the yield of H2SO4 by contact process?
Answer:
The main reaction in the manufacture of H2SO4 is the oxidation of SO2 into SO3 in the presence of catalyst.
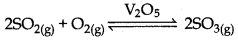
∆H = -196.6 kJ mol-1
The above reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield. But the temperature should not be very low otherwise the rate will become slow. Hence the reaction should be held at 2 bar and 720 K temperature for the maximum yield.

Question 7.25.
Why is Ka2 << K1 for H2SO4 in water?
Answer:
H2SO4 is a very strong acid it gives H+ ions readily in water, so the value of Ka1 is very large
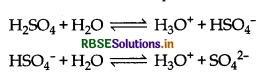
But HSO4- is stabilized by resonance and hence given second H+ ion with difficulty. Hence Ka2 << Ka1.
Question 7.26.
Considering the parameters such as bond dissociation energy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of F2 and Cl.
Answer:
The electrode potential of fluorine, Fe(+2.87 V) is much higher than the electrode potential of chlorine, Cl2 (+ 1.36V) so F is stronger oxidising agent than Cl.
Actually the value of electrode potential depends upon three parameters:
- Bond dissociation enthalpy
- Hydration enthalpy
- Electron gain erithalpy.
The value of these enthalpies are as follow:
From above data it is clear that electrode potential of F2 is higher than Cl, so F is stronger oxidising agent than Cl2,
Question 7.27.
Give two examples to show the anomalous behaviour of fluorine.
Answer:
Fluorine show anomalisms behaviour due to:
- Highest electronegativity.
- Small Size.
- Low F-F bond dissociation enthalpy.
- Absence of d-orbitals is its valence shell
The two examples of anomalous behaviour are:
(a) HF is liquid due to the presence of hydrogen
(b) Fluorine forms only one oxoacids other forms a number of oxoacids.
Question 7.28.
Sea is the greatest source of some halogens comment.
Answer:
Sea is a major source of halogens. Sea water contains chlorides, bromides and iodides of Na, K, Mg and Ca.But it mainly contains sodium chloride solution 2.5% by mass. Certain marine life contain iodine in their systems for example seaweeds contain 0.5% iodine chill salt peter contains about 0.2% of sodium iodate (NaIO3).

Question 7.29.
Give the reason for bleaching action of Cl2.
Answer:
In presence of moisture or in aqueous solution chlorine liberates nascent oxygen as follow:

This nascent oxygen oxidised coloured substances present in vegetables and organic matter to colourless substances.
Coloured substances + (O] Colourless substances. In this way, the bleaching action of chlorine is due to oxidation.
Question 7.30.
Name two poisonous gases which can be prepared from chlorine gas.
Answer:
The two poisonous gases are:
- Mustard gas (ClCH2CH2SCH2CH2Cl)
- Phosgene gas (COCl2)
Question 7.31.
Why is ICI more reactive than I2?
Answer:
ICI is more reactive than I because I-Cl bond is weaker than I-I bond. So ICI breaks easily to form halogen atom which bring about the reaction quickly.
Questions 7.32.
Why is helium used in diving apparatus?
Answer:
Helium is used as a diluent for oxygen in modern diving apparatus because it has very low solubility in blood.
Question 7.33.
Balance the following equation:
XeF6 + H2O → XeO2F2 + HF
Answer:
The balanced equation is as follow:
XeF6 + 2H2O → XeO2F2 + 4HF
Question 7.34.
Why has it been difficult to study the chemistry of radon?
Answer:
Radon (Rn) is radioactive with very short half life. Due to its radioactive nature the study of chemistry of radon is difficult.
RBSE Class 12 Chemistry The p-Block Elements Textbook Questions and Answers
Question 7.1.
Discuss the general characteristics of group15 elements with reference to their electronic configuration, oxidation state, atomic size, ionization enthalpy and electro-negativity.
Answer:
Electronic Configuration:
|
Trick for learning |
Element |
Atomic number |
Electronic configuration |
Inert gas core |
|
N (Nana) |
Nitrogen (N) |
7 |
1s2, 2s2, 2p3 |
[He] 2s2 2p3 |
|
P (Patekar) |
Phosphorous (P) |
15 |
1s2, 2p2, 2p6, 3s2, 3p3 |
[Ne] 3s2 3p3 |
|
As (Aaj) |
Arsenic (As) |
33 |
1s2, 2s2, 2p6, 3s2, 3p6, 3d10 4s2, 4p3 |
[Ar] 3d104s24p3 |
|
Sb (Sabke) |
Antimony (Sb) |
51 |
1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p6, 4d10, 5s2, 5p3 |
[Kr] 4d10 5s2 5p3 |
|
Bi (Beech hai) |
Bismuth (Bi) |
83 |
1s2, 2s2, 2p6, 3s3, 3p6, 3d10, 4s2, 4p6, 4d10 4f14, 5s2, 5p6, 5d10, 6s2, 6p3 |
[Xe] 4f14 5d10 6s2 6p3 |
Electron negativity:
Electronegativity decreases gradually on moving down the group from N to Bi. This is due to increase in atomic size from N to Bi.
Element N > P > As > Sb > Bi
Electronegativity -3.0 > 2.1 > 2.0 > 1.9
Ionisation Enthalpy:
On moving down the group the first ionisation enthalpy decreases regularly. It is because of increase in the size of atoms and screening effect of the intervening electrons. N > P > As > Sb > Bi (Ionisation Enthalpy)
Enthalpy of the elements of group 15 are much higher than those of corresponding elements of carbon family.
Oxidation State:
A chemical reaction which includes the movement of electrons is called oxidation. A substance which donates electrons is said to be oxidized. For example, The reaction between iron and oxygen - When iron (Fe) reacts with oxygen it forms rust because iron loses electrons and oxygen gains electrons.
Atomic size:
Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. In basic chemistry, the atomic radius is defined as the shortest distance between the atom's nuclei and the outermost shell of the atom.

Question 7.2.
Why does the reactivity of nitrogen differ from phosphorus?'
Answer:
Nitrogen is inert and unreative in the elemental state because it exist as diatomic molecule (N = N) and due to the presence of triple bond between two nitrogen atoms it has very high bond dissociation energy (941.4 kJ mol-1). While white phosphorus exists as a tetra atomic molecule (PA) with P-P single bond which is much weaker and have very low bond dissociation enthalpy (213 kJ mol-1) than nitrogen. Therefore, phosphorus is much more reactive than nitrogen.
Question 7.3.
Discuss the trends in chemical reactivity of group-15 elements.
Answer:
chemical reactivity of group-15 elements:
(1) Acidic nature: It forms sulphurous acid when dissolves in water
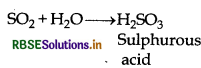
acid It turns moist blue litmus red. It is known as anhydride of sulphurous acid. It acidic behaviour can be shown by the following reactions.
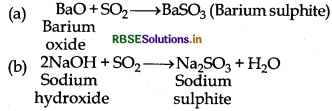
(2) Reaction with base: It readily reacts with sodium hydroxide and sodium sulphite is formed. Which again reacts with excess amount of sodium hydroxide then sodium hydrogen sulphite is formed.
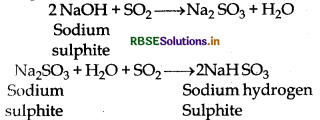
(3) Thermal decomposition: On heating strongly at 1473 K temperature it decomposed into SO, and S.
3SO3 → 2SO3 + S
On electric disintegeration it decomposes into SO and O2.
2SO2 → 2SO + O2
(4) Reaction with oxygen: SO2 reacts with oxygen when it is heated in presence of V2O5 or platinum asbestos catalysis, then SO3 is formed.
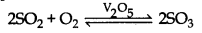
(5) Reaction with chlorine: SO2 reacts with chlorine in presence of charcoal then sulphuryl chloride (SO2Cl2) is fomed.

(6) Reducing properties: Sulphur-di-oxide behave as reclucing agent. Its some important properties are as follows
(a) Reduction of ferric salts into ferrous salts:
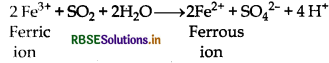
(b) Reduction of acidic MnO4- into Mn3+: In acidic medium sulphur-di-oxide decolourises pink colour of potassium permanganate solution.
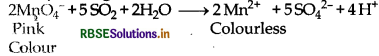
(c) Reduction of dichromate ion into chromium ion: It turn orange colour of acidic solution of potassium dichroinate into green.
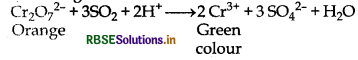
(d) Reduction of iodate ion into idine:
2IO-3 + 5SO2 + 4H2O → I2 + 5SO42- + 8H-
(e) Reduction of iodine into iodide:
I2 + SO2 + 2H2O → 2I- + SO2-4 + 4H+
(f) Reduction of halogen: It can reduce halogens into halogen acids or halide ions. As it can destroy the properties of chlorine so sulphur-di-oxide is known as Anti-chloride.
X + SO2 + 2H2O → 2HX + H2SO4 (where X = CI, Br Or 1)
(g) Reduction of lead dioxide into lead sulphate:
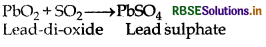
(7) Oxidising properties: It behave as mild oxidising agent. Some oxidising properties are given below:
(a) It oxidises magnesium into magnesium oxide.
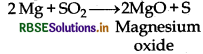
(b) It oxidises iron into iron oxide and iron sulphides.
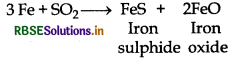
(c) It can oxidises H2S into S.
2H2S + SO2 → 3S + 2H2O
(d) It can oxidise carbon monooxide into carbon-dioxide.
2CO + SO2 → 2CO2 + S
(e) It can oxidise stannous and mercurous salts into stannic and mercuric salts in presence of HCl.
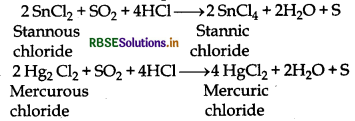
(8) Bleaching action: Moist sulphur-di-oxide behave as mild bleaching agent. It bleaches naturally coloured subtances like flowers, vegables, leaves etc. But its bleaching action is temporary. Becauses its bleaching action is due to reduction of the substances. When these bleached articles or substances is exposed to air then it regions its colour due to oxidation.
SO2 + 2H2O → H2SO4 + 2 [H]
Vegetable colouring matter + [H] → Colourless vegetable matter
(9) Reaction with hydrogen: SO2 oxidises hydrogen into water at 1000°C temperature,
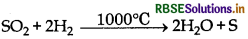
(10) Action of lime water: It can turns lime water milky, It is due to the formationof insoluble calcium sulphite. However, on passing lime water in excess milkiness disappears due to the formation of soluble sodium bisulphite.
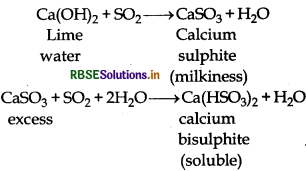
Question 7.4.
Why does NH3 form hydrogen bond but PH3 does not?
Answer:
The electronegativity of nitrogen (3.0) is much higher than hydrogen (2.1) hence the bond N-H becomes polar hence NH, undergoes hydrogen bonding, White the electronegativity of Pand His 2.1 therefore the bond P-H is not polar and hence PH3 does not forms hydrogen bonding.
Question 7.5.
How is nitrogen prepared in the laboratory? Write the chemical equation for the reactions involved.
Answer:
In laboratory nitrogen gas is prepared by heating equimolar aqueous solution of ammonium chloride and sodium nitrite.
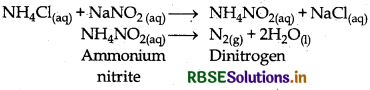
Question 7.6.
How is ammonia manufactured industrially?
Answer:
Ammonia can be manufactured industrially by Haber's process.
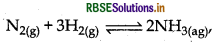
The conditions for the high yield are as follows:
- High pressure (200 to 300 atom)
- Low temperature (723 to 773K)
- Presence of catalyst Al2O3 with iron.
- The product should be collected time to time.

Question 7.7.
Illustrate how copper metal can give different products on reaction with HNO3.
Answer:
The reaction of copper with nitric acid:
(i) Reaction with nitric acid:
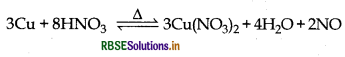
(ii) Reaction with concentrate nitric acid:

Question 7.8.
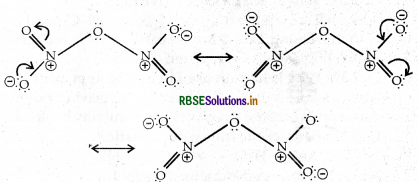
Give the resonating structures of NO and N2O5
Answer:
(i) Resonating Structure of NO2
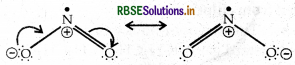
(ii) Resonating Structure of N2O5
Question 7.9.
The HNH angle value is higher than those HPH, HASH and HSbH angles. Why?
Answer:
Molecule NH3, PH3 ASH3 SbH3 BiH3
bond angle 107.8° 93.6° 91.8o 91.3° 90°
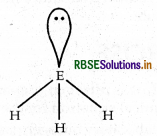
As we move down the group, the electronegativity of central atom decreases. The bond pair of electrons will lie much away from the central atom.In other words we can say that the force of repulsion between bond pairs goes on decreasing from NH3 to SbH3 hence bond angle also decreases regularly.

Question 7.10.
Why does R3 P = O exist but R3N = O does not (R = alkyl group)?
Answer:
Due to the absence of d-orbitals nitrogen cannot form pr-de multiple bonds hence nitrogen can not expand its covalency beyond four but the covalency of N in R3N = O is 5 so this compound does not exist. On the other hand Phas empty d-orbitals and hence can form pm-dm multiple bonds so it can expand its covalincy beyond 4 therefore P can form R3P = O.
Question 7.11.
Explain why NH3 is basic while BiH3 is only feebly basic.
Answer:
NH3 and PH3 both behave as Lewis bases due to the presence of lone pair of electron on central atom. However NH3 is more basic than PH3 because the atomic size of nitrogen is smaller than phosphorus. As a result the electron density on nitrogen atom in NH3 is higher than phosphorus. It means that the tendency to donate lone pair of electron is higher in ammonir therefore it is a strongr base than phosphine.
Question 7.12.
Nitrogen exists as diatomic molecule and phosphorus as P4 Why?
Answer:
Due to small size and high electronegativity nitrogen can form pn-pt multiple bonds thus it exists as a diatomic molecule with triple bond between two N-atoms. Phosphorus has larger size and lower electronegativity so it can not form pn-pit multiple bonding with itself hence it forms P-P single bond and exists as tetrahedral P4 molecule.
Question 7.13.
Write main differences between the properties of white phosphorus and red phosphorus.
Answer:
The main difference are as follows:
|
Property |
White phosphorus |
Red phosphorus |
|
(1) State |
Translucent |
Brittle substance |
|
(2) Colour |
White but gets yellowish on exposure to air |
It is red in colour. |
|
(3) Odour |
Garlic odour |
Odourless |
|
(4) Hardness |
Soft like wax and hence can be cut with knife |
Hard |
|
(5) Poisonous Nature |
It is poisonous and creates phossy jaw disease. |
Non-poisonous in nature |
|
(6) Solübility |
Soluble in Cs2 insoluble in Cs2 317 K |
It sublimes at 536K and melts at 862K and 43atm pressure. |
|
(7) Melting Point |
1.80 |
2.10 |
|
(8) Density |
Very reactive |
Less reactive |
|
(9) Reactivity |
Reacts with Cl2 and PCl3, PCl5 are formed. |
Reacts with Cl2 only on heating. |
|
(10) Reaction with chlorine |
White phosphorus |
Red phosphorus |
|
(11) Structure |
|
|
Question 7.14.
Why does nitrogen show catenation properties less than phosphorus?
Answer:
Catenation property is directly proportional ito bond dissociation energy. Since the bond dissociation energy of N-N is 159 kJ mol-1 which is much lesser than bond dissociation energy of P-P (213 kJ mol-1). Hence the catenation properties of nitroges is lesser than phosphorus.

Question 7.15.
Give the disproportionate reaction of H3PO3
Answer:
On heating H3PO3 disproportionate into PH3 and H3PO4
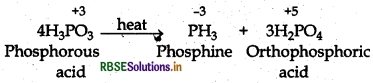
Question 7.16.
Can PCl5 act as an oxidising as well as reducing agent? Justify?
Answer:
In PCl the oxidation state is + 5. P has give electrons in its valence shell so it cannot increase its oxidation state beyond +5 by donating electrons. Therefore PCls cannot act as a reducing agent. However PCl, can decrease its oxidation state from +5 to +3 so it can act as an oxidising agent.
For Example:
(a) Oxidation of Silver:
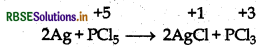
(b) Oxidation of Tin:
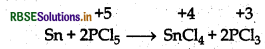
Question 7.17.
Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation
Answer:
(i) Electronic Configuration: The valence shell electronic configuration of O, S, Se, Te and Po is ns2 np, hence all the elements should be placed in same group i.e. in group-16.
8O - [He] 2s2, 2p4
16s - [Ne] 3s2, 3p4
34se - [Ar] 3d10, 4s2, 4p4
52Te - [Kr] 4d10, 5s2, 5p4
84Po - [Xe] 4f14, 5d10, 6s2, 6p4
(ii) Oxidation State: They required two extra electrons to acquire noble gas configuration hence there lowest oxidation state should be - 2. Oxygen due to high electro negativity show - 2 oxidation state. Sulphur to some extent being electronegative show - 2 oxidation state. As there are six electrons in their valence shell. Therefore there maximum value of oxidation state is +6. Other oxidation state shown by these elements are + 2 and + 4. Thus on the basis of minimum an maximum oxidation states, these elements are justified to be placed in the same group i.e. in group-16 of the Periodic table.
(iii) Formation of Hydrides: The elements O, S, Se, Te and Po complete their octets by sharing two electrons of their valence electrons with 1s-orbital of hydrogen to form hydrides of general formula EH, i.e. H2O, H2S, H2Se, H2 Te and H2PO. Therefore on the basis of their hydride formation with general formula EH2 these elements are justified to be placed in group-16.
Question 7.18.
Why is dioxygen a gas but sulphur a solid?
Answer:
Due to small size and high electronegativity, oxygen can form pn-pt multiple bonds hence exists as diatomic (O2) molecule. These molecules are held together by weak Vander Waal's force of attraction hence, O2 is gas at room temperature.
On the other hand, sulphur has bigger size and low electronegativity. It prefers to form S-S single bond, further more its catenation property is also higher than oxygen. Thus sulphur because of its higher tendency of catenation and lower tendency for the formation of pn-pt multiple bonding, it exist as Sg molecule with puckered ring like structure.
Hence due to bigger size, stronger force of attraction between S8 molecules it exists in solid state at room temperature.
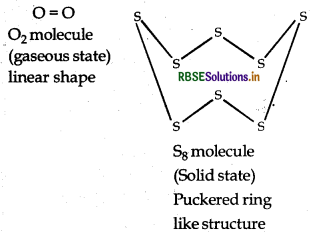
Question 7.19.
Knowing the electron gain enthalpy values for O → O- and O → O2- as - 141 and 702 kJ mol-1 respectivily. How can you account for the formation of a large number of oxides having O2- species and not O-?
Answer:
When oxygen reacts with metal then it froms folowng types of oxides:
- M2O
- MO
- MO2
The formation of these oxides involves the following steps.
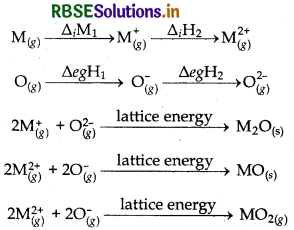
We know that the value of ∆iH2 is much higher than the value of ∆iH1 and the value fo∆egH2 is positive as compared to ∆egH1 but when MO and MO2 compounds re formed then the lattice energy released in case of MO is very much higher than the lattice energy released during the formation of MO2. it is due to the presence of higher charge on both the ions in case of MO. Hence the formation of MO is thermodynamically favourable. Hence due to above reason a large number of oxides haivng O2- species are formed.

Question 7.20.
Which aerosols deplete ozone?
Answer:
Areosols like chlorofluorocarbons (CFCs) i.e., freon (CCl2F2) depletes the Ozone (O3) layer by supplying Cl free radicals which can convert O3 into O2 as follow:
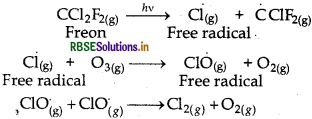
Question 21.
Describe the manufacture of H2SO4 by contact process?
Answer:
- Low temperature (about 720 K), high pressure about 2 bar and presence of catalyst (V2O5) are the favourable conditions for the manufacture of H2SO4 by contact process.
- H2SO4 is very strong acid in water largely because of its ionisation to H2O+ and H2SO4. Further, ionisation of H2SO4-, to SO42- and H3O+ is very small as losing H+ from a negatively charged H2SO4- is more difficult. That's why, Ką2 << Ka1
Question 22.
How is SO2 an air pollectant?
Answer:
- SO2 is a pungent smelling gas. It strongly irritates the respiratory tract. It also cause throat infection, irritation to eyes due to its poisonous as well as irritating nature.
- Even its very low concentration (0.03 ppm) has damaging effect on plant and vegetation. It causes chlorosis. Inchlorosis the formation of chlorophyll is slow down and it is also responsible gor the white colour of it is also responsible for the white colour of leaves.
- SO2 is responsible for acid rain i.e., SO2 dissolves in moisture/water of rain and forms H2SO3 which damages buliding materials especially marbles.
- CaCO3 + H2SO3 → CaSO3 + H2O + CO2
Question 7.23.
Why are halogens considered a strong oxidising agents?
Answer:
Halogens have strong tendency to accept electrons due to low bond dissociation enthalpy, high electronegativity and large negative value of electron gain enthalpy. Thus they get reduced easily.
X + e- → X
Thus halogens behave as strong oxidising agents. There oxidising power decreases down the group from F2 to I2.
Question 7.24.
Explain why fluorine forms only one oxo acid, HOF.
Answer:
Fluorine has highest electronegativity and does not have d-orbitals hence it can not form various oxoacids like HFO2 HFO3 and HFO4 in which the oxidation state of F is +3, +5 and +7. So It forms only one oxoacid i.e., in which it has oxidation state +1.
Question 7.25.
Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not?
Answer:
Yet both nitrogen and chlorine have same electronegativity i.e., 3.0, however only nitrogen involves in formation of hydrogen bonding. It is due to the small size of nitrogen as compared to chlorine. Due to small size N can cause greter polarisation of N-H bond. Consequently involves in hydrogen bonding.

Question 7.26.
Write two uses of ClO3.
Answer:
Two uses of ClO3 are:
- It acts as bleaching agent for paper pulp in paper industry and in textile industry.
- It acts as germicide for disinfecting water.
Question 7.27.
Why are halogens coloured?
Answer:
All the halogens are coloured. It is due to the reason that halogen molecules absorb the light in visible region and get exited to higher energy levels. The remaining energy is now transmitted. Actually the colour of halogens is due to this transmitted energy. As size of halogens increases the amount of energies required for exitation decreases, thus the colour deepends from F2 to I2. For example F2 absorbs violet light hence looks pale yellow, while I2 absorbs yellow light and appears deep violet. Similarly Cl2 emits greenish yellow and Br2 emits red light.
Question 7.28.
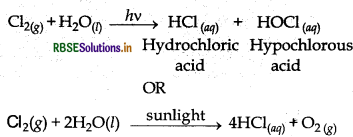
Write the reactions of F2 and Cl2 with water.
Answer:
(a) F2 oxidises H2O into O2 or O3.
2F2(g) + 2H2O(l) → 4H+(aq) + 4F-(aq) + O2(g)
3F2(g) + 3H2O(l) → 6H+(aq) + 6F-(aq) + O3(g)
(b) Cl2 reacts with water in presence of sunlight as follow:
Question 7.29.
How can you prepare Cl2 from HCl and HCI from Cl2? Write reactions only.
Answer:
(a) Cl2 from HCl: HCl can be oxidised into Cl2 in presence of varius oxidising agents like MnO2 KMnO4 and K2Cr2O7.
MnO2 + 4HCl → MnCl2 + 2H2O
(b) HCl from Cl2: Cl2 can be reduced into HCl by the reaction with H2 in presence of diffused sun light.
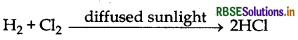
Question 7.30.
What inspired N. Bartlett for carrying out the reaction between Xe and PtF6?
Answer:
N. Bartlett observed that O2 can react with PtF6 and an ionic solid O2+ [PtF6]- is formed.
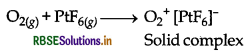
Solid complex In above reaction O2 gas is oxidised into O2+ by PtF6. He though that if the ionisation energy of Xe (1170 kJ mol) is quite close to the ionisation energy of O, molecule 1175 kJ mol-1), so Xe should react with PtF6 and also get oxidised into Xe+. This inspired him and he mixed Xe with PtF, then a rapid reaction took place. By the reaction a red solid with formula Xe + [PtF6]- was formed.
Xe + PtF6 → Xe+ [PtF6]-
It was the first compound of Xe.

Question 7.31.
What is the oxidation state of phosphorus in the following:
(i) H3PO3 (ii) PCl3 (iii) Ca3P2 (iv) Na3PO4 (v) POF3
Answer:
(i) H3PO3
3 x (+1) + x + 3 x (-2) = 0
+ 3 + x - 6 = 0
x = +3
(ii) PCl3
x + 3 x (-1) = 0
x - 3 = 0
x = + 3
(iii) Ca3P2
3 x (+2) + 2 x x = 0
+ 6 + 2x = 0
2x = -6
x = -3
(iv) Na3PO4
3 x (-1) + x + 4 x (-2) = 0
3 + x - 8 = 0
x - 5 = 0
x = +5
(v) POF3
x + (-2) + 3 x (-1) = 0
x - 5 = 0
x = +5
Question 7.32.
Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of MnO
(ii) Cl2 gas is passed into a solution of Nal in water.
Answer:
(i) Cl2 gas is produced.
[NaCl + H2SO4 → NaHSO4 + HCl] x 4
4HCI + MnO2 → MnCl2 + Cl2 + 2H2O
4NaCl + MnO2 + 4H2SO4 → MnCl2 + 4NaHSO4 + Cl2 + 2H2O
(ii) Cl2 oxidises Nal into I2.
Cl2(g) + 2NaI(aq) → 2NaCl(aq) + I2(s)
Question 7.33.
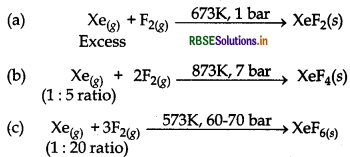
How are XeF2, XeF4 and XeF6 obtained?
Answer:
These xenon fluorides can be prepared as follow:
Question 7.34.
With what neutral molecule is ClO- isoelectronic? Is that molecule a Lewis base?
Answer:
If we replace O- (9 electron) in in ClO- with F (9 electron), the resulting neutral molecule will be CIF. Hence the isoelectronic molecule of ClO- is CIF. Since CIF can further combine with F to form CIF3 so CIF is a Lewis base.
Question 7.35.
How are XeO3 and XeOF4 prepared?
Answer:
On hydrolysis of XeF4 and XeF6, XeO3 is prepared.
6XeF4 +12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + 3H2O → XeO3 + 6HF
On partial hydrolysis of XeF6 the compound XeOF4 is prepared.
XeF6 + H2O → XeOF4 + 2HF

Question 7.36.
Arrange the following in the order of property indicated for each set:

Answer:
(i) As bond length increases, bond dissociation enthalpy decreases. Hence bond dissociation enthalpy decreases from F2 to I2. But the bond dissociation enthalpy of F2 is lower than Cl2 and Br2. It is due to the presence of lone pair of on fluorine atom which creates greater repulsion due to small size of fluorine henc the bond dissociation enthalphy of F2 decreases.
The increasing order of bond dissociation enthalpy is:
I2 < F2 < Br2 < Cl2
(ii) As the atomic size increases the bond dissociation enthalpy of H-X bond decreases and hence the acidic strength also increases. The increasing order of acidic strength is :
HF < HCl < HBr < HI
(iii) On moving down the group from N to Bi, the atomic size increases, consequently the electron density on the central atom decreases and hence the basic strength decreases. Therefore, the increasing order of basic strength is as follow:
BiH3 < SbH3 < AsH3 < PH3 < NH3
Question 7.37.
Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) CeF
Answer:
(ii) NeF2 does not exist because the ionisation enthalpy of neon (Ne) is very high so it can not oxidised into Ne2+ ion.
Question 7.38.
Give the formula and describe the structure of a noble gas species which is iso structural with:
(i) ICI4-
(ii) IBr2-
(iii) BrO3-
Answer:
(i) ICI4-: ICI4- has 36 [7 + (4 x 7) + 1] valence electrons similarly XeF4 a compound of noble gas species also has 36 valence electrons. ICI4- show sp3d2 hybridisation similarly XeF4 also show sp3d2 hybridisation. Hence XeF4 is iso-structural species with ICI4-
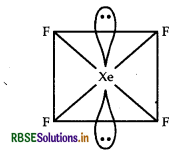
Square planar geometry sp3d2 - hybridisation of XeF4 compound.
(ii) IBr2-: IBr2- has 22[7+ (2 x 7) + 1] valence electrons. Similarly XeF2 compound of noble gas species also has 22 valence electrons. IBr2- show sp3d-hybridisation similarly XeF2 also show sp3d - hybridisation. Hence XeF2 is isostructural with IBr2- ion.
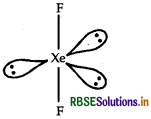
sp3d-hybridisation linear structure of XeF2 molecule.
(iii) BrO3-: BrO3- has 26 [7 + (3 x 6) + 1] valence electrons similarly XeO3 also has 26 valence electrons which is a noble gas compound. BrO3- show sp hybridisation. Similarly XeO3 also show sp3-hybridisation. Hence XeO3 is iso-structural species with BrO3- ion.
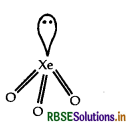
sp3 hybridisation triangular pyramidal geometry of XeO3 molecule
Question .7.39.
Why does noble gases have comparatively large atomic sizes?
Answer:
Noble gases have only Vander Waal's radii while other elements have covalent radii. As VanderWaal's radii are larger than covalent radii, hence noble gases have comparatively large atomic sizes among the period.
Question 7.40.
List the uses of neon and argon gases.
Answer:
Some important uses of neon and argon are as follow:
(a) Neon:
- Neon gas is used in voltage indicators and voltage regulators.
- The bulbs filled with neon gas are used in botanical gardens and in green houses.
- It is used in discharge tubes.
- It is used in fluorescent bulbs for advertisement display purposes.
(b) Argon:
- Argon gas is used in radio values and rectifiers.
- Argon gas is used in laboratory for handling air sensitive substances.
- It is used to provide an inert atmosphere in high temperature metallurgical processes for example arc welding of metals of alloys.
- It is used for filling electric bulbs.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम