RBSE Solutions for Class 12 Chemistry Chapter 5 Surface Chemistry
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 5 Surface Chemistry Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 5 Surface Chemistry
RBSE Class 12 Chemistry Surface Chemistry InText Questions and Answers
Question 5.1.
Why are substances like platinum and palladium often used for carrying out electrolysis of aqueous solutions?
Answer:
Substances like platinum and palladium are often used for carrying out electrolysis of aqueous solutions due to the following reasons:
- Pt and Pd have higher adsorbing power to adsorb gases like H. This H2 gas which is easily adsorbed on Pt and Pd works as catalyst in many chemical reactions.
- Pt and Pd form inert electrodes i.e., they are not attacked by the ions present in electrolyte.
Question 5.2
Why does physisorption decrease with increase of temperture?
Answer:
Physical adsorption is an exothermic process hence, on increasing temperature it decreases. Moreover, on increasing temperature kinetic energy of adsorbed molecules increases due to which weak van der Waals bonds break and hence physical adsorption decreases.
Question 5.3.
Why are powdered substances more effective adsorbents than their crystalline forms?
Answer:
In powdered form, the surface area of adsorbent increases hence, adsorption increases. The powdered charcoal, silica gel, finely divided metals are good adsorbent.

Question 5.4.
Why is it necessary to remove CO when ammonia is obtained by Haber's process?
Answer:
It is necessary to remove CO when ammonia is obtained by Haber's process because CO acts as poison for the catalyst iron as well as promoter MO.
Question 5.5.
Why is the ester hydrolysis slow in the beginning and becomes faster aftersome time?
Answer:
The reaction of ester hydrolysis is as follows:
RCOOR' + H2O = RCOOH + ROH
Ester Water Acid Alcohol
In this reaction the acid produced acts as autocatalyst. Hence, the reaction is slow in the beginning but as soon as acid is formed the reaction becomes faster.
Question 5.6.
What is the role of desorption in the process of catalysis?
Answer:
In the process of catalysis the reaction is completed on the surface of catalyst, theproduct formed during the process, is removed from the surface by the phenomenon of desorption. Due to desorption, surface of catalyst becomes free for fresh adsorption of the reactant.
Question 5.7.
What modification can you suggest in the Hardy-Schulze law?
Answer:
According to Hardy Schulze law the coagulation of sols is due to neutralization of their charges, since coagulation can also occur by mixing two oppositely charged sols so the law can be modified as. When oppositely charged sols are mixed in proper proportions to neutralise the charges of each other, coagulation of both the sols occurs.
Question 5.8.
Why is it essential to wash the precipitate with water before estimating it quantitatively?
Answer:
During filteration of precipate, some amount of electrolyte remains adsorbed on the surface of the particles of precipitate. Hence, it is essential to wash the precipitate with water to remove these adsorbed particles of electrolytes before estimating quantitatively.
RBSE Class 12 Chemistry Surface Chemistry Textbook Questions and Answers
Question 5.1.
Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
Answer:
Adsorption is a surface phenomenon in which concentration of gas or liquid increases on the surface of solid, while absorption is a bulk phenomenon in which molecule of substance is uniformly distributed at surface and in bulk of a solid. For example: Silica gel adsorbs water while anhydrous calcium chloride absorbs water.

Question 5.2.
What is the difference between physisiorption and chemisorption?
Answer:
In physical adsorption weak Vander Waal's forces are developed between adsorbate and adsorbent while in chemical adsorption strong chemical bonds are formed between adsorbate and adsorbent.
Question 5.3.
Give reason why a finely divided substance is more effective as an adsorbent?
Answer:
Finely divided substance has larger surface area and hence show larger extent of adsorption. For exampleCharcoal, silica gel, alumina and finely divided metals are good adsorbent when they are in powdered form.
Question 5.4.
What are the factors which influence the adsorption of a gas on a solid?
Answer:
The factors which influence the adsorption of a gas on a solid are as follows:
- Temperature
- Pressure
- Nature of gas
- Surface area of adsorbent
- Activity of adsorbate.
Question 5.5.
What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Answer:
The variaton of the mass of the gas adsorbed per gram of the adsorbent with pressure at constant temperature is termed as adsorption isotherm.
Freundlich adsorption isotherm: In the given curvey is the mass of adsorbed gas,'m' is the mass of adsorbent while 'p' is pressure.
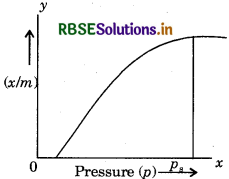
Here'p is the saturation pressure. Above this pressure extent of adsorption remains constant.
According to curve,
(i) At low pressure,
\(\frac{x}{m} \propto p^{-1}\)
\(\frac{x}{m}=k p^{-1}\)
(k - constant)
i.e., at low pressure, extent of adsorption (x/m) increases on increasing pressure.
(ii) At high pressure,
\(\begin{aligned} &\frac{x}{m} \propto p^{\circ} \\ &\frac{x}{m}=k \end{aligned}\)
i.e, at high pressure, extent of adsorption do not depend on pressure.
(iii) At intermediate pressure,
\(\frac{x}{m} \propto p^{1 / n}\)
where n > 1
Taking log on both sides.
log x/m = log k + 1/n log p
The above equation is known as Freundlich adsorption equation. It can be represented by the straight line equation
y = mx + c
where in curve, logis taken on y-axis and log pis taken on x-axis.
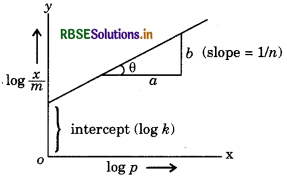
Above given curve represents the value of is in between 0 and 1. This curve also explain the Freundlich adsorption isotherm.

Question 5.6.
What do you understand by activation of adsorbent? How is it achieved?
Answer:
Activation of adsorbent means the increasing of the adsorbing power of the adsorbent. Generally, the adsorbing power can be increased by increasing surface area. Surface area of adsorbent can be increased by the following methods:
- By powdering the adsorbent.
- By making the surface of adsorbent rough
- By desorbing the adsorbed gases from the surface of adsorbent
Question 5.7.
What role does adsorption play in heterogeneous catalysis?
Answer:
In heterogeneous catalysis the reactants are gases while catlyst is solid in state. The reacting gaseous molecules get adsorbed on the surface of adsorbent by physical or chemical adsorption. Due to adsorption the concentration of reactants increases on the surface hence, rate of reaction increases. As adsorption is an exothermic process
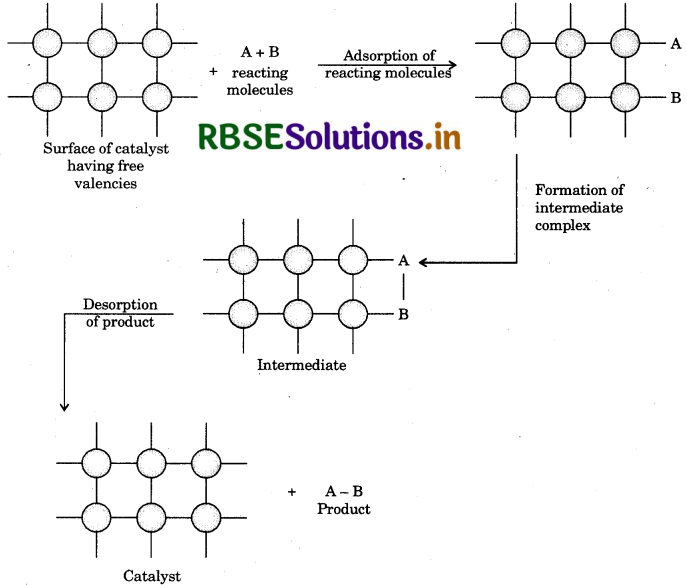
heat of adsorption is utilised in increasing the rate of reaction. The process of heterogeneous catalysis is completed on the surface of catalyst. It involves the following steps:
- Diffusion of reactants on the surface of catalyst.
- Adsorption of reacting molecule on the surface of catalyst.
- By the formation of intermediate, the chemical reaction proceeds on the surface of catalyst.
- Desorption of products from the surface of catalyst.
- After desorption the surface of catalyst is again available for the reaction.
- Diffusion of products far frmed the surface of catalyst.
In this way we can explain that there is an important role of adsorption in heterogeneous catalysis.
Question 5:8.
Why is adsorption always exothermic?
Answer:
When a gas (adsorbate) is adsorbed on the surface of a solid, its entropy decreases i.e., AS is-ve. For a spontaneous process, Gibb's Free energy must be-ve. Hence, according to gibb's Helmholtz equation,
∆G = ∆H - T∆S,
as T∆ S is-ve, therefore, ∆G can be ve only if ∆H is-ve. Hence, adsorption is always exothermic.

Question 5.9.
How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Answer:
On the basis of physical states of the dispersed phase and dispersion medium, colloidal solution can be classified as follows:
|
Dispersed phase |
Dispersion medium |
Type of colloid |
Example |
|
(1) Solid |
Solid |
Solid sol |
some coloured glasses, gems and precious stones etc. |
|
(2) Solid |
Liquid |
Sol |
Paints, cell fluids, gold sol, FeOH3 sol etc. |
|
(3) Solid |
Gas |
Aerosol |
Smoke, dust etc |
|
(4) Liquid |
Solid |
Gel |
Cheese, butter, jams and jellies etc. |
|
(5) Liquid |
Liquid |
Emulsion |
Milk, hair cream etc. |
|
(6) Liquid |
Gas |
Aerosol |
Fog, mist, cloud, insecticide, spray etc. |
|
(7) Gas |
Solid |
Solid sol |
Pumic stone, foam, rubber etc. |
|
(8) Gas |
Liquid |
Foam |
Froth, whipped cream, soap leather |
|
(9) Gas |
Gas |
No colloidal solution |
- |
Question 5.10.
Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Answer:
(i) Effect of Pressure: On increasing pressure the extent of adsorption increases. Freundlich observed that on increasing pressure the extent of adsorption increases up to particular limit. When staturation state is achieved then there is no effect on the extent of adsorption on further increasing the pressure i.e., extent of adsorption is indepedent of pressure. Freundlich gave a equation for it, which is known as Freundlich adsorption isotherm equation.
\(\log \frac{x}{m}=\log k+\frac{1}{n} \log p\)
(ii) Effect of Temperature: Adsorption is an exothermic process hence on increasing temperature
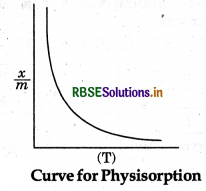
physisorption decreases because of breaking of weak Van der Waal's forces present between adsorbate and adsorbent. But it is observed that on increasing temperature the extent of adsorption for chemisorption first increases upto a particular limit then decreases, because chemisorption invovles chemical bonds between adsorbate and adsorbent.
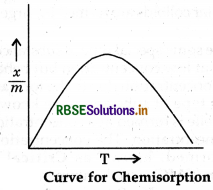
Question 5.11.
What are lyophilic and lyophobic sols? Give on example of each type. Why are hydrophobic sols easily coagulated?
Answer:
The differences between lyophilic and lyophobic sols:
|
Lyophilic Sol: |
Lyophobic Sol: |
|
(1) Particles of dispersed phase have great affinity for the dispersion medium. |
(1) Particles of dispersed phase have no affinity for dispersion medium, rather they hate despersion medium. |
|
(2) They are reversible in nature. |
(2) They are irreversible in nature. |
|
(3) They are self stabilised because of strong attractive forces operating between the dispersed phase and dispersion medium. |
(3) They are not easily stabilised as they need stabilizing agent for their preservation. |
|
(4) They can be prepared easily. |
(4) They cannot be prepared easily. |
|
(5) Example - Gum, gelatin, starch, albumin etc. |
(5) Example-Sols of gold, silver, Fe(OH), Aso, etc. |
|
(6) They are also known as intrinsic colloids. |
(6) They are also also known as extrinsic colloids. |
Stability of hydrophobic sol: The stability of hydrophobic sol is due to the presence of charge on the colloidal particles. If any how charge is removed then they will coagulate easily and will settle down. The charge can be removed by addition of suitable electrolytes.

Question 5.12.
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Answer:
The differences between multimolecular and macromolecular colloids:
|
Multimolecular Collids: |
Macromolecular Colloids: |
|
(1) They are formed by aggregation of small molecules (diameter <1 nm). |
(1) They are formed by large molecules (diameter is in between 1 nm to 1000 nm). |
|
(2) These colloids are generally lyophobic in nature. |
(2) These colloids are generally lyophitic in nature. |
|
(3) Example: Sulphur sol (ss) |
(3) Example-Starch, cellulose, Protein enzyme etc. |
Difference between associated colloids and multimolecular colloids and macromolecular colloids: Multimolecular colloids are formed by the aggregation of a large number of small molecules (e.g., Sg) whereas macromolecular colloids are formed by large sized molecules (e.g. starch). There are some special type of substances which behave as electrolyte at low concentration but behave as colloid at certain high concentration due to the formation of micelles. These special type of substances are known as associated colloids. Micelle is formed at a concentration higher than a particular concentration. The concentration, above which micelle is formed, known as Critical Micellisation Concentration (CMC), and the temperature at which CMC is calculated, known as Kraft temperature. For Example; the CMC value of soap is 10-4 to 10-3 mol L-1.
Question 5.13.
What are enzymes? Write in brief the mechanism of enzyme catalysis?
Answer:
Enzymes: Enzymes are the complex nitrogenous organic compounds which are produced in living cells of plants and animals. They are protenous molecules having high molecular weights and form colloidal solution in water. They are also called biochemical catalyst because of their importance in biochemical processes. Example: invertage, zymase, maltase, diastage, ureage etc.
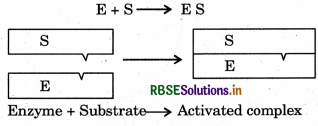
Mechanism of enzyme catalysis: There are a number of cavities present on the surface of colloidal particles of enzymes. These cavities are of characteristic shape and possess active grups such as-NH2 -COOH+ - SH,OH etc. These are active centres of enzyme catalyst. The molecules of the substrate which have complementary shape, fit into a lock. On account of the presence of active groups, an activated complex is formed which then decomposes to yield the products. The enzyme catalyse reaction is completed in the following steps.
Step 1: Binding of enzyme to substrate to form an activated complex
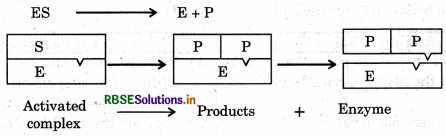
Step 2: Now activated complex is decomposed to form product.
Question 5.14.
How are colloids classified on the basis of:
(i) Physical states of components?
(ii) Nature of dispersed phase and
(iii) Interaction between dispersed phase and dispersion medium.
Answer:
(i) Physical states of components: For answer see question number 9.
(ii) Nature of dispersed phase: On the basis of dispersion medium colloidal solution are classified as follows:
|
Nature of dispersion medium |
Colloidal s.ol |
|
(1) Water |
Hydrosol or Aquasol |
|
(2) Alcohol |
Alcosol |
|
(3) Benzene |
Benzosol |
|
(4) Air |
Aerosol |
(iii) Classification on the basis of interaction between dispersed phase and dispersion medium:On the basis of it colloidal solution is classified into two groups.
- Lyophilic sol: The colloidal solution in which particles of dispersed phase have great affinity for the dispersion medium, is known as Lyophilic sol.
- Lyophobic Sol: The colloidal solution in which particles of dispersed phase no affinity for the dispersion medium, is known as Lyophobic sol.
[NOTE] If dispersion medium is liquid then they are called as hydrophilic and hydrophobic sols.
Question 5.15.
Explain what is observed when:
1. When a beam of light is based through a colloidal solution.
2. An electrolyte, NaCl is added to hydrated ferric oxide sol.
3. Electric current is passed throguh a colloidal sol.
Answer:
- When a beam of light is passed through a colloidal solution the scattering of light by colloidal particles takes place and the path of light becomes visible (Tyndall effect).
- When an electrolyte, NaCl is added to hydrated ferric oxide sol the positively charged colloidal particles of Fe(OH)3 get coagulated by the oppositely chargd Cl- ions provided by NaCl, (coagulation).
- On passing direct current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated (electrophoresis then coagulation)

Question 5.16.
What are emulsions? What are their different types ? Give example of each type.
Answer:
Emulsion: The colloidal system in which both the dispersed phase and the dispersion medium are liquid is known as a emulsion.
Emulsion are of two types:
- Oil in water type (O/W)
- Water in oil type (W/O).
1. Oil in water type emulsion: In this type of emulsion, water is dispersion medium while droplets of oil are dispersed phase. Example: Milk, vanishing cream etc.
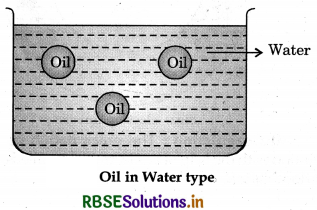
2. Water in oil type emulsion: In this type of emulsion oil works as dispersion medium while water as dispersed phase. For example: butter, cream etc.
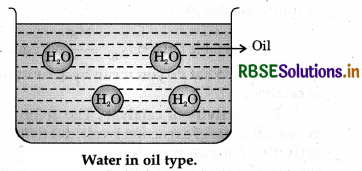
Question 5.17.
What is demulsification? Name two demulsifiers.
Answer:
The process of separation of dispersed phase from dispersion medium of an emulsion is called demulsification. i.e., separation of constituent liquids of an emulsion.
Demulsificaiton can be done by:
- Centrifuging
- Boiling
Question 5.18.
Action of soap is due to emulsification and micelle formation. Comment
Answer:
Soap is an emulsifier. It has two parts i.e., hydrophilic and hydrophobic. If a drop of oil is surrounded by soap solution then alkyl part of a soap remains in the oil and COO-Na part remains in water. Thus, soap molecules are concentrated over the surface of the drop of oil. As a result interfacial tension between oil and H2O decreases and hence they are intermixed into each other to form emulsion. In this way micelles are formed.
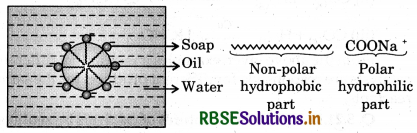
Question 5.19.
Give four examples of heterogeneous catalysis.
Answer:
Four examples of heterogeneous catalysis are:
(i) Haber process: In this process finely divided iron behave as catalyst which is in solid state. In Haber process, H and N are reactants while NH, is product. These all are in gaseous state.
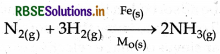
(ii) Ostwald's process: In this porcess platinum gauze behave as catalyst which is in solid state. In this process NH3 is oxidised into NO (nitric oxide) which is a gas.
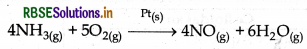
(iii) Oxidation of sulphur dioxide: In this process solid Pt catalyst is used and SO2 gas is oxidised into so, gas.
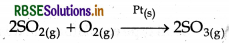
(iv) Hydrogenation of vegetable Oil: Here finely divided Nicatalyst is used for the hydrogenation of vegetable oil into vegetable ghee.
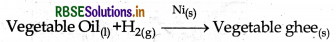

Question 5.20.
What do you mean by activity and selectivity of catalysts?
Answer:
Activity of catalyst: The ability of a catalyst to increase the rate of a reaction is called activity of catalyst. The activity of a catalyst depends upon the strength of chemisorption to a large extent. The reactant must adsorb resonably strongly for the catalyst to be active but must not adsorb so strongly that they become immobilise.
For example: The reaction between H2 and O2 is not possible at room temperature but in presence of Pt catalyst the reaction occurs with explosion.
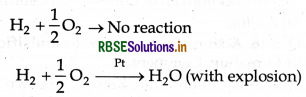
(i) Selectivity of catalyst: This is capability of catalysts to catalyse a specific reaction.
For example:
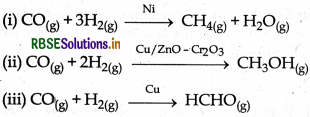
From the above given examples it is clear that different catalysts are responsible for different products for the same reactants.
Question 5.21.
Describe some features of catalysis by zeolites.
Answer:
Features of catalysis by zeolites:
- Zeolites are hydrated aluminosilicates having three dimensional network structure.
- The structure of zeolites is honey comb like i.e., they have pores. The size of their pore is in between 260 pm to 740pm
- Zeolite works as catalyst only for those molecules which can be adsorbed in these pores and catalysed whose size is small enough to entry these pores. Hence, zeolite acts as molecular sieves or shape selective catalysts.
For example: It is used in chemical industry for cracking and isomerisation of hydrocarbon. ZSM-5 is an important catalyst for petroleum industry.
It converts alcohols into petrol by first dehydrating them to form a mixture of hydrocarbons.
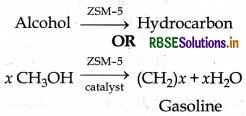
Question 5.22.
What is shape selective catalysis?
Answer:
Shape selective catalysis : Catalytic process which depends on the pore size of catalyst and the molecular size of reactants and products, is known as shape selective catalysis. The most important example of shape selective catalyst is zeolite. Zeolite has three dimensional network of Al-O-Si Linkage and it has honeycomb like structure. It has pores whose size is in between 260 pm to 740 pm Zeolite works as catalyst only for those reactants and products whose molecular size is lower than their pore size, because, if the molecular size of reactants and products are too large then they will not fit in their pores and the reaction will not be proceed. On the other hand, if the reactant molecules are too small, they would just slip through the pores in the catalyst without any interaction.
Example: ZSM-5

Question 5.23.
Explain the following terms:
(i) Electrophoresis,
(ii) Coagulation,
(iii) Dialysis,
(iv) Tyndall effect.
Answer:
(i) Electrophoresis: The migration of colloidal particles under the influence of electric field towards an oppositely charged electrode is known as electrophoresis. When the sol particles are negatively charged, the migration takes place towards anode and the phenomenon is called anaphoresis. In cataphoresis, the sol particle moves towards cathode in an electric field.
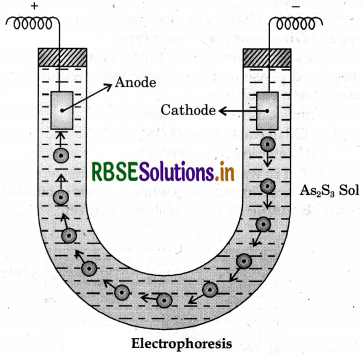
(ii) Coagulation: The precipitation of a colloidal solution with the help of an electrolyte is called coagulation or flocculation The reason for coagulation is that the oppositely charged ion of the electrolyte added, neutralizes the charge on the sol particles. The repulsive forces among the sol particles vanish, the aggregation of the particles takes place which causes the dispersed phase to settle.
Coagulation can be done by the following method:
(a) By electrophoresis,
(b) By mixing two oppositely charged sol,
(c) By boiling,
(d) By the addition of electrolyte.
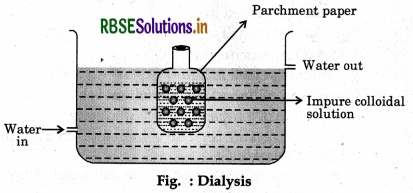
(iii) Dialysis: The process of separation of crystalloids from colloids by diffusion through parchment membrane is called dialysis. The process is very slow hence, electrodes are used for the separation of ions. It is konwn as electrodialysis.
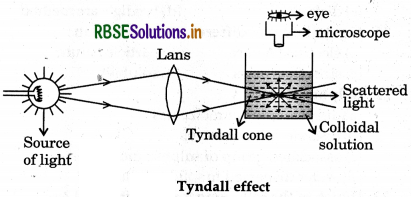
(iv) Tyndall effect: When a beam of light is passed through a colloidal solution, the path of the beam is illuminated and become visible as a blue bright streak (cone) when seen in a direction perpendicualr to the incedent beam. This phenomenon is called as Tyndall effect. The blue streak (cone) is known as Tyndall cone. This phenomenon is due to scattering of light by the sol particles.
Question 5.24.
Give four uses of emulsions. Ans. Four uses of emulsions are as follows:
(1) Milk is an emulsion of fat in water.
(2) Concentration of sulphide ores by froth floatation process is done by emulsificaiton.
(3) Various beauty products like cream, shampoo, vanishing cream,dye etc. and various drugs and syrups are good examples of emulsion.
(4) Cleansing action of soap and detergents is due to the formation of an emulsion between gas dirt and soap solution.
Question 5.25.
What are micelles? Give an example of a micelles system.
Answer:
Micelles: Micelles are the cluster or aggregated particles formed by association of colloids in solution. Example: micelles are soaps and detergents.
A concentrated solution of soap in water is a micellers system. Such substances are also called associated colloids. At low concentration micellers system (or associated colloid) behave as a true solution and dissociates into ion but at high concentration it behave as colloids. The concentration below which associated colloid behave as electrolyte i.e., form true solution and dissociates into ion while above which it behave as colloid is known as Critical Micellisation Concentration (CMC) and temperature is known as Kraft temperature.
Question 5.26.
Explain the terms with suitable examples:
(1) Alcosol,
(2) Aerosol and
(3) Hydrosol.
Answer:
(1) Alcosol: It is a colloidsol solution having alcohol as the dispersion mediume.g., collodion (a colloidal sol of cellulose nitrate in ethyl alcohol).
(2) Aerosol: It is a colloidal dispersion of a liquid in a gas.e.g., fog.
(3) Hydrosol: It is a colloidal sol of a solid in water as the dispersion mediume.g., starch sol or gold sol.

Question 5.27.
Comment on the statement that "Colloid is not a substance but state of a substance."
Answer:
Colloid is not a substance but state of a substance. This statement is true, because the same substance in one solvent may exist as a crystalloid while in the other as colloid. For example, sodium chloride (NaCl) in water behave as crystalloid while in benzene, it behaves as colloid. It happens due to the size of the particle. If the size of the particle lies in between 1 nm to 1000 nm it behave as colloid and if the particle size lie below 1 nm it behave as crystalloid.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम