RBSE Solutions for Class 12 Chemistry Chapter 3 Electrochemistry
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 3 Electrochemistry Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 3 Electrochemistry
RBSE Class 12 Chemistry Electrochemistry InText Questions and Answers
Question 3.1.
How would you determine the standard electrode potential of the system Mg2+Mg?
Answer:
To determine the standard electrode potential of Mg/Mg2+ electrode, a magnesium electrode is placed in 1.0 M Mg SO4 solution and is combined with S.H.E. as shown in Fig.
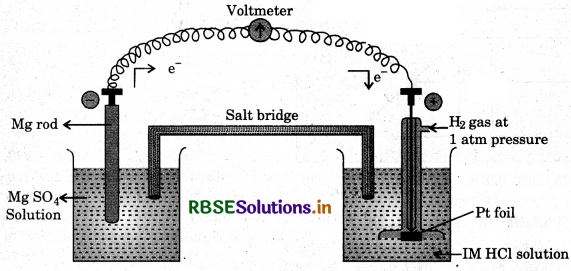
The E.M.F. of the cell is measured with the help of voltameter. In this case, the reading by the voltameter is 2.37 volt and the direction of current is from SHE to Mg / Mg2+ electrode. Thus, in this cell, Mg /Mg2+ electrode acts as anode and SHE acts as cathode.
The cell may be represented as:
Mg | Mg2+ (1M) | | H+ (1M)/H2 (1 atm), Pt
E°cell = E°H+/1/2H2 - E°Mg2+/Mg
= 0.0 - E°Mg2+|Mg
Thus,
E°Mg2+|Mg = -E°Cell
In this way, we can determine the electrode potential of Mg2+/Mg electrode.
Question 3.2.
Can you store copper sulphate solution in a zinc pot?
Answer:
E°zn2+| zn = -0.76 V
E°cu2+| CU = 0.34 V
This indicates that zinc is stronger reducing agent than copper. It displaces the copper from CuSO4 solution as
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Hence, we cannot store copper sulphate solution in a zinc pot.

Question 3.3.
Consult the table of standard electrode potentials and suggest three substances which can oxidise ferrous ions under suitable conditions.
Answer:
The oxidation of Fe2+ ions takes place as follows:
Fe2+ → Fe3+ + E°red = 0.77V
Only those substances can oxidise Fe2+ ions which have their reduction potential values higher than that of Fe2+ ions. For example: acidified KMnO4, acidified Cr2O72- , acidified H2O2.
Question 3.4.
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer:
pH = 10
PH = -log[H+]
[H+] = 10-PH
[H+] = 10-10 mol L-1
For hydrogen electrode
H+ + e- → 1/2 H2
Thus,
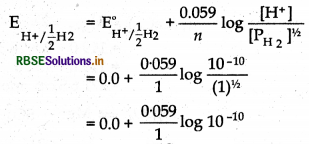
= 0.059 (-10 log 10)
= 0.059 x -10
= - 0.59 V
E°H+/1/2H2 = - 0.59 V
Question 3.5.
Calculate EMF of the cell in which the following reaction takes place:
Ni(s) + 2Ag+ (0.002M) → Ni2+ (0.160M) + 2Ag(s)
Given that E°ce11 = 1.05 V
Answer:
Nemst equation for the above cell,
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{\mathrm{o}}+\frac{0.059}{n} \log \frac{[\mathrm{Ni}]\left[\mathrm{Ag}^{+}\right]^2}{\left[\mathrm{Ni} i^{2+}\right][\mathrm{Ag}]^2}\)
Since [Ni] = [Ag] = 1
Thus

= 1.05 - 0.14
= 0.19 V
E°ce11 = 0.19 v
Question 3.6.
The cell in which the following reaction occurs:
2Fe3+(aq) + 2I-(aq) → 2Fe2+(aq) + I2(s)
has E°cell = 0.236V at 298 K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Answer:
2Fe3+ + 2e- → 2Fe2+
2I- → I2 + 2e-
Thus, for the given cell reaction n = 2
∆G° = -nFE°cell
= -2 x 96500 x 0.236J
= -45.55 kJ/mol
∆G° = -2.303 RT log Kc
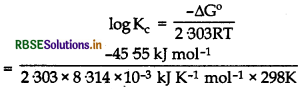
logKc = 7.983
Kc = Antilog (7.983)
= 9.616 x 107

Question 3.7.
Why does the conductivity of a solution decrea¬ses with dilution ?
Answer:
Conductivity of the solutionis the conductance of ions present per unit volume of electrolytic solution. On dilution, number of ions per unit volume decreases, hence, conductivity also decreases.
Question 3.8.
Suggest a way to determine value of water.
Answer:
Water is a weak electrolyte for water
can be obtained from the knowledge of molar conductance at infinite dilution of sodium hydroxide, hydrochloric acid and sodium chloride. (All are strong electrolyte)
\(\Lambda_{m\left(\mathrm{H}_2 \mathrm{O}\right)}^0=\Lambda_{m(\mathrm{NaOH})}^0+\Lambda_{m(\mathrm{HCl})}^0-\Lambda_{m(\mathrm{NaCl})}^0\)
Question 3.9.
The molar conductance of 0.05 mol L-1 of methanoic acid is 46.15 cm2 mol-1. Calculate its degree of dissociation and dissociation constant Given λ°(H+) = 349.6
S cm2 mol-1 and λ°(Hcoo-) = 54.6 S cm2 mol-1
Answer:
\(\Lambda_m^o(\mathrm{HCOOH})=\lambda_{\left(\mathrm{H}^{+}\right)}^o+\lambda_{\left(\mathrm{HCOO}^{-}\right)}^{\circ}\)
= 349.6 + 54.6
= 404.2 SCm2 mol-1
Given λm(HCOOH) = 461. S cm2 mol-1

= 0.114
\(\mathrm{K}=\frac{\mathrm{C} \alpha^2}{1-\alpha}=\frac{0.025 \times(0.114)^2}{1-0.114}\)
= 3.67 x 10-4
value of dissociation constant is 3.67 x 10-4
Question 3.10.
If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons flow through the wire?
Answer:
Q = It
I = 0.5 A
t = 2 x 60 x 60s
Q = 0.5 x 2 x 60 x 60 = 3600 C
96500 C is equivalent to flow of → 1 mol electrons
3600 C is equivalent to flow of → \(\frac{3600}{96500}\) mol electrons
= \(\frac{3600 \times 6.023 \times 10^{23}}{96500}\)
= 2.246 x 1022 electrons
Thus, 2.246 x 1022 electrons flow through the wire.
Question 3.11.
Suggest a list of metals which can be extracted electrolytically.
Answer:
Na, Ca, Mg and Al.
Question 3.12.
Consider the reaction:
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
What is the quantity of electricity in coulombs needed to reduce 1 mole of Cr2O72- ions?
Answer:
I mol of Cr2O72- need 6 mol of electrons for reduction to Cr3+
Thus, quantity of electricity = 6F
= 6 x 96500C = 579000C.

Question 3.13.
Write the chemistry of recharging the lead storage battery highlighting all the material that are involved during rechargin
Answer:
During charging. electrical energy is supplied to cell using an external source of electrical energy. It means cell behaves like an electrolytic cell. Reactions occuring
during the charging is opposite to the reaction occuring during the discharging of cell. Following reactions take place during charging:
PbSO4(5) + 2e- → Pb(s) + SO2-4(aq)
PbSO4(s) + 2H2O → PbO2(s) + SO2-4(aq) + 4H+(aq) + 2e-
2PbSO4(s) + 2H2O → Pb(s) + PbO2(s) + 4H+ + 2SO2-4(aq)
Question 3.14.
Suggest two materials other than hydrogen that can be used as fuels in the fuel cells.
Answer:
- Methanol (CH3OH)
- Methane (CH4)
Question 3.15.
Explain how rusting of iron can be envisaged as setting up of an electrochemical cell.
Answer:
Rusting of iron is an electrochemical process since it considers the formation of small electrochemical cell on the surface of iron in which pure iron acts as anode, impure iron acts as cathode and water drops containing dissolved CO2 and O2 acts as electrolyte. Reaction at anode: Iron is oxidised and Fe2+, ions go into the solution.
Fe → Fe2+ + 2e (oxidation)
Reaction at cathode: The electron released at anode are accepted by W ions which are formed by the ionisation of H2O and H2CO3 (formed by the reaction between CO2 and H2O)
\(\mathrm{H}_2 \mathrm{CO}_3 \rightleftharpoons 2 \mathrm{H}^{+}+\mathrm{CO}_3^{2-}\)
\(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
These hydrogen atoms reduce dissolved oxygen in water,
2H + 1/2 O2 → H2O
The over reaction at cathode can be written as
2H+ + 1/2O2 + 2e- → H2O
Thus, the overall reaction occuring in electrochemical cell is:
Fe(s) + 1/2O2 + 2H+ → Fe2+ + H2O
These Fe2+ ions are oxidesd by atom pherse or diisolved oxygen and form ferric oxide on the surface of iron.
4Fe2+ + O2 + 4H2O → 2Fe2O3 + 8H+
Fe2O3 + xH2O → Fe2O3. xH2O Rust
RBSE Class 12 Chemistry Electrochemistry Textbook Questions and Answers
Question 3.1.
Arrange the following metals in the order in which they displace each other from the solution of their salt. Al, Cu, Fe, Mg and Zn.
Answer:
Mg, Al, Zn, Fe, Cu
Question 3.2.
Given the standard electrode potentials
K+/K = 2.93 V,
Ag+/Ag = 0.80V
Hg22+/Hg = 0.79V,
Mg2+/Mg = -2.37V
Cr2+/Cr = -0.74V
Arrange these metals in increasing order of their reducing power
Answer:
Higher the value of oxidation potential, greater is the tendency of element to get oxidised and hence, greater is the reducing power. Thus, increasing order of reducing
power of metals is as follows:
Ag < Hg < Cr < Mg < K

Question 3.3.
Depict the galvanic cell in which the reaction
Zn(S) + 2Ag) → Zn2+(aq) + 2Ag(s)
takes place. Further show:
(j) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Answer:
(i) The electrode at which oxidation occurs i.e. anode is negatively charged. Hence, Zn electrode is negatively charged.
(ii) Electrons are the carrier of the current in the cell,
(iii) Reaction taking place at the electrodes are as
follows:
At anode Zn(s) → Zn2+(aq) + 2e-
At cathode Ag+(aq) + e- → Ag(s)
The cell can be represented by the following diagram.
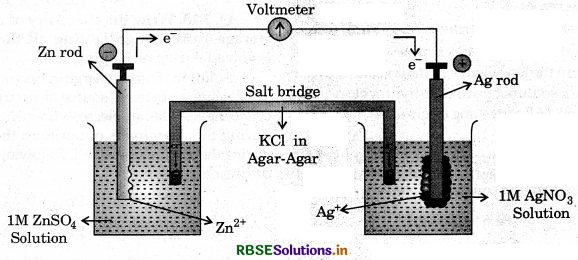
Question 3.4.
Calculate the standard cell potentials of galvanic cells In which the following reactions take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd(s)
Given that E°Cr3+/Cr = - 0.74V
E°Cd2+/Cd = -0.40 V
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Given E°Ag+/Ag = 0.80V, E°Fe3+/Fe2+ = -0.77 V
Also calculate ∆rG° and equilibrium constants of the reactions.
Answer:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd(s)
E°Cell = E°(Cathode) - E°(Anode)
= E°(Cd2+/Cd) - E°(Cr3+/Cr)
= - 0.40V - (- 0.74V)
= - 0.40V + 0.74V
= + 0.34V
∆G° = -nFE°Cell
= -6 x 96500 x 0.34
= - 196860 J mol-1
= - 196.860 kJ mol-1
∆G° = - 2.303 RT log Kc
-196.860 kJ = -2.303 x 8.314 x 298 x log Kc
or 196860J = 2.303 x 8.314 x 298 x 1ogKc
\(\frac{196860 \mathrm{~J}}{2.303 \times 8.314 \times 298}\) = logKc
logKc = 34.5014
Kc = Antilog 34.5014
= 3.173 x 1034
Gibbs energy of the cell (∆G°) = -19686 kJ/mol
Equilibrium cortant of the cell (Kc) = 3.173 x 1034
(ii) Fe2+(aq) + Ag+(aq) →
E°Cell = E°(cathode) - E°(Anode)
= 0.80V - 0.77V
= + 0.03 V
∆G° = -nFE°Cell
= -1 x 96500 x 0.03
= -2.895 kJ/mol
∆G° = -2.303RT logKc
-2895 = - 2.303 x 8.314 x 298 x log Kc
\(\frac{2895}{2.303 \times 8.314 \times 298}\) = log Kc
log Kc = 0.5074
Kc = Antilog 0.5074
Kc = 3.22
Gibbs free energy of the cell
= - 2.895kJ/mol
Equilibrium constant of the cell
= 3.22
Question 3.5.
Write the Nemst equation and the e.m.f. of the following cells at 298 K:
(i) Mg(s) | Mg2+ (0.001 M) || Cu2+ (0.0001M) | Cu(S)
(ii) Fe(s) | Fe2+(0.001M) || H+(1M) | H2(g) (lbar)/Pt(s)
(iii) Sn(s) | Sn2+(o.o50M) || H+(0.020M) | H2(g)(1bar) | Pt(s)
(iv) Pt(s) | Br2(I) | Br- (0.10M) || H+ (0.030M) | H2(g) (1bar) | Pt(s)
Answer:
(i) Mg(s) | Mg2+ (0.001 M) || Cu2+ (0.0001M) | Cu(S)
EMg2+/Mg = -2.37V, E(Cu2+/Cu) = + 0.34V
Cell reaction for the above cell is
Mg + Cu2+ → Mg2+ + Cu
Nemst equation is
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{\circ}+\frac{0 \cdot 059}{n} \log \frac{[\mathrm{Mg}]\left[\mathrm{Cu}^{2+}\right]}{\left[\mathrm{Mg}^{2+}\right][\mathrm{Cu}]}\)
Since [Mg] = [Cu] = 1 then
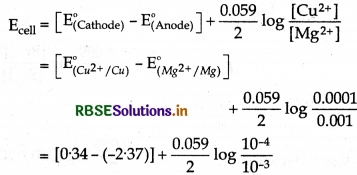
= 2.71 - 0.0295
= 2.6805
= 2.68 v
(ii) Fe(s) | Fe2+(0.001M) || H+(1M) | H2(g) (lbar)/Pt(s)
Cell reaction for the above cell is
Fe + 2H+ → Fe2+ + H2 (n = 2)
E(Fe2+/Fe) = - 0.44 V,
E°H+/1/2H2 = 0.0V
Nemst equation is
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{\circ}+\frac{0.059}{n} \log \frac{[\mathrm{Fe}]\left[\mathrm{H}^{+}\right]^2}{\left[\mathrm{Fe}^{2+}\right]\left[\mathrm{P}_{\mathrm{H}_2}\right]}\)
Here [Fe] = (pH2) = 1

= 0.44 + 0.0887
= 0.528V
(iii) Sn(s) | Sn2+(0.050M) || H+(0.020M) | H2(g)(1bar) | Pt(s)
Cell reaction for the above cell is
Sn + 2H+ → Sn2+ + H2, E(Sn2+/Sn) = 0.14v
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^o+\frac{0.059}{n} \log \frac{[\mathrm{Sn}]\left[\mathrm{H}^{+}\right]^2}{\left[\mathrm{Sn}^{2+}\right]\left(\mathrm{P}_{\mathrm{H}_2}\right)}\)
Since [Sn] = 1, (PH2) = 1
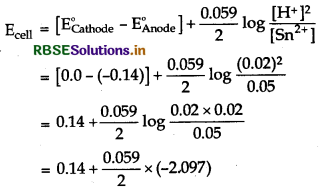
= 0.14 - 0.062 = 0.078 V
(iv) Pt(s) | Br2(I) | Br- (0.10M) || H+ (0.030M) | H2(g) (1bar) | Pt(s)
Cell reaction for the above cell is
2Br- + 2H+ → Br2 + H2 (n = 2)
E°H+/1/2H2 = 0.0v,
E°(1/2Br2/Br-) = +1.08 v
\(\mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^{\circ}+\frac{0.059}{n} \log \frac{\left[\mathrm{Br}^{-}\right]\left[\mathrm{H}^{+}\right]^2}{\left[\mathrm{Br}_2\right]\left[\mathrm{P}_{\mathrm{H}_2}\right]}\)
Here, [Br2] = 1,
[PH2] = 1
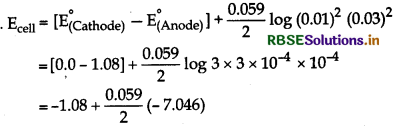
= -1.08V - 0.2078
= 1.2878 V
= 1.288 V
Question 3.6.
In the button cell widely used in watches and other devices, the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(I) → Zn2+(aq) + 2Ag(s) + OH-(aq)
Determine E° and ∆rG° for the reaction
Given Zn → Zn2+ + 2e-
E° = 0.76 V
Ag2O + H2O + 2e- → 2Ag + 2OH-, E° = 0.344 v
Answer:
Here Zn is oxidised hence, it acts as anode and AgO is reduced i.e., Ag+ ions are reduced into Ag. There¬fore, it acts as cathode.
E°(Zn2+/Zn) = -0.76 V' E°(Ag+/Ag) = 0.80 V
E°cell = [E°(Cathode) - E°(Anode)]
= E°(Ag+/Ag) - E°(Zn2+/Zn)
= 0.80 - (-0.76)
= 0.80 + 0.76
= 1.56 V
∆G° = -nFE°Cell
= - 2 x 96500 x 1.56
= 301080 J/mol
= -3.01 x 105 J/mol
= - 3.01 x 102 kJ/mol
∆G° = - 3.01 x 102 KJ/mol
Question 3.7.
Define conductivity and molar conductivity for solution of an electrolyte. Discuss their variaton with concentration. Discuss their variation with concentration.
Answer:
Let us explain some basic terms before we study the conductance of electricity through electrolytic solutions:
(1) Specific conductance or conductivity: The reciprocal of resistivity of a conductõr is called conductivity.
It is represented by the symbol ic(Greek : kappa).
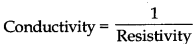
K = 1/p
or
K = 1/R x (l/A)
or
K = G x l/A
Its units are \(\Omega\)-1 cm-1 or Sm-1
if = 1 cm, A =1cm2 then K
Thus, conductivity is the conductance of solution of an electrolyte placed between two electrodes of 1cm2 area of cross-section separated by one cm i.e., conductance of one centimeter cube of the solution of an electrolyte is called conductivity.
(6) Molar conductivity: Molar conductivity is defined as conductance of the solution containing one mole of the electrolyte placed between the two electrodes lying 1 cm apart and having area of cross-section large enough to accommodate whole of the solution. It is represented by
Molar conductivity \(\left(\Lambda_m\right)\) = \(\frac{\kappa \times 1000}{C}\)
where, k is specific conductance or conductivity, C is concentration of solution in mol L-1 which is also called molar concentration and is denoted by M.
\(\Lambda_m=\frac{\kappa \times 1000}{\mathrm{M}}\)
Units of molar conductivity are ohm-1 cm2 mol-1 or SCm2 mol-1
Debye Huckel and Onsagar gave the following equation to show the variation of molar conductivity with concentration:
\(\lambda_m^c=\lambda_m^{\infty}-\mathrm{A} \sqrt{\mathrm{C}}\)
Here, \(\lambda_m^c\) = molar conductivity at concentration C
\(\lambda_m^{\infty}\) = molar conductivity at infinite dilution
C = molar concentration of solution
A = Constant which depends upon nature of solvent and temperature.
The value of constant A foragivenlvent and temperature depends upon the type of electrolyte i.e., charges on the cation and anion produced on the dissociation of electrolyte. Thus, KCl is konwn as 1-1 electrolyte, CaCl2 as 2-1 electrolyte, Na2SO4 as 1-2 electrolyte. The variation of molar conductivity with concentration can also be shown by plotting a graph between \(\Lambda_m\) and \(\sqrt{C}\) A graph between \(\Lambda_m\) and \(\sqrt{C}\) for KCI (a strong electrolyte) is shown in Fig. 3.4 in molar conductivity with dilution. When the graph is extrapolated to zero concentration, the intercept gives the molar conductivity atinfinite dilution \(\Lambda_m^{\circ}\)
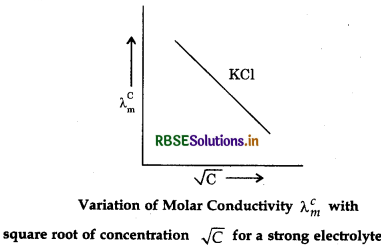
Question 3.8.
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm-1. Calculate its molar conductivity.
Answer:
Molarity (Q) = 0.02 M
Conductivity (K) = 0.0248 S cm-1
Molar Condcutivity imm
= 124 S cm2 mol-1
Molar conductivity of solution = 124 S cm2 mol-1

Question 3.9.
The resistance of a conductivity cell contain-ing 0.001 M KCl solution at 298 K is 1500 ft. What is the cell constant if conductivity of 0.001 M KCl solution at 296 K is 0.146 x 10-3 S cm-1 ?
Answer:
Molarity (M)= 0.001 M
Resistance (R) = 1500 Ω
Conductivity ( K ) = 0.146 x 10-3 S cm-1
Cell constant (G) = ?
Conductivity (K)= 1/R x G*
Cell Constant (G*) = K x R
= 0.146 x 10-3 x 1500
= 0.219 cm-1
Question 3.10.
The conductivity of NaCl at 298 K has been determined at different concentrations and the results are given below Calculate ∧m for all concentrations and draw a plot between ∧m and C1/2. Find the value of ∧m from the graph.
Answer:
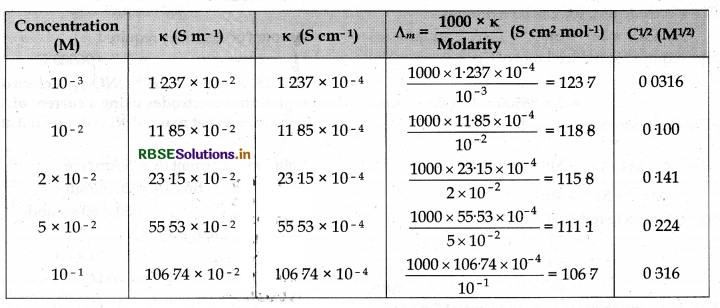
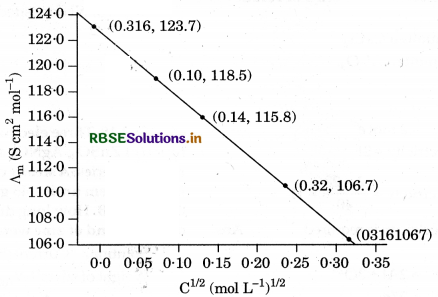
∧°m = intercept on ∧m axis = 124.0 S cm2 mol-1
Question 3.11.
Conductivity of 0.00241 M acetic acid is 7.896 x 10-5 S cm-1. Calculate its molar conductivity. If ∧m for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant ?
Answer:
Concentration (C) = 0.00241 M

Conductivity ( K ) = 32.76
Molar Conductivity (∧m) = ?
Molar conductivity at infinite dilution (∧m) = 390.5 S Cm2 mol-1
= 0.084
\(\begin{aligned} \alpha &=\frac{\Lambda_m^c}{\Lambda_m^{\circ}} \\ &=\frac{32.76}{390.5} \end{aligned}\)
= 1.86 x 10-5
Dissociation constant = 1.86 x 10-5
Question 3.12.
How much charge is required for the following reduction of:
(i) 1 mol of Al3+ to Al
(ii) 1 mol of Cu2+ to Cu
(iii) 1 mol of MnO-4 to Mn2+
Answer:
(i) The electrode reaction can be written as
Al3+ + 3e- → Al
Charge required to reduce 1 mol of Al3+
= 3F
= 3 x 96500 C
= 289500 C
(ii) The electrode reaction can be written as
Cu2+ + 2e- → Cu
Charge required to reduce 1 mol of Cu2+
= 2F
= 2 x 96500
= 193000
(iii) The electrode reaction can be written as
MnO-4 + 8H+ + 5e- → Mn2+ + 4H2O
Mn7+ + 5e- → Mn2+
Charge required to reduce 1 mol of Mn2+
= 5F
= 5 x 96500 C
= 482500 C

Question 3.13.
How much electricity in terms of Faraday is required to produce:
(i) 20.0 g of Ca from molten CaCl2
(ii) 40.0 g of Al from molten Al2O3.
Answer:
(i) In CaCl2
Ca2+ + 2e- → Ca
40 g of Ca require electricity = 2F
20 g of Ca require electricity = \(\frac{2 \times 20}{40}\) = IF
i.e., one Faraday of electricity is required.
(ii) In Al2O3
Al2O3 + 6e- → 2Al + 3O2-
3 mol 27g electrons
2 x 27 g Al require electricity = 6F
40 g Al require electricity = \(\frac{6 \times 40}{54}\)F
i.e., 4.44 Faraday of electricity is required.
Question 3.14.
How much electricity is required in Coulomb for the oxidation of:
(i) 1 mol of H2O to O2?
(ii) 1 mol of FeO to Fe2O3?
Answer:
(i) The electrode reaction can be written as
H2O → 2H+ + 1/2 O2 + 2e-
i.e, Amount of electricity required
= 2F
= 2 x 96500C
= 193000 C
(ii) The electrode reaction can be written as
2FeO + 1/2 O2 → Fe2O3
Fe2+ → Fe3+ + 1e-
Amount of electricity required
= 1F = 96500 C
Question 3.15.
A solution of Ni(NO3)2 is electrolysed be¬tween platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Answer:
Current (i) = 5 Ampere
time (t) = 20 minutes
= 20 x 60 seconds
Charge (Q) = If
= 5 x 20 x 60
= 6000 C
Mass of Ni deposited = 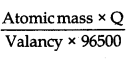
= \(\frac{58.7 \times 6000}{2 \times 96500}\)
= 1.825 g
Question 3.16.
Three electrolytic cells A, B, C containing so-lution of ZnSO4, AgNO3 and CuSO4 respectively are con-nected in series. A steady current of 1.5 ampere was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and of zinc were deposited ?
Answer:
Current (I) = 1.5 Ampere
Weight of silver (WAg) = 1.45 g
Ag+ + e- → Ag
108 g of Ag is deposited by = IF
= 96500 C electricity
1.45 g of Ag is deposited by
= \(\frac{96500 \times 1.45}{108}\)
Q = i x t
\(t=\frac{\mathrm{Q}}{\mathrm{I}}=\frac{1295.6}{1.5}\)
= 14 min 24 s
Cu2+ + 2e- → Cu 63.5 g
2 x 96500 C electricity deposit
= 63.5 g of Cu
1295.6 C electricity deposit
= \(\frac{63.5 \times 1295.6}{2 \times 96500}\)
= 0.426 g of Cu
Similarly,
Zn2+ + 2e- → Zn
2 x 96500 C electricity deposit = 65.4g of Zn
1295.6 C electricity deposit
\(=\frac{65.4 \times 1295.6}{2 \times 96500}\)
= 0.44g of Zn

Question 3.17.
Using the given standard electrode potential, predict if the reaction between the following is feasible:
(i) Fe3+(aq) and I-(aq)
(ii) Ag+(aq) and Cu (s)
(iii) Fe3+(aq) and Br-(aq)
(iv) Ag(s) and Fe3+(aq)
(v) Br2(aq) and Fe2+(aq)
Given standard electrode potentials:
\(\begin{aligned} &\mathrm{E}_{1 / 2 \mathrm{I}_2, \mathrm{I}^{-}}^{\Theta}=0.541 \mathrm{~V} \\ &\mathrm{E}_{\mathrm{Cu}^{2+}, \mathrm{Cu}}^{\Theta}=+0.34 \mathrm{~V} \end{aligned}\)
\(\begin{aligned} \mathrm{E}_{1 / 2 \mathrm{Br}_2, \mathrm{Br}^{-}}^{\Theta} &=+1.09 \mathrm{~V} \\ \mathrm{E}_{\mathrm{Ag}^{+}, \mathrm{Ag}}^{\Theta} &=0.80 \mathrm{~V} \end{aligned}\)
\(\mathrm{E}_{\mathrm{Fe}^{3+}, \mathrm{Fe}^{2+}}^{\Theta}=+0.77 \mathrm{~V}\)
Answer:
A reaction is feasible if EMF of the cell reaction is positive
(i) Reaction taking place between Fe3+(aq) and I-(aq) is
Fe3+(aq) + I-(aq) → Fe2+ + 1/2I2
Fe3+ is reduced and I" is oxidised
E°cell = E°(Cathode) - E°(Anode)
= E°(Fe3+/Fe2+) - E°(l/2I2/I-)
= 0.770 - 0.541
= 0.229 V
(Reaction is feasible)
(ii) 2Ag+(aq) + Cu → 2Ag(s) + Cu2+(aq)
Here, Ag+ is reduced and Cu is oxidised
E°cell = E°(Cathode) - E°(Anode)
E°cell = E°(Ag+/Ag) - E°(Cu2+/Cu)
= 0.80 - 0.34
= 0.46
(Reaction is feasible)
(iii) Fe3+(aq) + Br-(aq) → Fe2+(aq) + 1/2Br2
Here, Fe3+ is reduced and Br- is oxidised
E°cell = E°(Cathode) - E°(Anode)
= E°(Fe3+/Fe2+) - E°(l/2Br2/Br-)
= 0.77 -1.09
= - 0.32 V
(Reaction is not feasible)
(iv) Ag(s) + Fe3+(aq) → Ag+(aq) + Fe2+(aq)
Here, Ag is oxidised and Fe3+ is reduced
E°cell = E°(cathode) - E°(Anode)
= E°(Fe3+/Fe2+) - E°(Ag+/Ag)
= 0.77 - 0.80
= - 0.03 V
(Reaction is not f easible)
(V) 1/2Br2(aq) + Fe2+(aq) → Br- + Fe3+(aq)
Here, Br2 is reduced and Fe2+ is oxidised
E°cell = E°(Cathode) - E°(Anode)
= 1.09 - 0.77 v
= 0.32V
(Reaction is feasible)
Question 3.18.
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
(iii) A dilute solution of of H2SO4 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Answer:
(i) Electrolysis of aqueous solution of AgNO3 with silver electrodes:
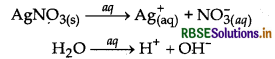
At cathode: Discharge potential of Ag+ ion is lower than that of H+ ions. Hence, Ag+ ions will be deposited in preference to H+ ions.
Ag+ + e- → Ag
At anode: As silver electrode is attacked by NO3 ions, Ag from anode dissolves to form Ag+ ions which go into the solution
Ag+ + e- → Ag
Thus, Ag is deposited at cathode and an equivalent amount of silver is oxidised to Age ions from anode.
(ii) Electrolysis of an aqueous solution of AgNO3 with platinum electrodes.
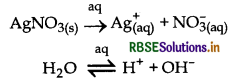
At cathode: Ag ions have lower discharge potential than H’ ions. Hence, Ag ions will be deposited at cathode in preference to H+ ions.
Ag+ + e- → Ag
At anode: Here, OH- ions have lower discharge potential than NO ions. Hence, OH- ions will decompose to give O2
OH- → OH + e-
4OH → 2H2O + O2
Thus, Ag is deposited at cathode and O2 is liberted at anode.
(iii) Electrolysis of dilute solution of H2SO4 with platinum electrodes
H2SO4(aq) → 2H+(aq) + SO2-4(aq)
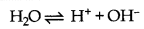
At Cathode: H+ + e- → 1/2H2
At anode: Since OH- has lower discharge pontential.
it decomposes to give O2
OH- → OH + e-
4OH → 2H2O + O2
Thus, H2 gas is liberated at cathode and O2 gas is liberated at anode.
(iv) Electrolysis of an aqueous solution of CuCl2 with platinum electrodes
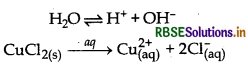
At Cathode: Cu2+ have lower discharge potential than H+ ions, hence Cu2+ ions are deposited at cathode.
Cu2+ + 2e- → Cu
At anode: Cl- ions has lower discharge potenetial than OH- ions. Hence CI ions are oxidised in preference to OH- ions
2Cl- → Cl2 + 2e-
Thus, Cu is deposited at cathode and Cl2 is liberated at anode.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम