RBSE Solutions for Class 12 Chemistry Chapter 15 Polymers
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 15 Polymers Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 15 Polymers
RBSE Class 12 Chemistry Polymers InText Questions and Answers
Question 15.1
What are polymers?
Answer:
Polymers are the backbone of modern civilization. In almost all walks of life, polymers are extensively used. The use of polymers in the manufacture of plastic buckets, cups, children's toys, packaging bags, synthetic clothing materials, automobile tyres, electrical insulating materials and machine parts, has completely revolutionised in daily life as well as the industrial scenario. In fact, polymers are the backbone of four major industries viz. plastics, elastomers, fibres and paints and varnishes. In this unit, we shall discuss.some important aspects of the chemistry of various polymers.
Question 15.2
Write the names of monomers of the follwing polymers:
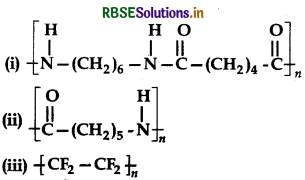
Answer:
(i) Hexamethylenediamine, H2N - (CH2)6 -NH2 and adipic acid, HOOC - (CH2)4 - COOH.
(ii) Caprolactam, (iii) Tetrafluroethane, F2C = CF2.

Question 15.3
Classify the following as addition and condensation polymers:
Terylene, Bakelite, Polythene Teflon.
Answer:
Addition polymers: Polythene, Teflon. Condensation polymers: Terylene, Bakelite.
Question 15.4.
Explain the difference between Buna-N and Buna-S.
Answer:
- Both Buna-N and Buna-S are synthetic rubber and copolymers. They differ in their constituents.
- Buna-N : Constituents are buta-1, 3-diene and arcylonitrile
- Buna-S: Constituents are buta-1,3-diene and styrene. They condense in the presence of sodium.
Question 15.5.
Arrange the following polymers in increasing order of their intermolecular forces:
(i) Nylon 6, 6, Buna-S, Polythene
Answer:
The increasing order of intermolecular forces of attraction follows the order :
Elastomer < Plastic < Fibre (i) Buna-S< Polythene < Nylon 6,6.
RBSE Class 12 Chemistry Polymers Textbook Questions and Answers
Question 15.1.
Explain the terms polymer and monomer.
Answer:
Polymers: Polymers are the backbone of modern civilization. In almost all walks of life, polymers are extensively used.
Monomers: Simple and reactive molecules from which the polymers are prepared either by addition or condensation are known as monomers. For example: Ethylene, vinylchloride, acrylonitrile, phenol and formaldehyde etc.
Question 15.2.
What are natural and synthetic polymers? Give two examples of each type.
Answer:
(1) Natural polymers: Polymers which are found in nature i.e., in plants and animals are called natural polymers. For example: proteins, cellulose, starch, some resins, rubber etc. Examples of natural polymers: Cellulose, proteins, nucleic acids and natural rubber.
(2) Synthetic polymers: A variety of synthetic polymers as plastics (polythene, polypropene), synthetic fibres (nylon, polyester) and synthetic rubbers (neoprene, polystyrene) are examples of man made polymers extensively used in daily life as well as in industry. Examples of synthetic polymers : PVC, polyester, synthetic rubber.

Question 15.3.
Distinguish between the terms homopolymer and copolymer and give an example of each.
Answer:
- Homopolymer: Polymers whose repeating structural units are derived from only one type of monomer units are termed as homopolymers.e.g., polyethylene, PVC, PAN, teflon, polystyrene etc.
- Copolymer: Polymers whose repeating structura units are derived from two or more different types of monomer units are termed as copolymers.e.g., Buna-S, BunaN, Nylon 6, 6, Phenol-formaldehyde resin (Bakelite).
Question 15.4.
How do you explain the functionality of a monomer?
Answer:
The number of bonding sites present in a molecule is called its functionality. For example, the functionality of ethene, propene, styrene, acrylonitrile is one, while that of 1, 3-butadiene, adipic acid, terepthalic acid, hexamethylene diamine is two.
Question 15.5.
Define the term polymerization.
Answer:
It is a process in which a large number of repeating structural units derived from one or more monomers join together in a regular fashion through covalent bonding to form a high molecular mass molecules i.e. polymers.
Question 15.6.
Is NH-CHR -Cota homopolymer or a copolymer?
Answer:
It is a homopolymer because of repeating structural unit has only one type of monomer unit i.e., NH2 - CHR - COOH.

Question 15.7.
In which classes, the polymers are classified on the basis of molecular forces?
Answer:
- Elastomers,
- Fibres,
- Thermoplastics and
- Thermosetting plastics.
Question 15.8.
How can you differentiate between addition and condensation polymerization?
Answer:
Addition Polymerization: In this type of polymerization, a large number of same or different monomers simply add one another leading to the formation of a polymer. In the addition polymerization the monomers used an addition unsaturated compounds, e.g., alkenes, alkadienes and their derivatives. For example, formation of polythene from ethene and poly acrylonitrile (PAN) from acrylonitrile.
Condensation Polymerization: In this type of polymerization, two or more bifunctional molecules undergo a series of independent condensation reactions usually with the elimination of simple molecules to form a polymer. For example, nylon 6,6 is a condensation polymer of hexamethylene diamine and adipic acid formed with elimination of H2O molecules.

Question 15.9.
Explain the term copolymerisation and give two examples.
Answer:
Copolymerisation is a process in which a mixture in one monomeric species is allowed to polymerise. The copolymor contains multiple units of each monomer in the chain. The examples are copolymers of 1,3 butadiene and styrene, hexomethylene diamine and adipic acid (Nylon 6,6).
Question 15.10.
Write the free radical mechanism for the polymerisation of ethene.
Answer:
In the chain initiation step, the decomposition of benzoyl peroxide gives benzoyl free radical which further gives phenyl free radical. Phenyl free radical adds to C = C double bond of ethene and generates a new free radical. In chain propagating step, the newly generated free radical adds to another ethene molecule to form larger free radical. This process repeats several times. In the chain termination step, two free radicals combine to form polyethene.
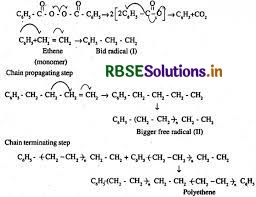
Question 15.11.
Define thermoplastics and thermosetting polymers with two examples each.
Answer:
|
Thermoplastic plastics |
Thermosetting plastics |
|
1. Such polymers or plastics are usually formed by addition polymerisation. |
1. These plastics are usually formed by condensation polymerisation. |
|
2. They are linear or slightly branched long chain polymers. |
2. They are cross linked or heavily branched. |
|
3. They can be easily soften on heating and hardened on cooling. |
3. It cannot be softened on heating. |
|
4. They are held together by Van der Waal forces of attraction. |
4. They are held together by strong hydrogen bonds. |
|
5. By nature they are soft, weak and less brittle. |
5. They are strong, hard and more brittle in nature. |
|
6. Their molecular weight is low. |
6. Their molecular weight is large. |
|
7. They are quite soluble in organic solvents. |
7. They are insoluble in organic solvents. |
|
8. They can be remoulded into desired shapes. |
8. They cannot be remoulded. |
|
9. Monomers used here do not have more than two reaction sites. |
9. Monomers used here have more than two reaction sites. |
|
10. Examples: polythene, polystyrene, polyvinyls, etc. |
10. Examples: bakelite, urea-formaldehyde resins, etc. |
Question 15.12.
Write the monomers used for getting the following polymers:
(i) Poly vinyl chloride,
(ii) Teflon,
(iii) Bakelite.
Answer:
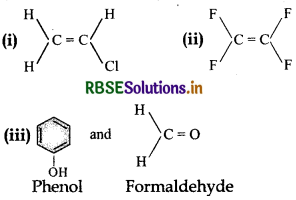

Question 15.13.
Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Answer:
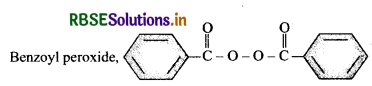
Question 15.14.
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Answer:
- Structure : All the double bonds present in natural rubber have cis-configuration which leads to a random coiled structure of rubber and provide elasticity to it.
- Reactivity: Presence of double bonds in natural rubber makes it reactive and it gets oxidised in presence of air and also makes vulcanization possible.
Question 15.15.
Discuss the main purpose of vulcanization of rubber.
Answer:
- Natural rubber is soft and sticky and becomes more plastic so at high temperature and brittle at low temperature. It has a large water absorption capacity, has low tensile strength and low resistance to abrasion. Natural rubber is not resistant to action of organic solvents and is easily attacked by O2 and other oxidising agents.
- To improve all these properties, natural rubber is vulcanized by heating it with sulphur at 373 - 415 K. The valcanized rubber thus obtained has excellent elasticity, low water absorption tendency and is resistant to the action of agents and oxidising agents.
Question 15.16.
What are the monomeric repeating units of Nylon 6 and Nylon 6, 6?
Answer:
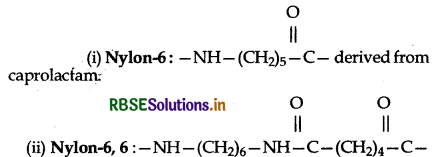
derived from two monomers, hexamethylene diamine and adipic acid.

Question 15.17.
Write the names and structures of the monomers of the following polymers:
(i) Buna-S,
(ii) Buna-N,
(iii) Neoprene,
(iv) Dacron.
Answer:
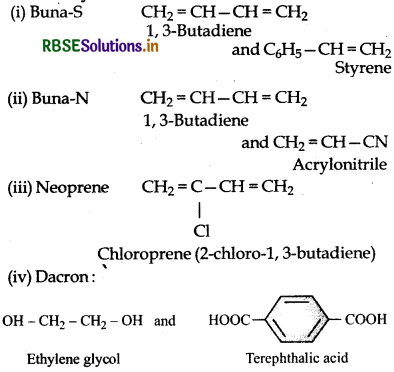
Question 15.18.
Identify the monomer in the following polymeric structures:
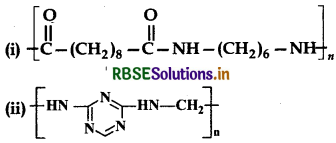
Answer:
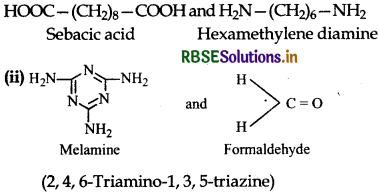
Question 15.19.
How is dacron obtained from ethylene glycol and terephthalic acid?
Answer:
Dacron or terylene (a polyester) is formed by the condensation polymerization of ethylene glycol and terephthalic acid with the elimination of water molecule. The reaction takes place at 420 - 460 K temperature in presence of zinc acetate and antimony trioxide as a catalyst.
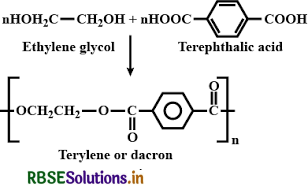
Question 15.20.
What is the biodegrable polymer? Given an example of a biodegradable aliphatic polyester.
Answer:
Polymers which disintegrate by themselves over a certain period of time by enzymatic hydrolysis and to some extent by oxidation are called biodegradable polymers. An example of biodegradable aliphatic polyester is PHBV i.e., Poly-β -hydroxybutyrate-co- β -hydroxy valerate.
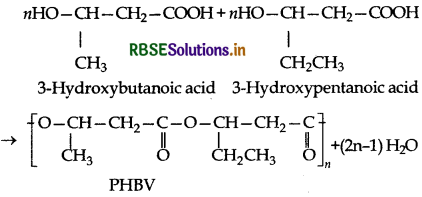

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम