RBSE Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
RBSE Class 12 Chemistry Alcohols, Phenols and Ethers InText Questions and Answers
Question 11.1.
Classify the following as primary, secondary and tertiary alcohols:
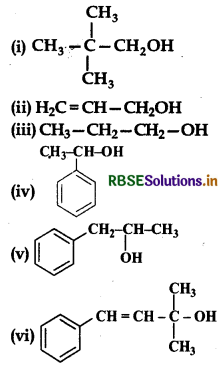
Answer:
Primary alcohols: (i), (i), (iii) Secondary alcohols : (iv), (v); Tertiary alcohol: (vi).

Question 11.2.
Identify allylic alcohols in the above examples.
Answer:
Allylic alcohols: (ii) and (vi)
Question 11.3.
Name the following compounds according to the IUPAC system:
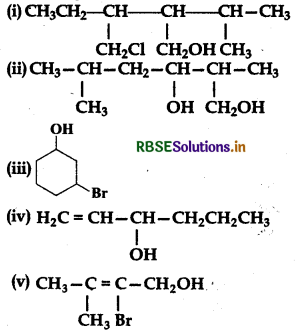
Answer:
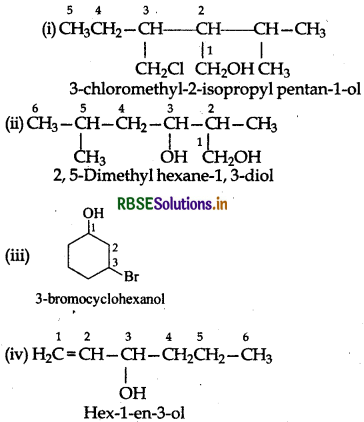
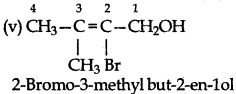
Question 11.4.
Show how are the following alcohols prepared by the reaction of suitable Grignard reagent on methanol?

Answer:
(i) The structure of given alcohol suggests that the Grignard reagent that reacts with methanol is isopropyl magnesium halide.

(ii) The structure of given alcohol suggests that the Grignard reagent that reacts with methanol is cyclohexyl magnesium halide.
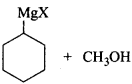

Question 11.5.
Write structure of products of the following reactions:
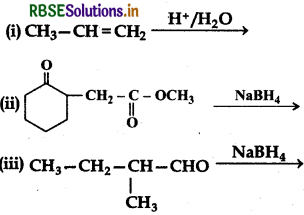
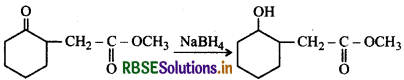
Answer:
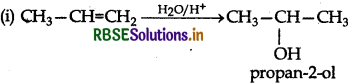
(ii) NaBH4 is a weak reducing agent. It reduces the aldehyde or ketones but not the esters.
(ii) NaBH reduces -CHO group to CH2OH.
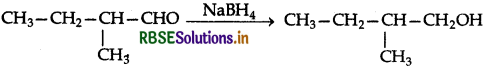
Question 11.6.
Give structures of the products you would expect when each of the following alcohol reacts with:
(a) HCl - ZnCl2, (b) HBr and (c) SOCl
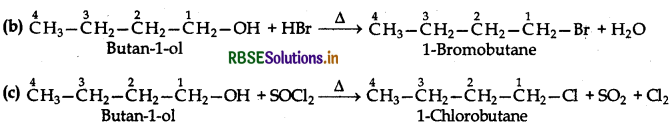
(i) Butan-1-ol
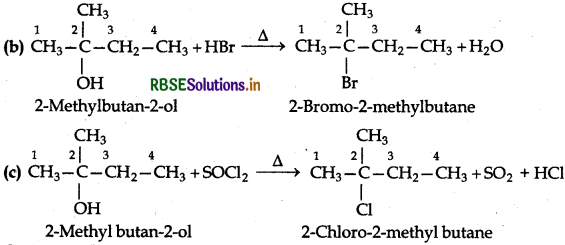
(ii) 2-Methylbutan-2-ol
Answer:
(i) Reactions of Butan-1-ol:
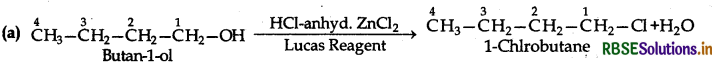
The reaction of butan-1-ol (1°) can take place only upon heating. At ordinary temperature, it does not take place.
(ii) Reactions of 2-Methyl butan-2-ol:

The alcohol being tertiary in nature reacts immediately with Lucas reagent at room temperature.
Question 11.7.
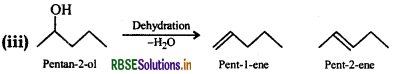
Predict the major product of acid catalysed dehydration of: (i) 1-Methylcyclohexanol and (ii) butan-1-ol
Answer:
(i) Acid catalysed dehydration of 1-methyl cyclohexanol can give two products i.e., 1-methylcyclohexene and 1methylene cyclohexene. However, methyleyclohexene will be preferably formed according to Saytzeff's rule since it is more substituted alkene.
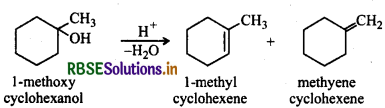
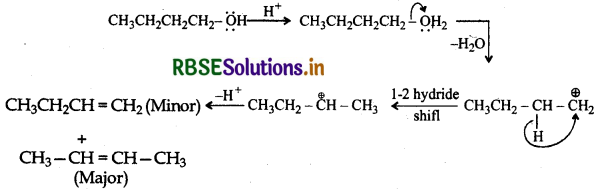
(ii) Butan-1-ol upon acid dehydration will give but-2-ene as the major product.
Question 11.8.
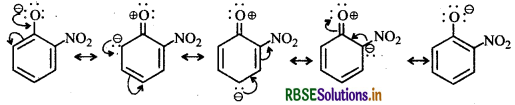
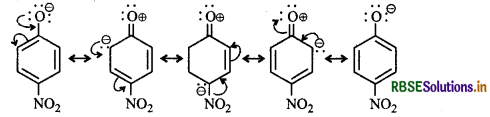
Ortho and para-nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Answer:
The resonance structure of o- and p-nitrophenoxide ions and phenoxide ions are given below:
(i) Resonance structure of o-nitro phenoxide ion:
(ii) Resonance structure of p-Nitrophenoxide ion :
(iii) Resonance structures of phenoxide ion:
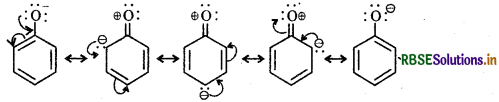
It is evident from the above resonance structures that due to - R effect of the -NO2 group, o-and p-nitrophenoxide ions are more stable than phenoxide ion. As a result o-and p-nitrophenols are more acidic than phenol.
Question 11.9.
Write the equations involved in the following reactions:
(i) Reimer-Tiemann reaction
(ii) Kolbe's reaction.
Answer:
(i) Reimer-Tiemann reaction: When phenol is treated with chloroform in the presence of sodium hydroxide, a −CHO group is introduced at the ortho position of the benzene ring. This reaction is known as the Reimer-Tiemann reaction. The intermediate is hydrolyzed in presence of alkalis to produce salicylaldehyde.
(ii) Kolbe's reaction: When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe's reaction.

Question 11.10.
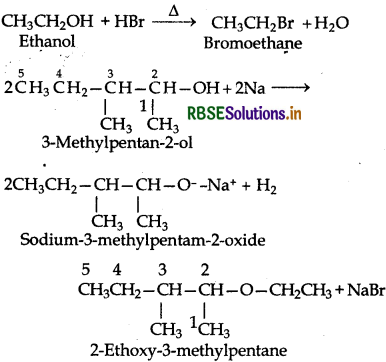
Write the reaction of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3methylpentan-2-ol.
Answer:
Williamson synthesis is a SN2 reaction therefore the alkyl halide should be primary (unhindered or least hindered). Thus, the alkyl halide should be derived from ethyl aclohol and the alkoxide ion from 3-methylpentan-2ol. Thus
Question 11.11.
Which of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?
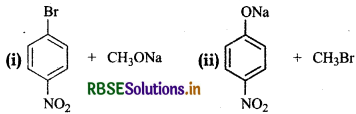
Answer:
(i) Chemically both sets are equally probable. In set (i), the Bratom is activated by the electron withdrawing effect of -NO2 group. Therefore, nucleophilic attack by CH3ONa followed by elimination of NaBr gives the desired ether.
Step 1:
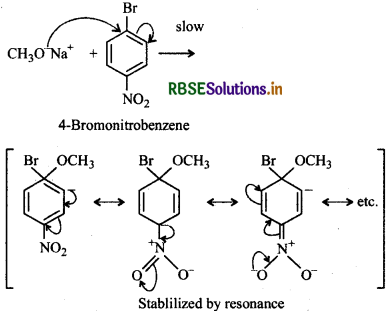
Step 2:
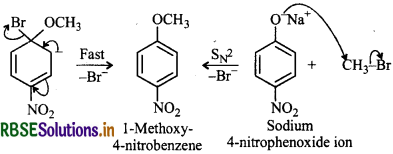
In set (ii), nucleophilic attack by 4-nitrophenoxide ion on methyl bromide gives the desired ether.
Question 11.12.
Predict the products of the following reactions:
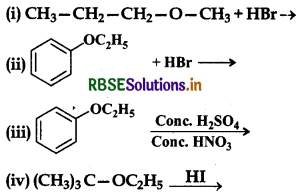
Answer:
(i) Both the alkyl groups attached to the oxygen atom are primary, therefore attack of Brion occurs on the smaller methyl group leading to the formation of propan-1ol and bromomethane.
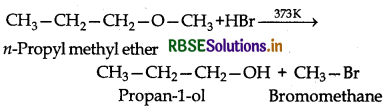
(ii) Due to resonance, C6H5-O bond has some double bond character and hence is stronger than O-C2H5 bond. Therefore, the cleavage of the weaker O-C2H5 bond occurs to yield phenol and bromoethane.
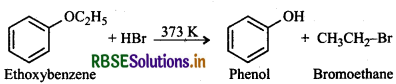
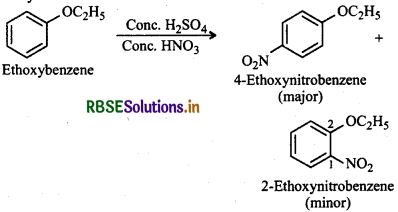
(iii) Due to strong + R-effect of the -OC2H5 group, it is activating as well as o, p-directing. Therefore, nitration of ethoxybenzene will give a mixture of 2- and 4ethoxynitrobenzene in which 4-ethoxynitrobenzene predominates due to steric hindrance at 2-position in 2ethoxynitrobenzene.
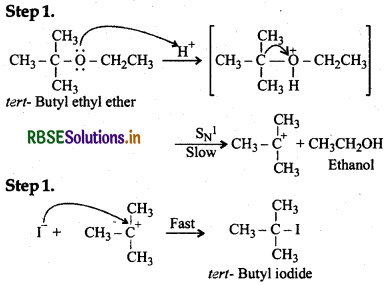
(iv) Since tert-butyl carbocation is much more stable than ethyl carbocation, therefore, reaction follows Sni mechanism leading to the formation of tert-butyl iodide and ethanol as shown below:
RBSE Class 12 Chemistry Alcohols, Phenols and Ethers Textbook Questions and Answers
Question 11.1.
Write the IUPAC names of the following compounds:
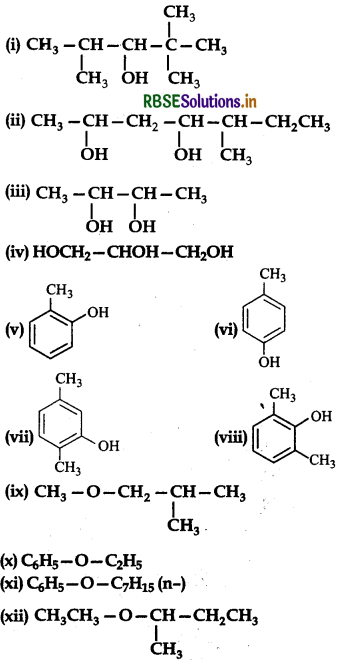
Answer:
(i) 2, 2, 4-Trimethylpentan 3-ol
(ii) 5-Ethylheptan-2,4-diol
(iii) Butane-2,3-diol
(iv) Propane-1, 2, 3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2,5-Dimethylphenol
(viii) 2, 6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane.
Question 11.2.
Write the structures of the compounds whose IUPAC names are as follows:
(i) 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5-Dimethylhexane-1,3,5-triol
(iv) 2,3-Diethylphenol
(v) 1-Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 3-Chloromethylpentan-1-ol
Answer:
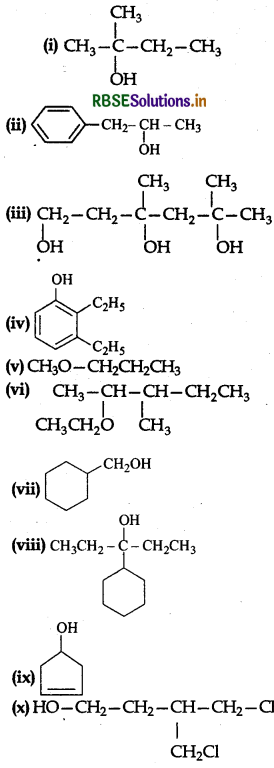

Question 11.3.
(i) Draw the structures of all isomeric alcohols of molecular formula CH2O and give their IUPAC names.
(ii) Classify the isomers of alcohols in question 11.3 (i) as primary, secondary and tertiary alcohols.
Answer:
Eight isomers are possible. These are:
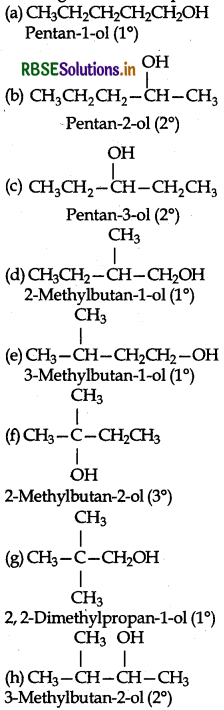
Question 11.4.
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Answer:
The molecules of butane are held together by weak van der Waals forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.
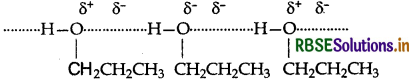
Therefore, the boiling point of propanol is much higher than that of butane.
Question 11.5.
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer:
Alcohols can form H-bonds with water and can also break the H-bonds already existing between water molecules. Therefore, they are soluble in water.
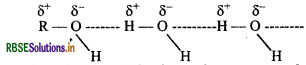
On the other hand, hydrocarbons can neither form Hbonds with water nor break the H-bonds already existing between water molecules and hence are insoluble in water.
Question 11.6.
What is meant by hydroboration-oxidation reaction? Illustrate with an example.
Answer:
The addition of diborane to alkenes to form trialkyboranes followed by their oxidation with alkaline hydrogen peroxide to form alcohols is called hydroborationoxidation. For example.
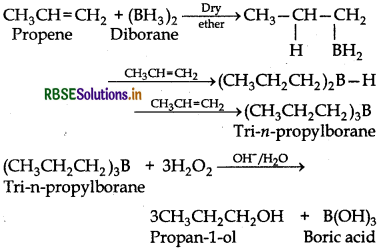
The alcohols obtained by this process appear to have been formed by direct addition of water to the alkene contrary to the Markovnikov's rule.
Question 11.7.
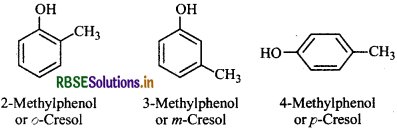
Give the structures and IUPAC names of monohydric phenols of molecular formula C7H8O.
Answer:
The three isomers are:
Question 11.8.
While separating a mixture of ortho-and-paranitrophenols by steam distillation, name the isomer which will be steam volatile. Give reasons.
Answer:
o-Nitrophenol is steam-volatile due to chelation (intramolecular H-bonding) and hence can be separated by steam distillation from p-nitrophenol which is not steam volatile because of intermolecular H-bonding.

Question 11.9.
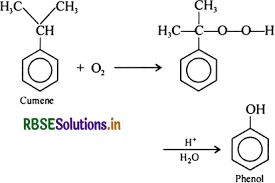
Give the equations of reactions of preparation of phenol from cumene.
Answer:
To prepare phenol, cumene is first oxidized in the presence of air of cumene hydro-peroxide at 368 to 408 K. Then, cumene hydroxide is treated with dilute acid to prepare phenol and acetone as by-products at 323 to 363 K.
Question 11.10.
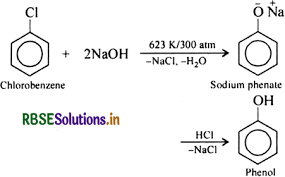
Write chemical reaction for the preparation of phenol from chlorobenzene.
Answer:
Chlorobenzene is fused with NaOH (at 623 K and 320 atm pressure) to produce sodium phenoxide, which gives phenol on acidification.
Question 11.11.
Write the mechanism of hydration of ethene to form ethanol.
Answer:
Direct addition of H2O to ethene in presence of an acid does not occur, Indirectly, ethene is first passed through conc H2SO4, when ethyl hydrogen sulphate is formed.
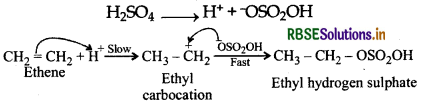
Ethyl hydrogen sulphate carbocation Ethyl hydrogen sulphate when boiled with water undergoes hydrolysis to form ethanol.
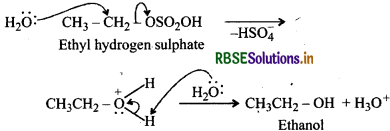
Question 11.12.
You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Answer:
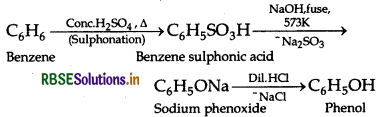
Question 11.13.
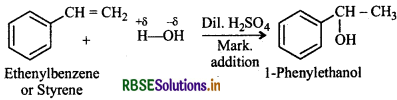
Show how will you synthesize:
(i) 1-phenylethanol from a suitable alkene?
(ii) Cyclohexylmethanol using an alkyl halide by an SN2 reaction?
(iii) Pentan-1-ol using a suitable alkyl halide?
Answer:
(i) Addition of H2O to ethenylbenzene (or styrene) in presence of dil. H2SO4 gives 1-phenylethanol.
(ii) Hydrolysis of cyclohexylmethyl bromide by aqueous NaOH gives cyclohexylmethanol.
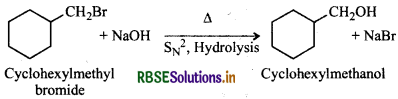
(iii) Hydrolysis of 1-bromopentane by aqueous NaOH gives pentan-1-ol.
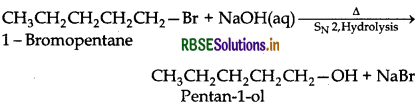
Question 11.14.
Give two reactions that show the acidic nature of phenol. Compare its acidity with that of ethanol.
Answer:
The reactions showing acidic nature of phenol are:
(i) Reaction with sodium: Phenol reacts with active metals like sodium to liberate H2 gas.

(ii) Reaction with NaOH: Phenol dissolves in NaOH to form sodium phenoxide and water.
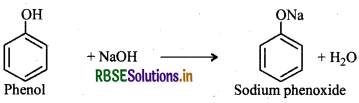
Comparison of acidic character of phenol and ethanol: Phenol is more acidic than ethanol. This is due to the reason that phenoxide ion left after the loss of a proton from phenol is stabilized by resonance while ethoxide ion (left after loss of a proton from ethanol) is not.
Question 11.15.
Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol?
Answer:
Due to strong -R and I- effect of the -NO2 group, electron density in the O-H bond decreases and hence the loss of a proton becomes easy.
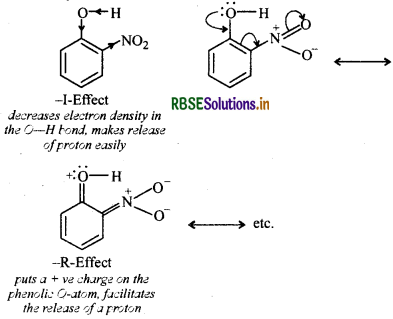
Further, after the loss of a proton, the o-nitrophenoxide ion left behind is stabilized by resonance, thereby making onitrophenol a stronger acid.
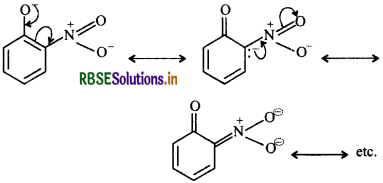
In contrast, due to +R effect of the OCH3 group, the electron density in the O-H bond increases thereby making the loss of a proton difficult. OH

Furthemore, the o-methoxyphenoxide ion left after the loss of a proton is destabilized by resonance because the two negative charges repel each other thereby making omethoxyphenol a weaker acid. Thus, o-nitrophenol is more acidic than o-methoxyphenol.

Question 11.16.
Explain how does-OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Answer:
Phenol may be regarded as a resonance hybrid of structures. As a result of +R-effect of the OH group, the electron density in the benzene ring increases thereby facilitating the attack by an electrophile. In other words, presence of OH group, activates the benzene ring towards electrophilic substitution reactions. Further since the electron density is relatively higher at the two o-and one p-position, therefore, electrophilic substitution occurs mainly at o-and P-positions.

Question 11.17.
Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline KMnO4 solution,
(ii) Bromine in CS2 with phenol,
(iii) Dilute nitric acid with phenol
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Answer:
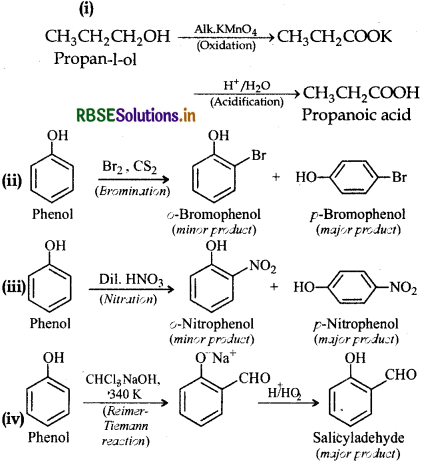
Question 11.18.
Explain the following with an example:
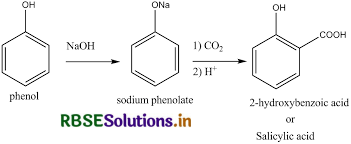
(i) Kolbe's reaction,
(ii) Reimer-Tiemann reaction,
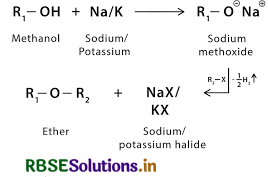
(iii) Williamson ether synthesis,
(iv) Unsymmetrical ether.
Answer:
(i) Reimer-Tiemann reaction: When phenol is treated with chloroform in the presence of sodium hydroxide, a −CHO group is introduced at the ortho position of the benzene ring. This reaction is known as the Reimer-Tiemann reaction. The intermediate is hydrolyzed in presence of alkalis to produce salicylaldehyde.
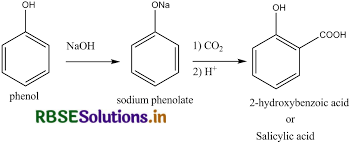
(ii) Kolbe's reaction: When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe's reaction.
(iii) Williamson ether synthesis is a laboratory method to prepare symmetrical and unsymmetrical ethers by allowing alkyl halides to react with sodium alkoxides. This reaction involves SN2 attack of the alkoxide ion on the alkyl halide. Better results are obtained in case of primary alkyl halides. If the alkyl halide is secondary or tertiary, then elimination competes over substitution.
(iv) If the alkyl or aryl groups attached to the oxygen atom are different, ethers are called unsymmetrical ethers. For example, ethyl methyl ether, methyl phenyl ether, 4chlorophenyl-4-nitrophenyl ether, etc. Write the structures of these ethers yourself.
Question 11.19.
Wrtie the mechanism of acid-catalysed dehydration of ethanol to ethene.
Answer:
Mechanism of acid catalysed dehydration of ethanol
Step I: Protonation of ethanol
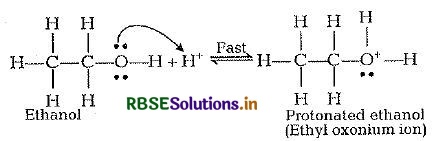
Step II: Formation of carbocation

Step III: Elimination of a proton
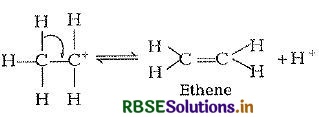
Note The acid used in Step I is released in Step III. To drive the equilibrium to the right, ethene is removed as it is formed.
Question 11.20.
How are the following conversions carried out?
(i) Propene propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethylmagnesium chloride - Propan-1-ol
(iv) Methylmagnesium bromide → 2-Methylpropan-2-ol.
Answer:
(i) Propan-2-ol can be prepared from propene by hydration as shown below:
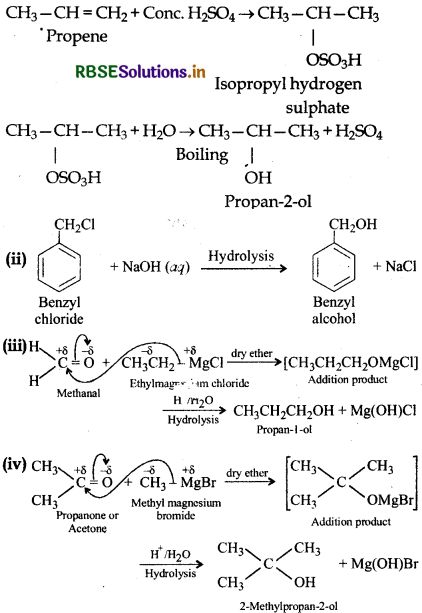
Question 11.21.
Name the reagents in the following reaction:
(i) Oxidation of a primary alcohol to a carboxylic acid
(ii) Oxidation of a primary alcohol to an aldehyde
(iii) Bromination of phenol to 2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid
(v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Answer:
(i) Acidified potassium dichromate or, neutral, acidic or alkaline potassium permanganate (followed by hydrolysis with dil. H2SO4).
(ii) Pyridinium chlorochromate
(PCC), C5H5NH+ CrO3Cl- in CH2Cl2
Or
Pyridinium dichromate
(PDC), (C5H5NH)2 Cr2O72- in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate (followed by hydrolysis with dil. H2SO4).
(v) 85% H2SO4 at 440 K.
(vi) Ni/H or NaBH4 or LiAlH4.

Question 11.22.
Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer:
Ethanol undergoes intermolecular H-bonding due to the presence of a hydrogen attached to the electronegative oxygen atom. As a result, ethanol exists as associated molecules.
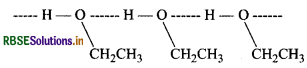
Consequently, a large amount of energy is required to break these hydrogen bonds. Therefore, the boiling point of ethanol is higher than that of methoxymethane which does not form H-bonds.
Question 11.23.
Give the IUPAC names of the following ethers:
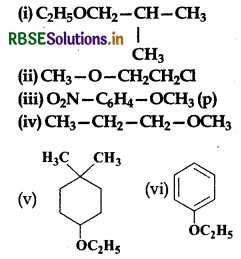
Answer:
(i) 1-Ethoxy-2-methylpropane
(ii) 2-Chloro-l-methoxyethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 4-Ethoxy-1, 1-dimethylcyclohexane (lowest locant rule is followed)
(vi) Ethoxybenzene
Question 11.24.
Write the names of reagents and equations for the preparation of the following ethers by Williamson synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methyl-2-methoxypropane
(iv) 1-Methoxyethane
Answer:
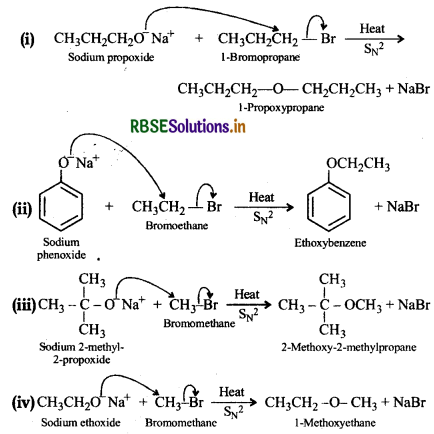
Question 11.25.
Illustrate with examples the limitations of Williamson synthesis for the preparation of certain type of ethers.
Answer:
There are few limitations of Williamson Ether Synthesis.
- Tertiary alkyl halides or sterically hindered primary or secondary alkyl halides tend to undergo E2 elimination in the presence of the alkoxide that in addition to being a nucleophile also act as a base.
- Alkali phenoxides may undergo C-alkylation in addition to expected O-alkylation.
- This process for preparing ethers is too limited to be of any practical value for synthetic organic chemists.

Question 11.26.
How is 1-propoxypropane synthesized from propan-1-ol?
Answer:
The following two methods can be used:
(a) Williamson synthesis:
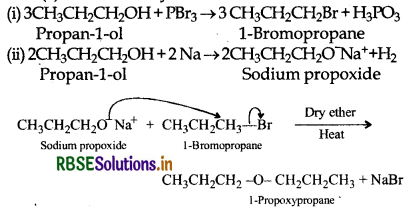
(b) By dehydration of 1-propanol with conc. H2SO4 at 413 K
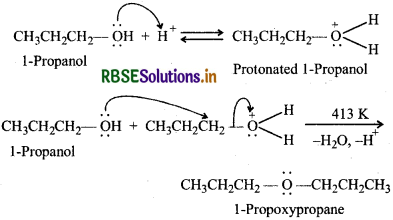
Question 11.27.
Preparation of ethers by acid dehydration of secondary and tertiary alcohols is not a suitable method. Give reason.
Answer:
Acid-catalysed dehydration of 1° alcohols to ethers occurs by SN2 reaction involving nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.
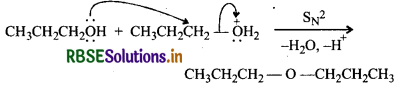
Under these conditions, 2 and 3 alcohols, however, give alkenes rather than ethers. The reason being that due to steric hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not occur. Instead protonated 2 and 3° alcohols lose a molecule of water to form stable 2 and 3° carbocations. These carbocations prefer to lose a proton to form alkenes rather than undergoing nucleophilic attack by alcohol molecule to form ethers.
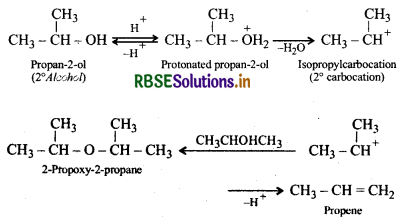
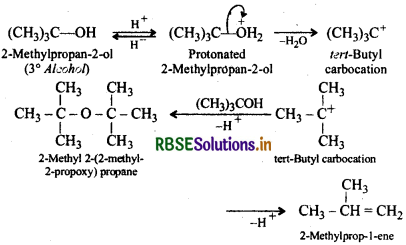
Similarly, 3 alcohols give alkenes rather than ethers.
Question 11.28.
Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane,
(ii) methoxybenzene
(iii) benzyl ethyl ether
Answer:
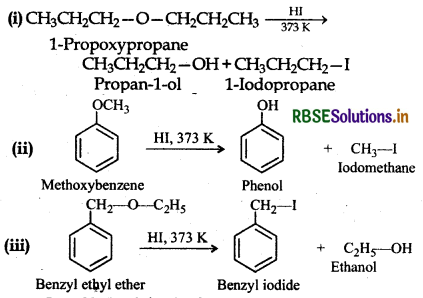

Question 11.29.
Explain the fact that in aryl alkyl ethers:
(i) the alkoxy group activates the benzene ring towards electrophilic substitution and
(ii) it directs the incoming substituents to ortho-and para-Positions in benzene ring.
Answer:
(i) The alkoxy group activates the benzene ring towards electrophilic substitution and
(ii) It directs the incoming substituents to ortho and para positions in benzene ring.
Question 11.30.
Write the mechanism of the reaction of HI with methoxymethane.
Answer:
With equimolar amounts of HI and methoxymethane, a mixture of methyl alcohol and methyl iodide is formed by the following mechanism:
Step I
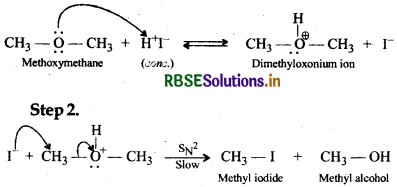
If, however, excess of HI is used, methyl alcohol formed in step 2 is also converted into methyl iodide by the following mechanism:
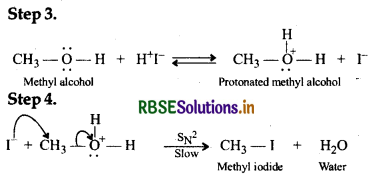
Question 11.31.
Write equations of the following reactions:
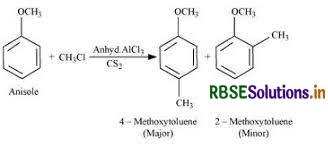
(i) Friedel-Crafts reaction-alkylation in anisole
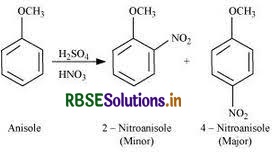
(ii) Nitration of anisole
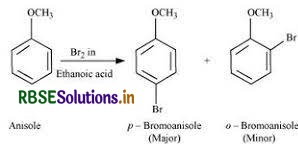
(iii) Bromination of anisole in ethanoic acid medium
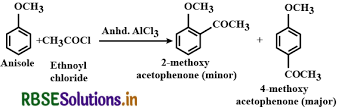
(iv) Friedel-Crafts acetylation of anisole.
Answer:
(i) Friedel-Crafts reaction-alkylation in anisole:
- In order to attach substituents to an aromatic ring, Charles Friedel and James Crafts created a series of reactions in 1877 known as the Friedel-Crafts reactions.
- Alkylation reactions and acylation reactions are the two primary categories of Friedel-Crafts processes.
- Both of these reactions work by substituting electrophilic aromatics.
(ii) Nitration of anisole:
- Methoxybenzene, often known as anisole, is a chemical substance having the formula C7H8O.
- It is a white liquid with an anise-seed aroma, and both natural and synthetic scents frequently contain its derivatives.
- The substance, which is a precursor to other synthetic chemicals, is primarily synthesized.
- It is an ether.
Alkylation of Anisole:
The following reaction is followed as the alkylation of Anisole
Nitration of Anisole:
The following reaction is followed as the Nitration of Anisole
Bromination of Anisole in Ethanoic acid medium:
The following reaction is followed as Bromination of Anisole in Ethanoic acid medium,
Acetylation of Anisole:
The following reaction is followed as Acetylation of Anisole,
Question 11.32.
Show how would you synthesize the following alcohols from appropriate'alkenes?

Answer:
Since addition and elimination are reverse of each other, therefore, the general strategy is to first dehydrate a suitable alcohol to give either a single alkene or a mixture of alkenes. If a mixture of alkenes is possible, then find out which of the alkenes will give the desired alcohol. Please note that acid-catalysed addition of H2O to alkenes occurs in accordance with Markovnikov's rule.
Addition of H2O to 4-methylhept-3-ene in presence of an acid gives the desired alcohol.
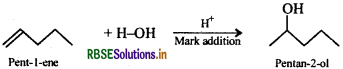
Now addition of H2O to pent-1-ene gives the desired alcohol
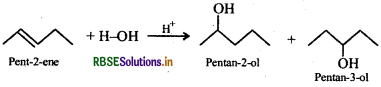
However, addition of H2O to pent-2-ene gives a mixture of two alcohols, i.e., pentan-2-ol and pentan-3-ol.
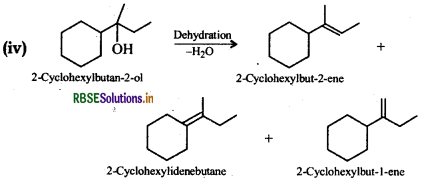
Now addition of H2O to either of the three alkenes in presence of an acid gives the desired alcohol.
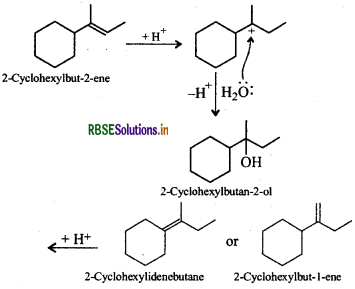
Thus, the desired alkene is 2-cyclohexylbut-1-ene or 2cyclohexylbut-2-ene or 2-cyclohexylidenebutane.
Question 11.33.
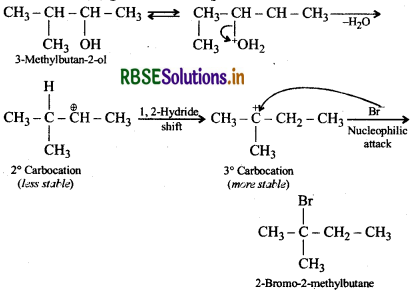
When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place:
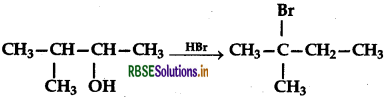
Give a mechanism for this reaction.
Answer:
Protonation of the given alcohol followed by loss of water gives a 2° carbocation (I) which being less stable rearranges by 1, 2-hydride shift to form the more stable 3° carbocation (II). Nucleophilic attack by Br- ion on this carbocation (II) gives the final product.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम