RBSE Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
RBSE Class 12 Chemistry Haloalkanes and Haloarenes InText Questions and Answers
Question 10.1.
Write the structures of the following compounds:
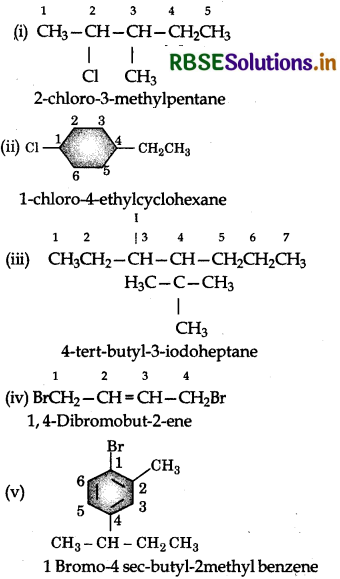
(i) 2-chloro-3-methylpentane
(ii) 1-chloro-4-ethylcyclohexane
(iii) 4-tert-Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec-butyl-2-methyl benzene
Answer:
Question 10.2
Why is sulphuric acid not used during the reaction of alcohols with KI?
Answer:
Sulphuric acid (H2SO4) is an oxidising agent. It oxidess HI produced during the reaction to I2 and thus prevents the reaction between an alcohol and HI to from an haloalkane.
KI + H2SO4 → KHSO5 + HI
2HI + H2SO4 → 2H2O + I2 + SO2
To solve the problem H2SO4 is replaced by phosphoric acid (H3PO4) which provides HI for the reaction and does not give I2 as is done by H2SO4.
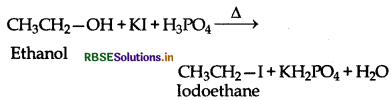

Question 10.3
Write structure of different dihalogen derivates of propane.
Answer:
propane (CH3CH2CH3) has two primary (1) and one secondary (2) H-atoms. Four isomeric dihalogen derivates are possible for each halogen. For example:
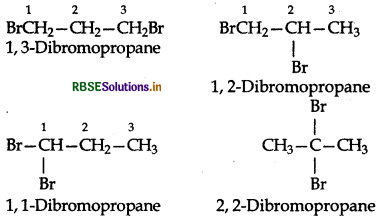
Similarly we can write four isomeric dihalogen derivatives for other halogens.
Question 10.4
Among the isomeric alkanes of molecular formula C5H12. Identify the one which on photochemical chlorination yields:
Answer:
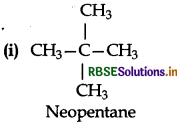
All the hydrogen atoms are equivalent. Therefor replacement of any one of them will give the same product.
(ii) CH3CH2CH2CH2CH3
There are three sets of equivalent H -atoms designated as a, b and c. The replacement of any one set of the equivalent H -atoms of each set will give the same product. Thus, three isomeric monochlorides are possible.
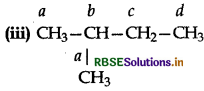
There are four types of equivalent H-atoms designated as a,b,c and d. Thus, four isomeric monochlorides are possible.
Question 10.5
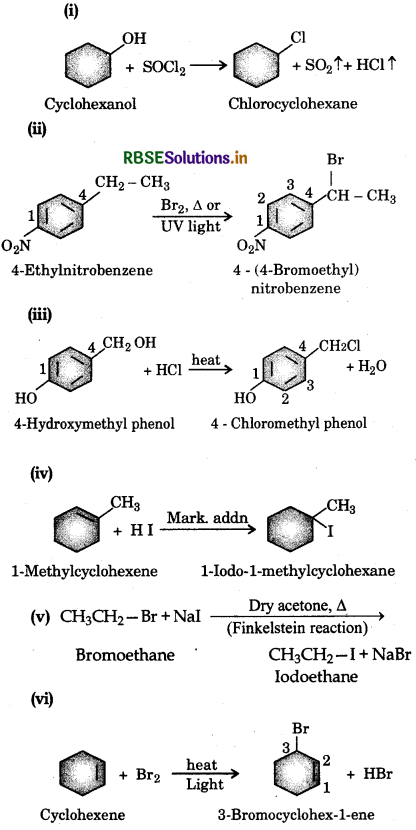
Draw the structures of major monohalo products in each of the following reactions:
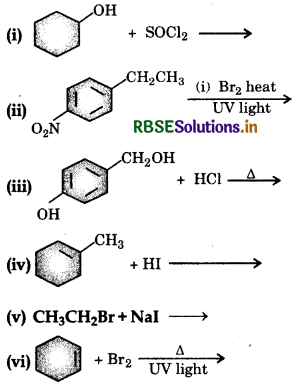
Answer:
Question 10.6.
Arrange each set of compounds in order to increasing boiling points:
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane
(ii) 1-Chloropropene, Isopropyl chloride, 1-Chlorobutane.
Answer:
(i) Boiling points of organic compounds are associated with the van der Waals attraction which depends upon the size of molecules. In the given case, all the compounds contain only one carbon atom. Therefore, the size of molecules depends upon the size of halogen atom present and also upon the number of halogen atoms present in different molecules. Thus the increasing order of boiling points is: Chloromethane (CH3CI) < Bromomethane (CH3Br) < Dibromomethane (CH2Br2) < Bromoform (CHBr3).
(ii) The branching of the carbon chain decreases the size of isomer and this decreases the boiling point as compared to straight chain isomer. The increasing order of boiling point is:
Isopropyl chloride [(CH3)2CHCI)] < 1-Chloropropane (CICH2CH2CH3) < 1-Chlorobutane (CICH CH2CH3).

Question 10.7.
Which alkyl halide from the following pairs would you expect react more rapidly by SN2 mechanism? Explain your answer.
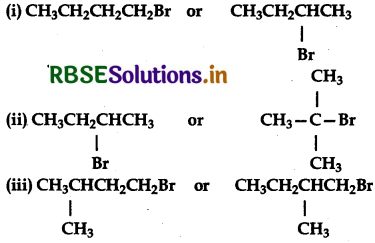
Answer:
(i) CH3CH2CH2CH2Br is a primary (1°) alkyl halide while CH3 CH2 - CHBr - CH3 is a secondary (2°) alkyl halide. Since there will be some steric hindrance in secondary (2°) halides than in primary (1°) alkyl halides, therefore CH3CH2CH2Br will react faster than CH3CH2 CHBrCH3 in SN2 reaction.
(ii) CH3CH2CHBCH3 is a secondary halide while (CH3)3C-Br is tertiary (3°) alkyl halide. Since due to steric hindrance secondary (2°) alkyl halides react faster than tertiary (3°) alkyl halides, therefore, CH3CH2CHBrСH3 will react faster than (CH3)3CBr in SN2 reaction.
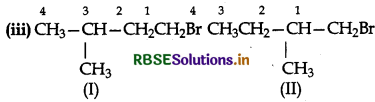
Both I and II are primary (1°) alkyl halides. But in alkyl halide (II) the-CH3 group at C2 is closer to Bratom while in alkyl halide (I) the-CH3 group at C3 is little away from the Br atom. As a result alkyl halide (II) will suffer greater steric hindrance than (I). Therefore (CH3)2CHCH2CH2Br (I) will react faster than CH3CH2CH(CH3)3CH2Br is SN2 reaction.
Question 10.8.
In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
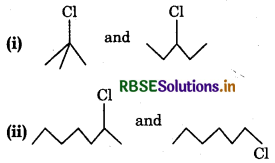
Answer:
The reactivity in SN1 reaction depends on stability of the carbocation intermediate which an alkyl halide gives on ionization. Thus
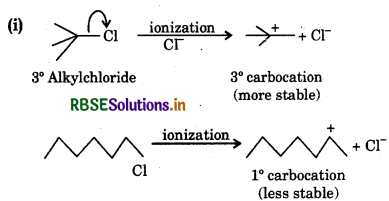
since the stability of 3 carbocations is more than 2 carbocations, therefore  will react faster than
will react faster than 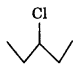 in SN1 reaction.
in SN1 reaction.
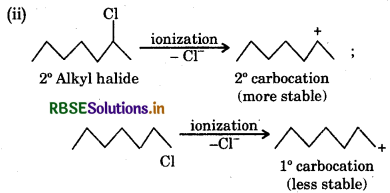
Since the stability of 2 carbocations is more than 1 carbocation therefore 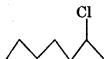 will react faster than
will react faster than 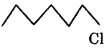 in SN1 reaction.
in SN1 reaction.
Question 10.9.
Identify A,B,C,D,E,R and R1 in the following:
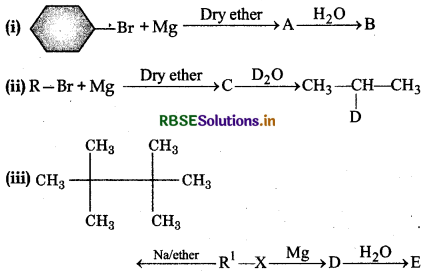
Answer:
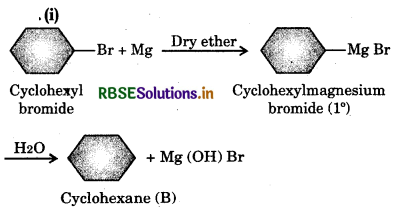
(ii) In this reaction the alkyl group Ris (CH3)2CH. The reaction proceeds as follows:
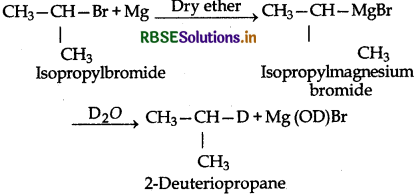
(iii) In this reaction, R1 is (CH3)3C group. The reaction proceeds as follows:
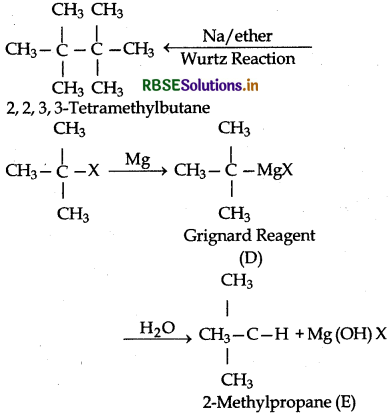
RBSE Class 12 Chemistry Haloalkanes and Haloarenes Textbook Questions and Answers
Question 10.1.
Name the folowing halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides.
(i) (GH3)2 CHCH(CI)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)Cl
(iii) CH3CH2C(CH3)2CH2I
(iv) (CH3)3CCH2CH(Br)CH5
(v) CH3CH(CH3)CH(Br)CH3
(vi) CH3C(C2H5)2CHBr
(vii) CH3C(CI)(C2H5)CH2CH3
(viii) CH3CH = C(Cl)CH2CH(CH3)2
(ix) CH3CH = CHC(Br)(CH3)2
(x) p-ClC6H4 CH2 CH(CH3)2
(xi) m-ClCH2C6H4 C(CH3)3
(xi) o-Br-C6H4CH(CH3)CH2CH3
Answer:
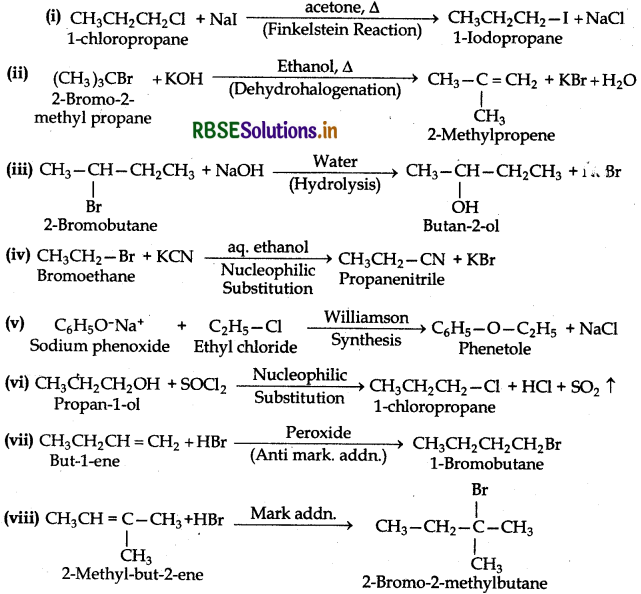
(i) 2-Chloro-3-methylbutane, 2° alkyl halides,
(ii) 3-Chloro-4-methylhexane, 2° alkyl halide,
(iii) 1-Iodo-2, 2-dimethylbutane, 1° alkyl halide,
(iv) 1-Bromo-3, 3-dimethyl1-phenylbutane, 2° benzylic halide,
(v) 2-Bromo-3methylbutane, 2o alkyl halide,
(vi) 1-Bromo-2--ethyl-2methylbutane, 1° alkyl halide,
(vii) 3-Chloro-3-methylpentane, 3° alkyl halide,
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide,
(ix) 4-Bromo-4-methylpent-2-ene, allylic halide,
(x) 1-Chloro-4-(2-methylpropyl) benzene, aryl halide,
(xi) 1-Chloromethyl-3-(2, 2-dimethylpropyl) beznene, 1° benzylic halide,
(xii) 1-Bromo-2-(1-methylpropyl)benzene or 1-bromo-2-sec-butylbenzene, aryl halide.

Question 10.2.
Give the IUPAC names of the following compounds:
(i) CH3CH(CI)CH(Br)CH3
(ii) CHF2CBrCIF
(iii) ClCH2C = C CH2 Br
(iv) (CCl3)3 CCl
(v) CH3C(p-ClC6H4)2CH(Br)CH3
(vi) (CH3)3CCH = C(Cl)C6H4I-p
Answer:
(i) 2-Bromo-3-chlorobutane,
(ii) 1-Bromo-1-chloro1, 2, 2-trifluoroethane,
(iii) 1-Bromo-4-chlorobut-2-yne,
(iv) 2-(Trichloromethyl)-1,1,1,2,3,3,3-heptachloropropane,
(v) 2-bromo, 3-bis (4-chlorophenyl) butane,
(vi) 1-chloro-1(4-iodophenyl)-3, 3-dimethylbut-1-ene.
Question 10.3.
Write the structures of the following organic halogen compounds:
(i) 2-Chloro-3-methylpentane
(ii) p-Bromexchlorobenzene
(iii) 1-Chloro-l-ethylcyclohexane
(iv) 2(2-Chlorophenyl)-1-iodooctane
(v) Perfluorobenzene
(vi) 4-tert-Butyl-3-iodoheptane
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene.
Answer:
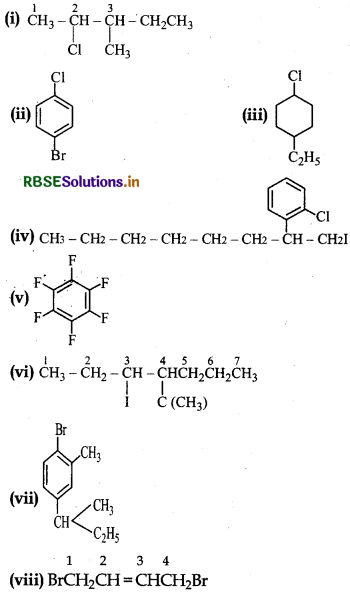
Question 10.4.
Which one of the following has highest dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4
Answer:
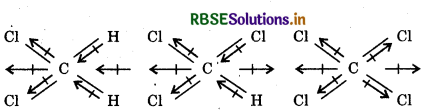
(i) In dichloromethane (CH2Cl2), the resultant dipole moment of two C-Cl and two C-H bonds reinforce another. The molecule has maximum dipole moment (μ) = 1 62D.
(ii) Inchloroform (CH3CI) the resultant dipole moment of two C-abonds is opposed by resultant dipole moment of C-Cland C-H bonds. Since the latter resultant dipole moment is smaller, the molecule as a whole has dipole moment (μ) = 103 D.
(iii) Tetrachloromethane (CCl4) is a symmetrical molecule and has dipole moment (μ) = 0. Thus, dichloromethane (CH2Cl) has the maximum dipole moment value.
Question 10.5.
A hydrocarbon does not react with chlorine but gives a single monochloro compound, C5H9Cl in bright sunlight. Identify the hydrocarbon.
Answer:
- The hydrocarbon with molecular formula C5H10 is either an alkene or cycloalkane.
- Since the hydrocarbon does not react with chlorine (Cl2) in the dark, it cannot be an alkane but must be a cycloalkane.
- Since the compound reacts with Cl2 in presence of sunlight, to give a single monachloro-compound, C5H9Cl, therefore all the ten H-atoms of the cycloalkane must be equivalent. Thus, the cycloalkane is cyclopentane.
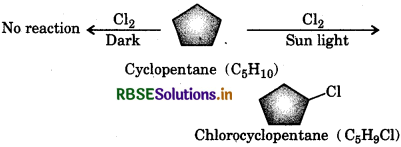
Question 106.
Write the isomers of the compound having formula C4H9Br.
Answer:
The given compound has the following structural isomers:
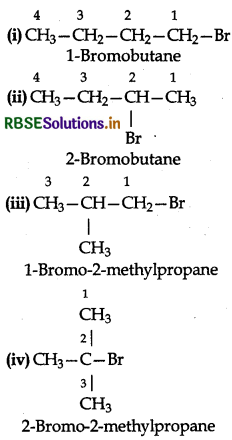
Question 10.7.
Write equations for the preparation of 1lodobu tane from:
(a) Butan-1-ol (2) 1-Chlorobutane (3) But-l-ene
Answer:
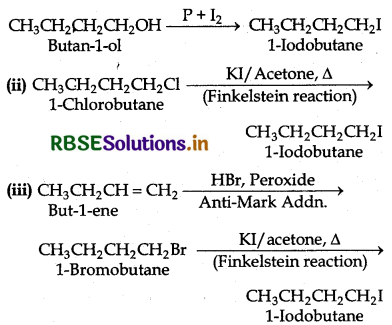
Question 10.8.
What are the ambident nucleophiles? Explain with an example.
Answer:
Núcleophiles which can attack through two different sites are known as ambident nucleophiles. For example, cyanide lon is a resonance hybrid of the following two structures:
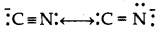
It can attack either through carbon to form cyanides or nitriles or through nitrogen to form isocyanides or carbylaminss.

Question 10.9.
Which compound in each of the following pairs will reacts faster in SN2 reaction with -OH?
1. CH3Br or CH3I
2. (CH3)3CCI or CH3Cl
Answer:
- CH3-I will reacts faster in SN2 reaction than CH3-Br because I- -ion is a better leaving group than Br- ion
- CH3Cl will reacts faster in SN2 reaction than (CH3)3CI because of less steric hindrance.
Question 10.10.
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ctharde in ethanol and identify the major alkene.
(i) 1-Bromo-1-methylcyclohexane,
(ii) 2-Chloro-2-methyl butane,
(iii) 3-Bromo-2,2 rimethylpentane.
Answer:
(i) In 1-bromo-1-methylcyclohexane the β - hydrogen atoms on either side of the Bratom are equivalent. therefore only 1-alkene is formed.
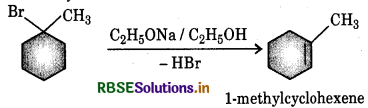
(ii) 2-Chloro-2-methylbutane has two different sets of equivalent β-hydrogen atoms and hence it can give twoalkenes I and II). But according to Say rule, more highly substituted allene being more stable is the main product.
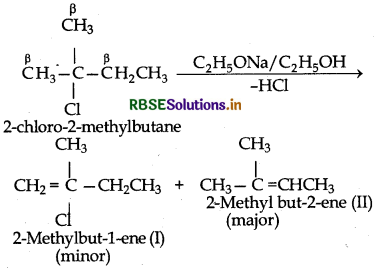
(iii) 3-Bromo-2, 2, 3-trimethylpentane has two differentsites of β - hydrogen atoms and hence, it can give two alkanes (I and II) But according to Saytzeff rulemore highly substituted alkene (II) being more stable is the major product
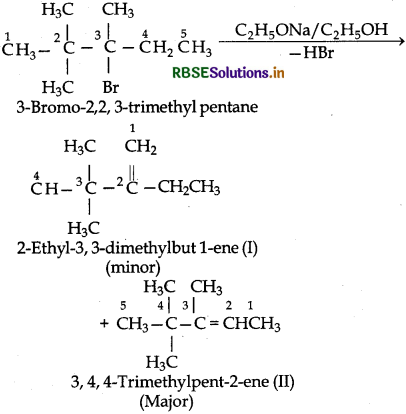
Question 10.11.
How will you bring about the following conversions?
(i) Ethanol to but-1-yne
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-1-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl
Answer:
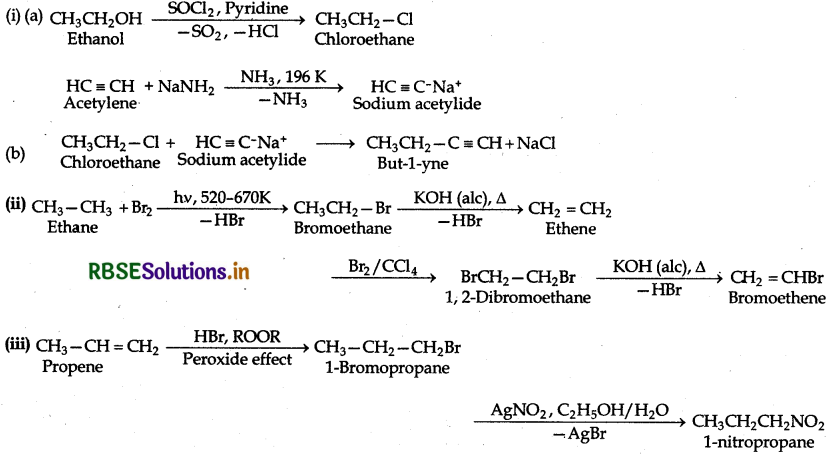
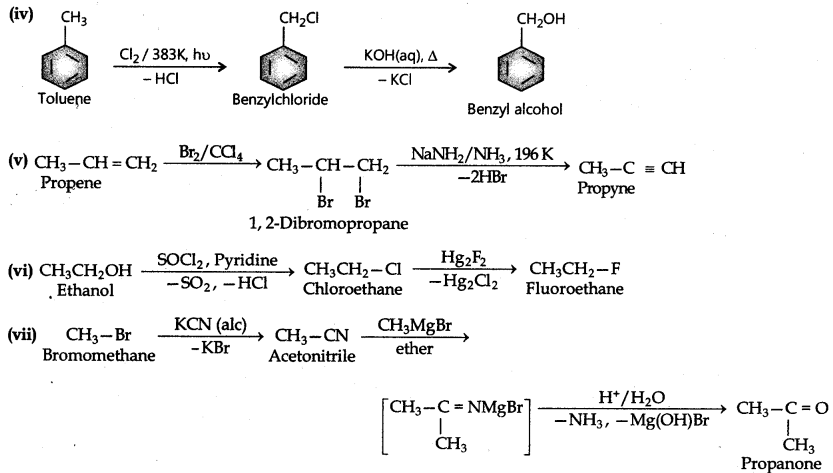
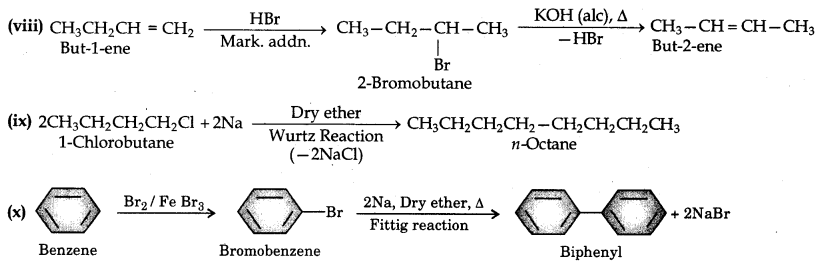
Question 10.12.
Explain why:
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) Alkyl halides, though polar are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Answer:
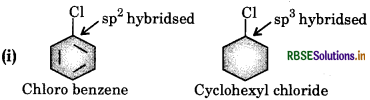
The polarity of C- bond in chlorobenzene is less than that of same bond in cyclohexyl chloride because of carbon atom involved in chlorobenzene is more electronegative due to greater scharacter as compared to the carbon atom in cyclohexyl chloride with lesser character. Therefore, the dipole moment of chlorobenzene is less than cyclohexyl chloride.
(ii) Alkyl halides or halalkanes are polar molecules, therefore their molecules are held together with dipole-dipole interactions. In H2O the molecules are held together by intermolecular H-bonding Since the new forces of attraction between H2O and haloalkane molecules are weaker than the forces of attraction already existing between haloalkane hakalkane molecules and water water molecules, therefore haloalkans or alkyl halides are immiscible with water.
(iii) Grignard reagents (R-Mg-X) should be prepared under anhydrous conditions because these are readily decomposed with water to give alkanes

That is why the used as solvent in the preparation of Grignard reagents in completely anhydrous in nature.

Question 10.13.
Give the uses of freon-12, DDT, carbon tetrachloride and iodoform
Answer:
Uses of freon 12 (dichlorodifluoromethane):
In refrigerators and air conditioners, freon 12 is used as a refrigerant. Also, freon 12 finds applications in aerosol spray propellants such as body sprays and hair sprays. Since, freon 12 damages ozone layers, it’s use should not be allowed.
Uses of DDT (p,p-dichlorodiphenyltrichloroethane):
DDT is an insecticide and is effective against mosquitoes and lice. But it is banned in some countries due to its harmful effects.
Uses of carbon tetrachloride:
Carbon tetrachloride is used in the manufacture of refrigerants and propellants for aerosol cans. In the industrial preparation of pharmaceuticals, carbon tetrachloride is used as a solvent. In the synthesis of chlorofluorocarbons and other chemicals carbon tetrachloride is used as feedstock. Earlier (upto 1960’s), carbon tetrachloride was used as a cleansing fluid, a fire extinguisher and a degreasing agent.
Uses of Iodoform:
When iodoform comes in contact with skin, it liberates free iodine. Hence, iodoform was used as an antiseptic. Nowadays, other formulations containing iodine are used in place of iodoform due to the objectionable smell of iodoform. Molecules of Freon 12, carbon tetrachloride and iodoform, mostly contain carbon and halogen as major elements. Whereas, molecules of DDT contain more number of carbon and hydrogen atoms than chlorine atoms.
Question 10.14
Write the structure of the major organic product in each of the following reactions:
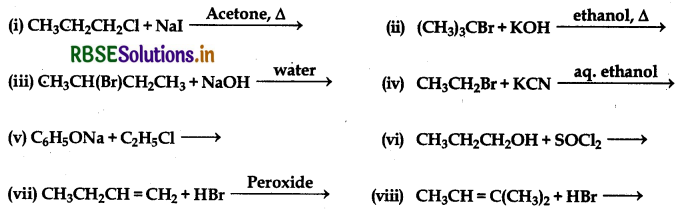
Answer:
Question 10.15.
Explain the following reaction:
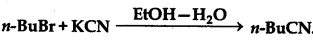
Answer:
KCN is a resonance hybrid of two contributing structures.
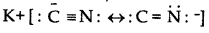
The structures show that the cyanide ion is an ambident nucleophile and the nucleophile attack is possible either through C-atomir Natom resulting the formation of cyanides or nitrilesor isocyanides or cartylamines respectively. In the given case, in the presence of polar solvent, KCN readily lonises to give ions. The nucleophile attack take place through atom and not through N-atomas C-Cbond is more stable than C-N bond.
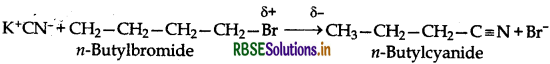

Question 10.16.
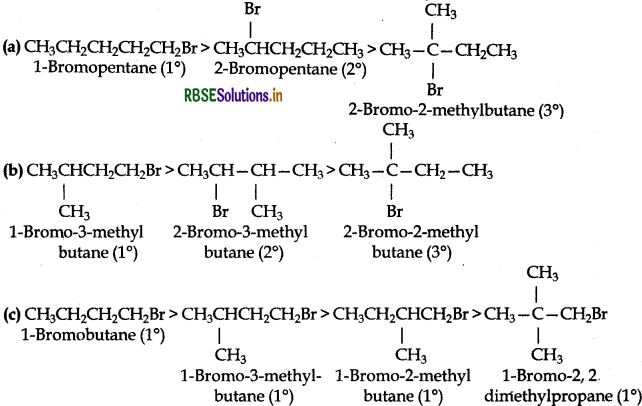
Arrange the compounds of each set in order of decreasing reactivity towards (SN2) displacement.
(a) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(b) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
(c) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Answer:
The reactivity of a particular haloalkane towards SN2 reaction is inversely proportional to the steric hindrance around the C-atom involved in C-Xbond. More the steric hindrance lesser will be the reactivity of haloalkanes towards SN2 reaction. In the light of this, the decreasing order of reactivity in all the three given cases in as follow:
Question 10.17.
Out of C6H5CH2Cl and C6H5CH(Cl)C6H5 which is more easily hydrolysed by aqueous KOH?
Answer:
C6H5CH2Cl is a primary (1 °) aralkyl halide while C6H5CH(Cl)C6H5 is a secondary (2 °) aralkyl halide.
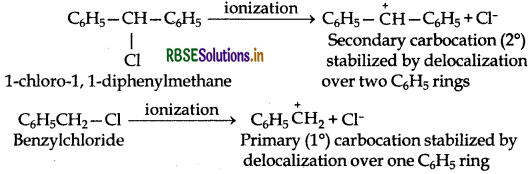
In SN1 reactions, the reactivity depends upon the stability of carbocations. Since the carbocation C6H5CHC6H5 is more stable than C6H5CH2 therefore C6H5CHClC6H5 gets hydrolysed more easily than C6H5CH2Cl under Seconditions.
Question 10.18.
p Dichlorobenzene has higher melting point and lower solubility than those of o-and m-isomers. Discuss.
Answer:
The given three isomers are position isomers which differ in the relative positions of the Cl- atom in the ring.
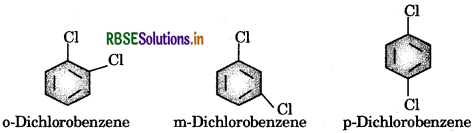
p - isomer is more symmetrical thane σ - and m - isomers. This means that in a crystal lattice the p owers are more closely packed as compared to s-and-isomers. As a result, it has higher melting point and lower solubility as compared to σ- and m-isomers. As a result, it a has higher melting point and lower solubility as compared to σ - and m - isomers.
Question 10.19.
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-2-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylehanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3,4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyl iodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenyl isocyanide.
Answer:
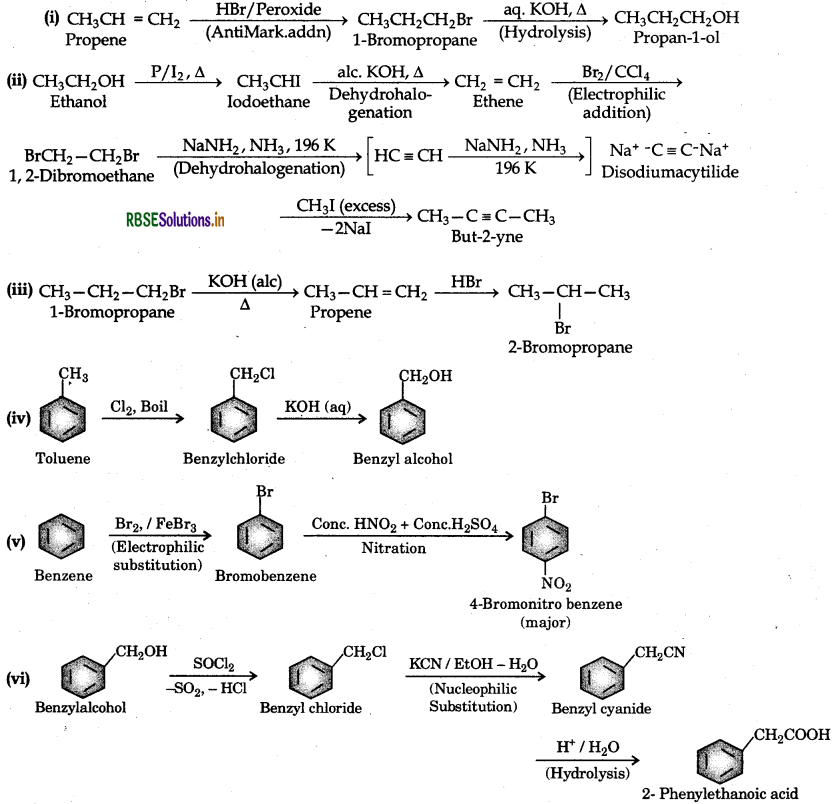
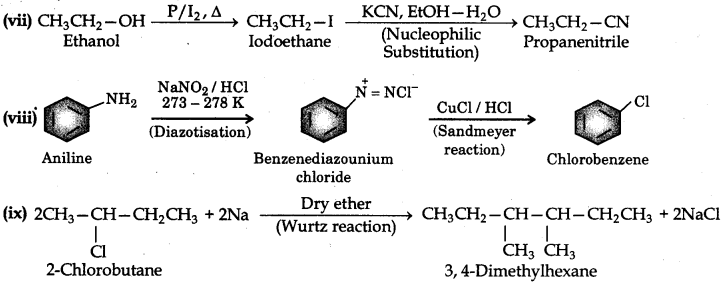
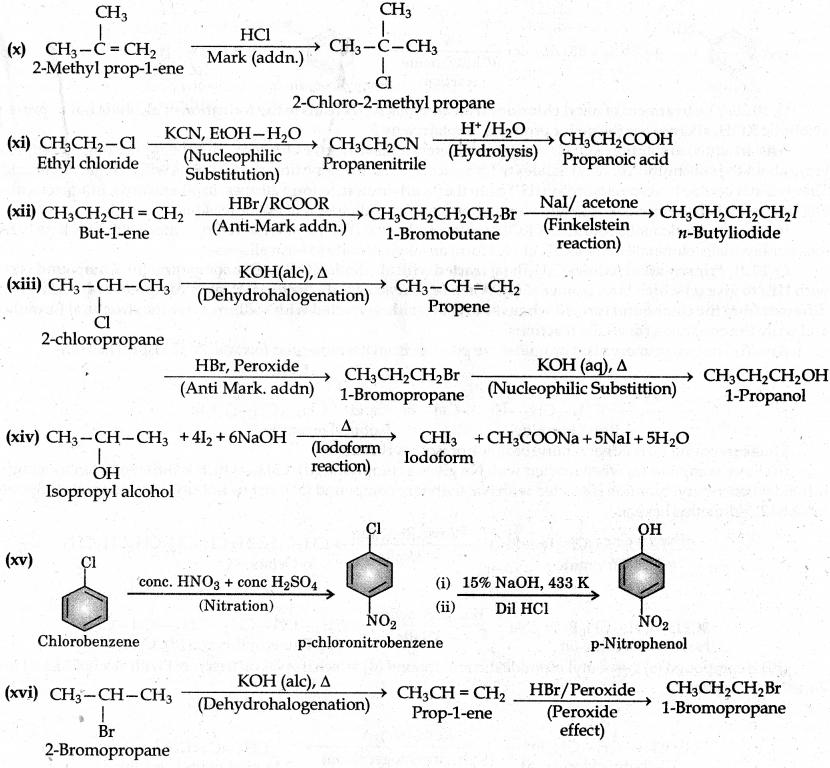
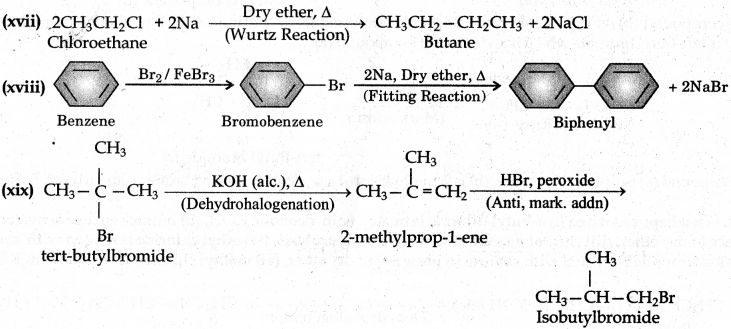
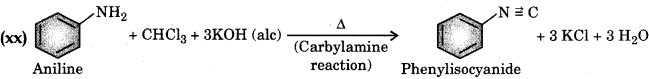
Question 10.20.
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH, alkanes are the major products. Explain why?
Answer:
In aqueous solution KOH is almost completely ionised to give OH- ions. They being a strong nucleophile will bring about the substitution of alkyl halides to form alcohols. At the same time the OH- ions will be highly hydrated also. They will not be able to abstract a proton (H+) from the B-carbon atom to form alkanes. In other words, in aqueous medium OH- ions with behave as weak base and elimination leading to alkanes will not be feasible. In contrast, an alcoholic solution of KOH contains alkoxide (RO-) ions which being a much stronger base than OH- ions perferentially eliminate of molecule of HCl form an alkyl chloride to form alkenes.

Question 10.21.
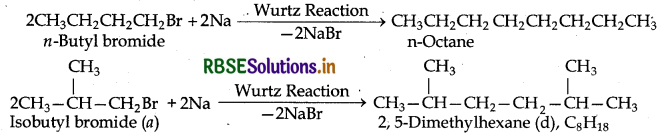
Primary alkyl halides C4H9Br (a) reacted with alcoholic KOH to give compound (b) Compound is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with Na metal it gives compound (d) C9H18 is which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Answer:
(i) The two primary alkyl bromides are possible from the molecular formula (a) C4H9Br. These are:

Thus compound (a) is either n-butyl bromide or isobutyl bromide.
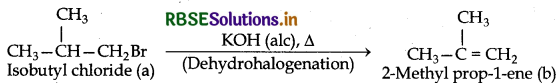
(ii) Since compond (a) when reacted with Na gives a compound (d) C8H18 which is different from the compound formed when n-butyl bromide is reacted with Na, therefore compound (a) must be isobutyl bromide and compound (d) must be 2,5-dimethyl hexane.
(iii) If compound (a) is isobutyl bromide then compound (d) which it gives on treatment with alcoholic KOH must be 2-methyl-1-propene.
(iv) The compound (b) on treatment with HBr gives compound (c) according to Markownikov's rule. Therefore, compound (c) is tert-butyl bromide which is an isomer
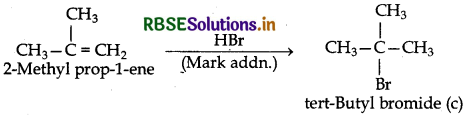
Thus, compound (a) is isobutyl bromide, (b) is 2-methylprop-l-ene, (c) is tert-butyl bromide and (d) is 2,5-dimethyl hexane.
Question 10.22.
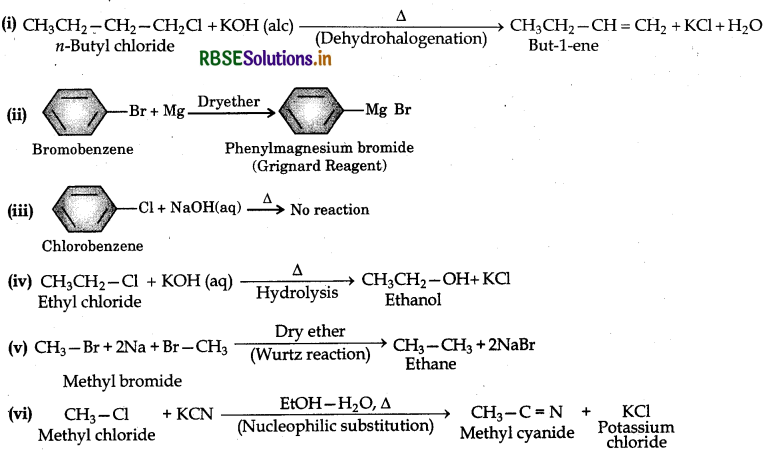
What happens when (i) n-butylchloride is treated with alcoholic KOH, (ii) bromobenzene is treated with Mg in presence of dry ether, (iii) chlorobenzene is subjected to hydrolysis, (iv) ethyl chloride is treated with aqueous KOH, (v) methyl bromide is treated with sodium in presence of dry ether, (vi) methyl chloride is treated with KCN?
Answer:

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम