RBSE Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
Rajasthan Board RBSE Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Physics in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Physics Important Questions for exam preparation. Students can also go through RBSE Class 11 Physics Notes to understand and remember the concepts easily.
RBSE Class 11 Physics Solutions Chapter 11 Thermal Properties of Matter
RBSE Class 11 Physics Thermal Properties of Matter Textbook Questions and Answers
Question 11.1.
The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales.
Solution:
Relation between Kelvin and Celsius scales is
TC + 273.15 = TK ....................(1)
where, TK = Temperature in Kelvin and
TC = Temperature in Celsius
Putting the respective values in equation (1)
For Neon:
TC = (24.57 - 273.15)°C
TC = -248.58°C
For CO2:
TC = (216.55 - 273.15)°C
TC = -56.60°C
Relation between Kelvin and Fahrenheit scales
⇒ \(\frac{T_F-32}{180}=\frac{T_K-273.15}{100}\)
For Neon:
TF = \(\frac{180}{100}\)(TK - 273.15) + 32
TF =(24.57 - 273.15) \(\frac{18}{10}\) + 32
TF = 415.44°F
For CO2:
TF = \(\frac{18}{10}\)(216.55 - 273.15) + 32
TF = -69.88°C

Question 11.2.
Two absolute scales A and B have triple points of water defined to be 200 A and 350 B. What is the relation between TA and TB?
Solution:
Triple point of water on scale A = 200 A
Triple point of water on scale B = 350 B
According to the question
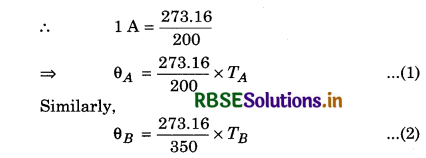
Since θA and θB represent the triple point of water.

Question 11.3.
The electrical resistance in ohms of a certain thermometer varies with temperature according to the approximate law:
R = R0 [1 + α x 10-3 (T - T0)]
The resistance is 101.6 Ω at the triple point of water, 273.16 K and 165.5 Ω at the normal melting point of lead (600.5 K). What is the temperature when the resistance is 123.4 Ω?
Solution:
Given: R = [1 + α x 10-3(T - T0)] ....................(1)
RTr = 101.6 Ω;RN = 165.5 Ω
TN = 600.5 K
To find, T = ? at R = 123.4 Ω
Let R0 be the resistance at temperature T0. From the given equation (1),
R0 [1 + 0.005 (273.16 - T0)] = 101.6 Ω .................(2)
Also resistance at melting point of lead
R = 165.5 Ω
⇒ R0 [1 + 0.005 (600.5 - T0)] = 165.5 ......................(3)
Dividing equations (2) by (3),
⇒ \(\frac{1+0.005\left(273.16-T_0\right)}{1+0.005\left(600.5-T_0\right)}=\frac{101.6}{165.5}\)
⇒ \(\frac{1+1.366-5 T_0 \times 10^{-3}}{1+3.003-5 T_0 \times 10^{-3}}\) = 0.614
⇒ T0 = -50.33 K
Substituting for T0 in equation (2), we get
R0 [1 + 0.005 (273.16 - (-50.33)] = 101.6
⇒ 2.617 R0 = 101.6
R0 = \(\frac{101.6}{2.617}\) = 38.823 Ω
Now, resistance at the unknown temperature T is 123.4 Ω.
Substituting for R1, R2 and T0 in equation (1),
⇒ 123.4 = 38.823 [1 + 5 x 10-3 (T - (-50.33)]
⇒ 123.4 = 38823 + 0.194T + 9.71
On simplifying
⇒ T = 385.3 K
Question 11.4.
Answer the following:
(a) The triple point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and the boiling point of water as standard fixed points (as was originally done in the Celsius scale)?
(b) There were two fixed points in the original Celsius scale as mentioned above which were assigned the number 0°C and 100°C respectively. On the absolute scale, one of the fixed points is the triple-point of water, which on the Kelvin absolute scale is assigned the number 273.16 K. WTiat is the other fixed point on this (Kelvin) scale?
(c) The absolute temperature (Kelvin scale) T is related to the temperature tc on the Celsius scale by
tc = T - 273.15
Why do we have 273.15 in this relation and not 273.16?
(d) What is the temperature of the triple-point of water on an absolute scale whose unit interval size is equal to that of the Fahrenheit scale?
Solution:
(a) This is because of the fact that the triple point of water occurs at a unique point, where there exists unique set of values of pressure and volume. On the contrary the melting and boiling points do not have unique set of values of pressure and volume.
(b) The other fixed point on the Kelvin absoute scale is the absolute zero itself.
(c) 273.16 K is the triple point of water. The relation gives the temperature on absolute scale which corresponds to 0.01°C (on putting the value of Ttr)
(d) The size of degree on centrigrade and absolute scales are equal and difference of temperature between melting point of ice and steam point have been divided into 100 divisions. However, this temperature range has been divided into 180 divisions on Fahrenheit scale.
⇒ 1° on abslute scale = \(\frac{180}{100}\)
= 1.8 times the size of degree on Fahrenheit scale.
⇒ Temperature of triple point of water (273.16 K) on absolute scale haveing a degree equal to 1.8.
= 273.16 x 1.8
T'= 491.69
Question 11.5.
Two ideal gas thermometers A and B use oxygen and hydrogen respectively. The following observations are made:
|
Temperature |
Pressure thermometer A |
Pressure thermometer B |
|
Triple point of water |
1.250 x 105 Pa |
0.200 x 105 Pa |
|
Normal melting point of sulphur |
1.797 x 105 Pa |
0.287 x105 Pa |
(a) What is the absolute temperature of normal melting point of sulphur as read by thermometers A and B?
(b) What do you think is the reason for the slightly different answers from A and B'? (The thermometers are not faulty). What further procedure is needed in the experiment to reduce the discrepancy between the two readings?
Solution:
(a) Let T be the melting point of sulphur for water Ttr = 273.16 K
For thermometer A
T = \(\frac{P}{P_{t r}}\) x 273.16
Putting respective values
T = \(\frac{1.797 \times 10^5}{1.250 \times 10^5}\) x 273.16
T = 392.69 K
For thermometer B
T = \(\frac{P}{P_{t r}}\) x 273.16 = \(\frac{0.287 \times 10^5}{0.200 \times 10^5}\) x 273.16
T = 391.98 K
Difference in two values is due to the fact that oxygen and hydrogen do not behave strictly as perfect gases.
(b) The discrepancy arises because the gases are not perfectly ideal. To reduce the discrepancy, readings should be taken for lower temperature and lower pressures and the plot between temperature measure in the limit pressure tends to zero, when the gases approach ideal gas behaviour.

Question 11.6.
A steel tape 1 m long is correctly calibrated for a temperature of 27.0°C. The length of a steel rod measured by this tape is found to be 63.0 cm on a hot day when the temperature is 45.0°C. What is the actual length of the steel rod on that day? What is the length of the same steel rod on a day when the temperature is 27.0°C? Coefficient of linear, expansion of steel = 1.20 x 10-5 K-1.
Solution:
If l1 and l2 be the length of the steel tape at temperature T1 and T2, then
l2 = l1(l + α∆T) ......................(a)
where, ∆T = T2 - T1
At T = 27° C, steel tape shows correct magnitude.
At T = 45° C, the size of 1 cm as marked on the tape increases
Let it increases to l'.
Then l' = 1 cm x (1 + α∆T) ..................(b)
Here, a = 1.20 x 10-5°C-1
Putting respective values in equation (b)
l' = [1 + 1.20 x 10-5 x (45 - 27)]
l' = 1.000216 cm
The size of the rod at 45°C is 63 cm
⇒ Actual length of the rod at 45°C
= 63 x 1.000216
LA = 63.0136 cm
The length of the rod on a day when the temperature is 27°,
= 63 x 1 = 63 cm
Question 11.7.
A large steel wheel is to be fitted on to a shaft of the same material. At 27°C, the outer diameter of the shaft is 8.70 cm and the diameter of the central hole in the wheel is 8.69 cm.The shaft is cooled using ‘dry ice’. At what temperature of the shaft does the wheel slip on the shaft? Assume coefficient of linear expansion of the steel to be constant over the required temperature range ; αsteel = 1.20 x 10-5 K-1.
Solution:
Here, T1 = 27° C = 27 + 273 = 300 K
Length at temperature, T1K = lT1 = 8.70 cm
Length of temperature, T2K = lT2 = 8.69 cm
Change in length = lT2 - lT1 = lT1 α (T2 - T1)
or 8.69 - 8.70 = 8.7 x (1.2 x 10-5) x (T2 - 300)
or T2 - 300 = \(-\frac{0.01}{8.70 \times 1.2 \times 10^{-5}}\) = -95.8
or T2 = 300 - 95.8 = 204.2 K
= - 68.8°C
Question 11.8.
A hole is drilled in a copper sheet. The diameter of the hole is 4.24 cm at 27.0°C. What is the change in the diameter of the hole when the sheet is heated to 227°C? Coefficient of linear expansion of copper = 1.70 x 10-5°C-1.
Solution:
D1 = 4.24 cm
D1 = 4.24 x 10-2 m at T1 = 27.0°C
D2 = ? at P2 = 227°C
αcu = 1.70x 10-5°C-1
Here, the difference in temperature
∆T = T2 - T1 = 227 - 27
∆T = 200°C
Coefficient of superficial expansion, ß = 2α
⇒ ßcu = 2 x 1.70 x 10-5 x C-1 = 3.4 x 10-5°C-1
Surface area of hole at T2 = 227°C.
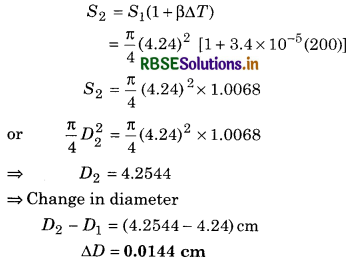
Question 11.9.
A brass wire 1.8 m long at 27°C is held taut with little tension between two rigid supports. If the wire is cooled to a temperature of -39°C, what is the tension developed in the wire, if its diameter is 2.0 mm?
Coefficient of linear expansion of brass = 2.0 x 10-5° K-1; Young’s modulus of brass = 0.91 x 1011 Pa.
Solution:
Given: L = 1.8 m, T1 = 27°C
T2 = -39°C, F = ?, r = 1 mm = 10-3 m
α = 2 x 105°C-1, Y = 0.91 x 1011 N/m
From Y = \(\frac{F L}{a \Delta L}, ∆L = \frac{F L}{\alpha Y}\)
Also ∆L = αL∆T
∴ \(\frac{F L}{a Y}\) = αL∆T
⇒ F = -3.8 x 102 N
Negative sign shows that the force is inwards due to contraction of wire.
(Please note that superficial expansion at the joint has been neglected because its effect will be negligible.)
Question 11.10.
A brass rod of length 50 cm and diameter 3.0 mm is joined to a steel rod of the same length and diameter. What is the change in length of the combined rod at 250°C, if the original lengths are at 40.0°C? Is there a ‘thermal stress’ developed at the junction? The ends of the rod are free to expand (Coefficient of linear expansion of brass = 2.0x 10-5K-1, steel = 1.2 x 10-5 K-1).
Solution:
Given: L1 = 50 cm
α1 = 2.10 x 10-5
∆T = (250 - 40) = 210°C
We know that
⇒ ∆L1 = L1α1∆T
⇒ ∆L1 = 50 x 2.10 x 10-5 x 210 = 0.220 cm
∆L2 = L2α2∆T
= 50 (1.2 x 10-5) (250 - 40)
= 0.126 cm
∴ Change in length in the combined rod.
∆L = ∆L + ∆L2 = 0.220 + 0.126
∆L = 0.346 cm
No thermal stress is developed at the junction since the rod is free to expand.

Question 11.11.
The coefficient of volume expansion of glycerine is 49x 10-5K-1. What is the fractional change in its density for a 30°C rise in temperature?
Solution:
Given: γ = 49 x 10-5°C-1
∆T = 30°C
As V' = V + ∆V = V (1 + γ∆T)
∴ V'= V (1 + 49 x 10-5 x 30) = 1.0147 V
Since ρ = \(\frac{m}{V}\), ρ' = \(\frac{m}{V^{\prime}}=\frac{m}{1.0147 \mathrm{~V}}\)
ρ' = 0.98559 ρ
x, fractional change in density
x = \(\frac{\rho-\rho^{\prime}}{\rho}=\frac{\rho-0.955 \rho}{\rho}\) = 0.0145
x = 0.0145 or x = 1.5 x 10-2
Question 11.12.
A 10 kW drilling machine is used to drill a bore in a small aluminium block of mass 8.0 kg. How much is the rise in temperature of the block in 2.5 minutes, assuming 50% of power is used up in heating the machine itself or lost to the surroundings? Specific heat of aluminium = 0.91 J g-1K-1.
Solution:
Work done by the drilling machine per second = 10 kW
∴ Work done in 2.5 minutes (150 s)
= 10 x 103 x 150 = 1.5 x 106 J
Out of this, only 50% is used to heat the aluminium block. Let s be specific heat of aluminium block and ∆T be the rise in its temperature.
∴ ms∆T = 50% of 1.5 x 106 J
= 0.75 x 106 J
or ∆T = \(\frac{0.75 \times 10^6}{m s}\)
Now, m = 8.0 kg = 8000 g
and s = 0.91 J/g°C
∆T = \(\frac{0.75 \times 10^6}{8000 \times 0.91}=\frac{750}{8 \times 0.91}\) = 103.2°C
∴ The rise in temperature of the block in 2.5 minutes is 103.2°C
Question 11.13.
A copper block of mass 2.5 kg is heated in a furnace to a temperature of 500°C and then placed on a large ice block. What is the maximum amount of ice that can melt? (Specific heat of copper = 0.39 J g-1K-1; heat of fusion of water = 335 J/g).
Solution:
When the cooper block is kept on ice, it cools from 500°C to 0°C.
The amount of heat lost by copper
= m x s∆T = 2.5 x 103 x 0.39 x 500 J.
This heat is utilised in melting ice. If M is mass of ice melted, it will required M x L amount of heat, where L is latent heat of fusion of ice.
∴ 2.5 x 103 x 0.39 x 500 = M x 335
or M = \(\frac{2.5 \times 10^3 \times 0.39 \times 500}{335}\) g
= \(\frac{2.5 \times 500 \times 0.39}{335}\) kg = 1.4552 kg
∴ The maximum amount of ice that can melt is 1.46 kg provided heat energy of the copper block is not allowed to be radiated away to the surroundings.
Question 11.14.
In an experiment on the specific heat of a metal, a 0.20 kg block of the metal at 150°C is dropped in a copper calorimeter (of water equivalent 0.025 kg) containing 150 cm of water at 27°C. The final temperature is 40°C. Compute the specific heat of the metal. If heat losses to the surroundings are not negligible, is your answer greater or smaller than the actual value for specific heat of the metal?
Solution:
Here, heat lost by the block of metal
= m x s x (150 - 40) = 0.20 x s x 110 calorie
Heat gained by calorimeter + Water
= (0.025 + 0.150) x 103 x (40 - 27)
= 0.175 x 13 x 103
(∵ Mass of water = 0.150 kg and specific heat capacity of water = 103 cal/kg °C)
Now, heat lost = heat gained
∴ 0.20 x s x 110 = 0.175 x 13 x 103
or s = \(\frac{0.175 \times 13 \times 10^3}{0.2 \times 110}\)
or s = 0.103 x 103 cal/kg°C
= 0.103 x 4.2 J/g°C = 0.43 J/g°C
∴ Specific heat of metal is 0.43.
If some heat is lost to the surroundings, the final temperature will be less than 40°C and the rise in temperature of water would be less than 13°C (= 40°C - 27°C). Therefore, specific heat of metal would be found smaller than 0.43.
Question 11.15.
Given below are observations on molar specific heats at room temperature of sorpe common gases.
|
Gas |
Molar specific heat (Cv ) (cal mol-1 K-1) |
|
Hydrogen |
4.87 |
|
Nitrogen |
4.97 |
|
Oxygen |
5.02 |
|
Nitric Oxide |
4.99 |
|
Carbon monoxide |
5.01 |
|
Chlorine |
6.17 |
The measured molar specific heats of these gases are markedly different from those for monoatomic gases. Typically, molar specific heat of a monatomic gas is 2.92 cal/mol K. Explain this difference. What can you infer from the somewhat larger (than the rest) value for chlorine?
Solution:
All the gases listed in the table are diatomic gases. Whereas monoatomic gases have three degrees of freedom, a molecule of a diatomic gas, besides having three degrees of freedom can have rotational and vibrational degrees of freedom also. In other words, a molecule of a diatomic gas can rotate about an axis. It can also vibrate about an axis joining the two atoms. Thus, a diatomic molecule will have translational, rotational and vibrational energies. Now, to raise the temperature of a diatomic gas, heat is to be supplied not only to provide translational energy but also rotational and vibrational energies. Hence, molar specific heat of a diatomic gas is greater than that for monoatomic gas. The higher value of molar specific heat of chlorine than that of hydrogen, nitrogen, oxygen etc. shows that in case of chlorine molecules, there are not only rotational modes but vibrational modes also present at the room temperature. Vibrational modes are not present at the room temperature for other gases.

Question 11.16.
A child running a temperature of 101°F is given an antipyrin (i. e., a medicine that lowers fever) which causes an increase in the rate of evaporation of sweat from his body. If the fever is brought down to 98°F in 20 minutes, what is the average rate of extra evaporation caused by the drug? Assume the evaporation mechanism to be the only way by which heat is lost. The mass of the child is 30 kg. The specific heat of human body is approximately the same as that of water, and latent heat of evaporation of water at that temperature is about 580 cal/g.
Solution:
The fall in temperature of the body in 20 min
= 101 - 98 = 3°F = 3 x \(\frac{5}{9}\) = \(\frac{15}{9}\)°C
∴ Fall of temperature per minute
= \(\frac{15}{9} \times \frac{1}{20}=\frac{1}{12}\)°C
∴ The amount of heat lost by the body per minute = Mass of the body x Sp. heat x Decrease in temperature per minute
= 30 x 103 x 1 x \(\frac{1}{12}\) cal.
If M is mass of the extra sweat evaporated per minute, the heat of vapourisation taken from the body = 580 x M.
∴ 580 x M = \(\frac{30 \times 10^3}{12}\)
or M = \(\frac{30 \times 10^3}{12 \times 580}\) = 4.31 g
∴ Average rate of extra evaporation caused by the drug is 4.31 g/min
Question 11.17.
A ‘thermacole’ icebox is a cheap and efficient method for storing small quantities of cooked food in summer in particular. A cubical icebox of side 30 cm has a thickness of 5.0 cm. If 4.0 kg of ice is put in the box, estimate the amount of ice remaining after 6 h. The outside temperature is 45°C, and coefficient of thermal conductivity of thermacole is 0.01 Js-1m-1K-1. [Heat of fusion of water = 335 x 103 J/kg].
Solution:
Let x be the amount of ice remaining after 6 hours. Then (4.0 - x) kg of ice requiring (4.0 - x) x 335 x 10 J of heat conducted from outside to the inside of the box remains in 6 hours (∵Heat of fusion of water = 335 x 103 J/kg).
Here, Q = (4.0 - x) x 335 x 103 J
t = 6 hours = 6 x 60 x 60 = 2.16 x 104 s
A = 6 x (30 x 30 cm2) = 5400cm2 = 0.54 m2.
(There are six faces of the box through which heat is conducted.)
l = 5.0 cm = 5 x 10-2 m
θ1 = 45° C, θ2 = 0°C and K = 0.01 J/s m°C
Using Q = \(\frac{K A\left(\theta_1-\theta_2\right)}{l}\)t
and substituting the values, we get
(40 - x) x 335 x 103
= \(\frac{0.01 \times 0.54 \times 45 \times 2.16 \times 10^4}{5 \times 10^{-2}}\)
On solving, x = 3.69 = 3.7 kg
∴ The amount of ice remaining in the box after 6 hours is 3.7 kg.
Question 11.18.
A brass boiler has a base area of 0.15 m2 and thickness 1.0 cm. It boils water at the rate of 6.0 kg/min when placed on a gas stove. Estimate the temperature of the part of the flame in contact with the boiler. Thermal conductivity of brass = 109 Js-1 K-1m-1. (Heat of vaporisation of water = 2256 x 103 J/kg).
Solution:
We know that flow of heat Q is given by
Q = \(\frac{K A\left(\theta_1-\theta_2\right)}{l}\)t
Now, the amount of water boiling per second
= \(\frac{6.0 \mathrm{~kg}}{\min }=\frac{6.0}{60}\) kg/s = 0.1 kg/s
and the amount of heat required per second
= 0.1 x 2256 x 103 = 2.256 x105 J.
Now this heat is being conducted by the boiler of base thickness 1.0 cm (1 = 1.0 cm = 10-2 m).The temperature of boiling water (inside of the boiler) θ2 = 100° C. The temperature θ1 of the part of the boiler in contact with flame is to be calculated.
Substituting the values, we get
2.256 x 105 = \(\frac{109 \times 0.15\left(\theta_1-100\right) \times 1}{10^{-2}}\)
or θ1 = 100 + \(\frac{2.256 \times 10}{1.09 \times 0.15}\)
= 100 + 137.99 = 237.99 = 238°C
∴ The required temperature is 238°C.
Question 11.19.
Explain why:
(a) A body with large reflectivity is a poor emitter.
(b) A brass tumbler feels much colder than a wooden tray on a chilly day.
(c) An optical pyrometer (for measuring high temperatures) calibrated for an ideal black body radiation gives too low a value for the temperature of a red hot iron piece in the open, but gives a correct value for the temperature when the same piece is in the furnace.
(d) The earth without its atmosphere would be inhospitably cold.
(e) Heating systems based on circulation of steam are more efficient in warming a building than those based on circulation of hot water.
Solution:
(a) A body with large reflectivity will be poor absorber of radiant energy. Poor absorbers are poor radiators and hence a body with large reflectivity will be poor radiator or emitter.
(b) Brass being a good conductor of heat readily conducts away body heat when it is touched by hand. Thus, it feels colder than a wooden tray which does not conduct body heat because it is a bad conductor of heat.
(c) When the red hot iron piece is in the oven, its temperature is given by E = σT4. But, if it is in the open having the
temperature T0, its energy is radiated according to E' = σ (T4 - T04). Hence, it gives too low a value for temperature.
(d) The atmosphere around earth acts like a one-way valve. It lets sun’s radiations (which are high frequency) come to earth (during day time); but does not let the radiation being emitted by earth during night go past it. Hence because of its atmosphere, greenhouse effect is observed over. Had it not been so, there would have continuous drain of energy from earth’s surface making it inhospitably cold.
(e) The amount of heat contained in steam at 100°C is more than that in water at 100°C because of latent heat of evaporation of water which is 540 cal/g. Thus, one gram of steam has 540 calories more heat than water at the same temperature. Hence heating system based on circulation of steam is more efficient.
Question 11.20.
A body cools from 80°C to 50°C in 5 minutes. Calculate the time it takes to cool from 60°C to 30°C. The temperature of the surroundings is 20°C.
Solution:
\(\frac{\text { Change in temp. }}{\text { Time interval }}=-K\left[T_{\text {mean }}-T_0\right]\) ..................(1)
∴ Step - I: (80°C to 50°C)
Tm = \(\frac{T_1+T_2}{2}=\frac{80+50}{2}=\frac{130}{2}\) = 65
T0 = Surrounding temp. = 20°C
From equation (1),
∆t = 5 min.
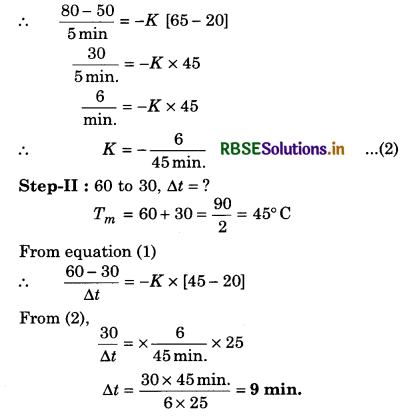
Additional Exercises
Question 11.21.
Answer the following questions based on the P-T phase diagram of carbon dioxide:
(i) At what temperature and pressure can the solid, liquid and vapour phases of CO2 co-exist in equilibrium?
(ii) What is the effect of decrease of pressure on the fusion and boiling point of CO2?
(iii) What are the critical temperature and pressure for CO2? What is their significance?
(iv) Is CO2 solid, liquid or gas at (a) -70°C under 1 atm, (b) -60°C under 10 atm, (c) 15°C under 56 atm?
Solution:
(i) At the triple point, i.e., temperature = -56.6°C and pressure = 5.11 atm, the vapour, liquid and the solid phase of carbon dioxide exist in equilibrium.
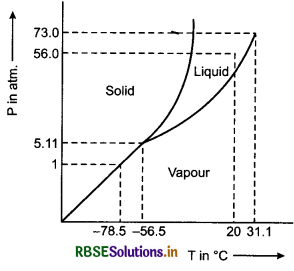
(ii) If the pressure decreases, both fusion and boiling points of carbon dioxide decrease.
(iii) The critical temperature and pressure of carbon dioxide are 31.1°C, it cannot be liquefied, howsoever large pressure we may apply to it.
(iv) (a) CO2 will be a vapour at -70°C at a pressure of 1 atm.
(b) CO2 will be solid at -60°C at a pressure of 10 atm; and
(c) It will be a liquid at 15°C at a pressure of 56 atm.
Question 11.22.
Answer the following questions based on the P-T phase diagram of CO2:
(i) CO2 at 1 atm pressure and temperature -60°C is compressed isothermally. Does it go through a liquid phase?
(ii) What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure?
(iii) Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature -65°C as it is heated up to room temperature at constant pressure.
(iv) CO2 is heated to temperature 70°C and compressed isothermally. What changes in its properties do you expect to observe?
Solution:
(i) Carbon dioxide will be solidified from the vapour without becoming liquid. This is so because the temperature -60°C lies in the region of solid phase.
(ii) CO2 condenses to solid without passing through the liquid phase the reason being the same as in (i) above.
(iii) Solid CO2 is first converted into the liquid and then because the horizontal line corresponding to 10 atm. pressure intersects the liquid and the vapour phase of the phase diagram of CO2.
(iv) Since 70°C is higher than the critical temperature of CO2, the gas cannot be liquified by pressure alone. It will remain in the vapour form. However, since the pressure is increasing, the behaviour of the gas will deviate more and more from the behaviour of a perfect gas.

- RBSE Class 11 Physics Important Questions Chapter 4 Motion in a Plane
- RBSE Solutions for Class 11 Physics Chapter 15 Waves
- RBSE Solutions for Class 11 Physics Chapter 14 Oscillations
- RBSE Solutions for Class 11 Physics Chapter 13 Kinetic Theory
- RBSE Solutions for Class 11 Physics Chapter 12 Thermodynamics
- RBSE Solutions for Class 11 Physics Chapter 10 Mechanical Properties of Fluids
- RBSE Solutions for Class 11 Physics Chapter 9 Mechanical Properties of Solids
- RBSE Solutions for Class 11 Physics Chapter 8 Gravitation
- RBSE Solutions for Class 11 Physics Chapter 7 System of Particles and Rotational Motion
- RBSE Solutions for Class 11 Physics Chapter 6 Work, Energy and Power
- RBSE Solutions for Class 11 Physics Chapter 5 Laws of Motion