RBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry
These comprehensive RBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry will give a brief overview of all the concepts.
RBSE Class 12 Chemistry Chapter 3 Notes Electrochemistry
→ Electrochemical cell: A cell or device which converts the chemical energy into electrical energy by redox reaction is called galvanic or galvani or voltic or electrochemical cell.
→ Half cell: Those cells in whcih oxidation or reductions reactions occurs are called half cells. If in the cell oxidation reaction prodceeds then it is called oxidation half cell or if reduction reaction proceeds then it is called reduction half cell.
→ Inert electrolyte: Those electrolytes, which do not show any chemical change during the reaction known as inert electrolyte. Example: KCl, KNO3, NH4NO3 etc.
→ Salt Bridge: It is U-shaped glas tube containing saturated solution of inert electrolyte.
→ Electrode potential: The electrical potential difference develops between metal and its ions in solution is called electrode potential.
→ Oxidation potential: If oxidation occurs on metal rod, then it is called oxidation potential.
→ Reduction potential: If reduction occurs on metal rod, then it is called reduction potential.
→ Standard reduction potential: The tendency of an electrode to accept electrons or to get reduced in called standard reduction potential.
→ Standard electrode potential: The standard electrode potential is defined as the electrode potential, when the concentration of the ions in solution is 1 mole per litre and temperature is 25°C (298 K).
→ Standard oxidation potential: The tendency of an electrode to lose electrons to get oxidised at 298 temperature and 1 mol L-1 concentration is called standard oxidation potential.

→ Electromotive force or cell potential: The difference between the electrode potentials of two electrodes of an electrochemical cell is called cell potential. It is called electromotive force or EMF of the cell if no current is allowed to flow in circuit.
→ Electrochemical series: A series in which various elements are placed in order of increasing reduction potential values is called electrochemical series.
→ Electrolytic conduction: The conduction of electricity through the aqueous or in the molten state of any electrolyte is called electrolytic conduction.
→ Ohm's Law: Current passing through a conductor is directly proportional to the potential difference across it.
→ Specific resistance or resistivity: Specific resistance of a conducforts equal to its resistance when length of the conductor is 1 cm and area of cross-section is 1 cm2 i.e., the resistance, of one centimeter cube of conductor is called specfic resistance or resistivity.
→ Conductivity or specific conductance : The reciprocal or resistivity of conductor is known as conductivity or conductance of one centimeter cube of the solution of an electrolyte is called conductivity.
→ Conductance: It is the measure of the ease with which current flows through a conductor. It is reciprocal of electrical resistance.
→ Cell constant: The ratio of distance between two electrodes of a cell and area of cross-section of electrode is called cell constant.
→ Molar conductivity: The conductance of the solution containing one mole of the electrolyte placed between the two electrolytes lying 1 cm apart and having area of cross-section large enough to accommodate whole of the solution.
→ Equivalent conductivity: It is the conductance of solution containing 1 gram equivalent of an electrolyte placed between two electrodes lying 1 cm a part and having area of cross section large enough to accomodate whole of the solution.
→ Conductors: The substances which allow the electricity to pass though them are called conductors.
→ Metallic conductors: The metallic substances which allow the electricity to flow through them without undergoing any chemical change are called metallic conductors. Example: Cu, Ag, Au etc.
→ Electrolyte: The substances which allow the passage of electricity through them in the form of their aqueous solution or in their molten state are called electrolytes or electrolytic conductors. Example: CaCl2, KCl, KNO3 etc.
→ Insulators: The substances which do not allow the passage of electricity through them are called insulators. Example: plastic, glass etc.
→ Semiconductors: The substance which have their conductivity between conductors and insulators are called semi-conductors. Example: silicon (Si), germanium) (Ge) etc.
→ Superconductors: The substances which have zero resistivity or infinite conductivity are called superconductors.

→ Strong electrolyte: The electrolytes which dissociate completely into ions in their aqueous solutions are called strong electrolytes. Example: NaCl, KCl, NaOH etc.
→ Weak electrolyte: The electrolytes which do not ionise completely into ions in their aqueous solution are called weak electrolytes. Example: CH3COOH, NH4OH etc.
→ Kohlrausch's law: Limiting molar conductivity of an electrolyte may be represented as the sum of the individual contributions of the cation and anion of the electrolyte.
→ Electrolytic cell: A cell or device which converts the electrical energy into chemical energy is called electro chemical cell or electrolytic cell.
→ Faraday's first law of electrolysis : The mass of the substance deposited or liberated at any electrode is directly proportional to the quantity of electricity passed through the electrolyte.
→ Faraday's second law of electrolysis: When same quantity of electricity is passed through the solutions of different electrolytes connected in series the weights of the substances deposited at respective electrodes are directly proportional to their equivalent weights.
→ Battery: When a number of cells are connected in series, the arrangmeent is called a battery. They are used as a source of electrical enenergy in which chemical energy of a spontaneous redox reaction is converted into electrical energy.
→ Primary Batteries: In a primary battery, the reaction takes place only once and after using over a period of time, it becomes dead and connot be used again.
→ Secondary Batteries: In these batteries, the electrode reactions can be reversed by using an external source of electrical energy. Thus, these cells can be recharged on passing electric current through them and can be used again and again.
→ Fuel cells: The galvanic cells which convert the energy of combustion of fuels like hydrogen, methane, methanol directly into electrical energy are called fuel cells.

→ Corrosion: When the metals are exposed to atmosphere, the gases and water vapours present in the atmosphere attack the surface of metal and form some undesirable compounds like metal oxide, carbonate, sulphide, sulphate which result deterioration of the metal. This process is called corrosion. Example: Rusting of iron, tarnishing of silver.
→ Conductance (G) = \(\frac{1}{R}\) where R = resistance.
→ Specific resistance (κ) = G × \(\frac{l}{A}\)
where l = length, A = area, G = conductance.
→ Cell constant (G*) = \(\frac{l}{A}\)
→ Equivalent conductance (Λeq) = \(\frac{1000 \times \kappa}{N}\)
where N = normality (Unit = S cm2 equi-1)
→ Molar conductance (Λm) = \(\frac{1000 \times \kappa}{M}\)
wehre M = molarity (unit = S cm2 mol-1)
→ Debye-Huckel-Qnsagar equation
Λmc = Λm∞ - A√C
where C = concentration, Λmc = molar conductivity at infinite dilution
→ Degree of dissocation: a = \(\frac{\Lambda_m^c}{\Lambda_m^{\infty}}\)
→ Mathematical expression of Kohlrausch's law:
Λm∞(AxBy) = xλ(Ay+)∞ + yλ(By-)∞
→ According to Nernst equation:
Mn+ + ne- → M
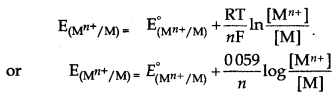
where, n = number of electron.
→ Nernst equation for the general cell reaction
aA + bB → cC + dD
Ecell = E°cell + \(\frac{0.0591}{n} \)log\(\frac{[\mathrm{A}]^a \cdot[\mathrm{B}]^b}{[\mathrm{C}]^c[\mathrm{D}]^d}\)
→ For concentration cells
E = \(\frac{0.0591}{n} \)log\(\frac{\mathrm{C}_1}{\mathrm{C}_2}\)
Where, C1 > C2
→ Free energy (ΔG°) = -nF E°cell
→ Fre energy (ΔG°) = -2.303 RT log K

→ At equilibrium E°cell = \(\frac{0.0591}{n} \) log Kc
→ Effeciency of fuel cell (T) = \(\frac{\Delta \mathrm{G}}{\Delta \mathrm{H}}\) × 100
→ According to first law of Faraday
W = ZIt
→ According to second law of Faraday
\(\frac{W_1}{W_2}=\frac{E_1}{E_2}\)
