RBSE Class 12 Chemistry Important Questions Chapter 9 Coordination Compounds
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 9 Coordination Compounds Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 9 Important Questions Coordination Compounds
Multi Choice Questions (MCO):
Question 1.
Which of the following is a tridentate ligand?
(a) NO2-
(b) oxalate ion
(c) glycinate ion
(d) dien
Answer:
(d) dien
Question 2.
An example of ambidentate ligand is:
(a) Ammine
(b) Aquo
(c) Oxalato
(d) Thiocyanato
Answer:
(d) Thiocyanato

Question 3.
The number of possible isomers for the complex
[CO(C2O4)2 (NH3)2] is:
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(d) 4
Question 4.
The complexes [CO(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [CO(CN)6] are the examples of which type of isomerism?
(a) Geometrical isomerism
(b) Linkage isomerism
(c) Ionisation isomerism
(d) Coordination isomerism
Answer:
(d) Coordination isomerism
Question 5.
Which kind of isomerism is extibited by octahedral
[CO(NH3)4Br2]Cl?
(a) Geometrical and ionisation
(b) Geometrical and optical
(c) Optical and ionisation
(d) Geometrical only.
Answer:
(b) Geometrical and optical
Question 6.
The ionisation isomer of [Cr(H2O)4 Cl(NO)2]Cl is:
(a) [Cr(H2O)4 (O2N)]Cl2
(b) [Cr(H2O)44Cl2](NO2)
(c) [Cr(H2O)4 Cl(ONO)]CI
(d) [Cr(H2O) Cl2(NO2)]H2O
Answer:
(b) [Cr(H2O)44Cl2](NO2)
Question 7.
Which one of the following has an optical isomer?
(a) [CO(H2O)4 (en)]3+
(b) [Zn(en)2]2+
(c) [Zn(en) (NH3)2]2+
(d) [CO(en)3]3+
Answer:
(d) [CO(en)3]3+
Question 8.
Which of the following has highest molar conductivity?
(a) Diamminedichloro platinum (II)
(b) Tetraamminedichlorocobalt (IV) chloride
(c) Potassium hexacyanoferrate (II)
(d) Hexaaquochromium (III) chloride
Answer:
(b) Tetraamminedichlorocobalt (IV) chloride
Question 9.
The primary and secondary valencies of chromium in the complex ion dichloridoxalatochromium (III) are respectively?
(a) 3,4
(b) 4,3
(c) 3,6
(d) 6,3
Answer:
(c) 3,6

Question 10.
The hybridisation of Fe in K4[Fe(CN)6] is
(a) dsp2
(b) sp3
(c) d2sp3
(d) sp3d2
Answer:
(c) d2sp3
Question 11.
Amongst the following ion which one has the highest paramagnetism?
(a) [Cr(H2O)6]3+
(b) [Fe(H2O)6]2+
(c) [Cu(H2O)6]3+
(d) [Zn(H2O)6]2+
Answer:
(b) [Fe(H2O)6]2+
Question 12.
Which of the following is an outer orbital complex?
(a) [Fe(CN)6]4-
(b) [FeF6]3-
(c) [CO(NH3)6]2+
(d) [Cu(CN)6]2+
Answer:
(b) [FeF6]3-
Question 13.
Which of the following compound has tetrahedral geometry?
(a) [Ni(CN)4]2-
(b) [Pd(CN)4]2-
(c) [PdCl4]2-
(d) [NiCl4]2-
Answer:
(d) [NiCl4]2-
Question 14.
What is shape of Fe(CO)5 molecule?
(a) octahedral
(b) tetrahedral
(c) square pyramidal
(d) trigonal bipyramidal
Answer:
(d) trigonal bipyramidal
Question 15.
The oxidation state of nickel in [Ni(CO)4] is:
(a) 4
(b) 0
(c) 2
(d) 3
Answer:
(b) 0
Very Short Type Answer Questions:
Question 1.
What is the difference between a complex and a double salt?
Answer:
Double salt is combination of (+) ve and (-) ve ions, which completely dissociates into its ions, when dissolved in water. Whereas complex is a salt, in which molecular structure of complex ion retain itself in aqueous solution, i.e., do not dissociates into its ions completely.
Question 2.
Write the coordination isomer of [Cu(NH3)4] [PtCl4].
Answer:
[Pt(NH3)4] [CuCl1].
Question 3.
Write the coordination number and oxidation state of platinum in the complex [Pt(en)2 Cl2].
Answer:
Coordination number of platinum in the complex, [Pt (en)2 Cl2] is 6 as en is a bidentate ligand. Let the oxidation state of Pt is x.
∴ x + 0 +2 (-2) = 0 ⇒ x = +2
Thus, oxidation state of Pt be + 2

Question 4.
Write down the IUPAC name of the complex [Pt (en)2Cl2]2+. What type of isomerism is shown by this complex.
Answer:
IUPAC name of [Pt(en)2Cl2]2+ is dichlorobis (ethane 1,2-diamine) platinum (IV) ion. The given complex show geometrical isomerism and optical-isomerism as follows.
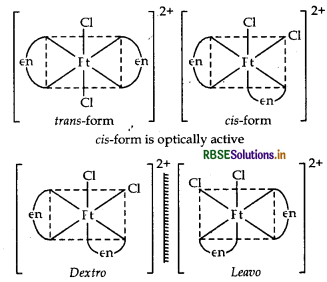
Question 5.
What is the IUPAC name of the complex [Ni (NH3)6] Cl2?
Answer:
The IUPAC name of the complex, [Ni(NH3)6] Cl2 is hexaamminenickel (II) chloride.
Question 6.
What type of isomersion is exhibited by the complex [CO(NH3)5 NO2]2+?
Answer:
The complex, [CO(NH3)5 NO2]2+ exhibits linkage isomerism as NO2 group being an ambidentate ligand can bind to a and, hence two different isomers are formed. These are [CO(ONO) (NH3)5]2+ and [CO(NO2) (NH3)5]2+
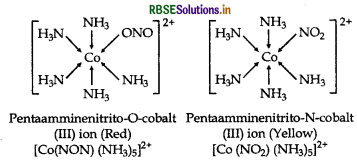
Question 7.
What type of isomerism is exhibited by the follow- ing complex [CO(NH3)5 SO4]Cl?
Answer:
The complex, [CO(NH3)5 SO4] Cl exhibit ionisation isomerism as it gives two different ions when dissolve in water. Its another ionisation isomer is [CO(NH3)5]
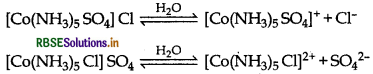
Question 8.
What type of isomerism is shown by the following complex [CO(NH3)6] [Cr(CN)6]?
Answer:
The complex, [CO(NH3)6] [Cr(CN)6] shows coordination isomerism which is caused by the interchange of ligands between the two complex ions. Its another coordination isoner is [Cr(NH3)6] [CO(CN)6].
Question 9.
What do you understand by denticity of a ligand?
Answer:
The number of coordinating or ligating sites present in a ligand is called the denticity of that ligand. e.g.
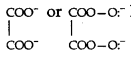
Question 10.
Name the following coordination compound:
K3 [CrF6].
Answer:
The IUPAC name of the complex, K3[CrF6] is potassium hexafluorochromate (III).
Question 11.
Write the IUPAC name of [Pt(NH3)2]Cl.
Answer:
The IUPAC name of the complex, [PtCl (NH2CH3)2] Cl is diamminechlorido methylamine platinum (II) chloride.
Question 12.
Write the IUPAC name of [Pt(NH3)4Cl2]Cl2.
Answer:
The IUPAC name of the complex, [Pt (NH3)4 Cl2] is tetraamminedichlorido polatinum (IV) chloride.

Question 13.
Write the IUPAC name of [Cr(NH3)6] [CO(CN)6].
Answer:
The IUPAC name of [Cr (NH3)6] [CO(CN)6] is hexaamminechromium (III) hexacyano cobaltate (III)
Question 14.
Write the IUPAC name of [Pt(NH3)3 (NO)Cl2]Br2.
Answer:
The IUPAC name of [Pt (NH3)3 (NO) Cl2] Br2 is triamminedichloridenitrosylplatinum (IV) bromide.
Question 15.
Write the IUPAC name of [CO(CN)2 (NH3)4]Cl.
Answer:
The IUPAC name of [CO(CN)2 (NH3)4] Cl is tetraamminedicyanocobalt (III) chloride.
Question 16.
Write the IUPAC name of [Cr(NH3)5 (NCS)][ZnCl].
Answer:
The IUPAC name of [Cr (NH3)5 (NCS)] [ZnCl] is pentaammineisothiocyanato chromium (III) tetrachlorozincate (II).
Question 17.
Give an example of linkage isomerism.
Answer:
[CO (NH3)5 (NO2)] Cl1⁄2 and [CO (NH3)5 (ONO)] Cl2 are examples of linkages isomerism.
Question 18.
Illustrate the following with an example: 'coordination isomerism'.
Answer:
Coordination isomerism occurs when cation and an ion both are complex ions and differ in the distribution of ligands in the cation and anion. e.g.
(i) [CO(NH3)6] [Cr (CN)6] and [Cr (NH3)6] [CO (CN)6]
(ii) [Cr (NH3)6] [CO(C2O4)3] and [CO(NH3)6] [Cr (C2O4)3]
Question 19.
Given an example of coordination isomerism.
Answer:
[CO (en)3] [Cr (CN)6] and [Cr (en)3] [CO (CN)6] is an example of ccordination isomerism.
Question 20.
Give an example of ionisation isomerism.
Answer:
[CO(NH3)5 CI] SO4 and [CO(NH3)5 (SO4)] Cl is an example of ionisation isomerism.
Question 21.
Write the IUPAC name of [CO(NH3)5CI]Cl2 (Atomic no. of CO = 27).
Answer:
The IUPAC name of [CO(NH3)5 CCl2 is pentaamminechloridocobalt (III) chloride.
Question 22.
What is ambidentate ligand? Give an example.
Answer:
Ambidentate ligand Ligands which can ligate through two different atoms (with any one at a time) present in it are called ambidenatate ligands. e.g. NO2, SCN, CNO, etc. NO2 can ligate through two sites, e.g.
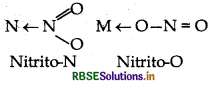
Question 23.
When a coordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound write structural formula of the complex.
Answer:
When one mole of CrCl3.6H2O is mixed with AgNO3, two moles of AgCl are precipitated which indicates the two ionisable chloride ions in the complex are present. Hence, its structural formula is [Cr (H2O)5 Cl] Cl2 H2O.
Question 24.
When a coordination compound COCl3.6NH3 is mixed with AgNO3, 3 moles of AgCl are precipitated per mole of the compound. Write structural formula of the complex.
Answer:
When one mole of COCl3.6NH3 is mixed with AgNO3, two moles of AgCl are precipitated which indicates that two ionisable chloride ions in the complex are present. Hence, its structural formula is [CO(NH3)6] Cl3.
Question 25.
What is meant by chelate effect?
Answer:
When a didentate or a polydentate ligand containing donor atoms positioned in such a way that, they coordinate with the central metal ion forming a five or six membered ring the effect is called chelate effect. As a result of chelate effect, the stability of the complex increases.

Question 26.
Which of the following is more stable complex and why?
[CO(NH3)6]3+ and [CO(en)g]3+
Answer:
[CO(en)3]3 is more stable complex because of chelation.
Question 27.
Explain the following.
[Fe(CN)6] and [Fe(H2O)2+] are of different colours in dilute solutions. Ans. In both the complexes, Fe is in + 2 state with the configuration 3d, i.e. it has four unpaired electrons. As the ligands H2O and CN-possess different crystal field splitting energy (40) they absorb different components of visible light (VIBGYOR) for d-d transition. Hence, the transmitted colours are different.
Question 28.
Why is CO a stronger ligand than Cl-?
Answer:
Ligand such as CO has empty л-orbitals which overlap with the filled d-orbitals (t2g orbitals) of transition metals forming p-bonds (back bonding). Hence, CO is а л-ассеptor ligand which can accept the electron density from the metal atom into their л oг л+ orbitals. These ллinteractions increase the value of Ap. This accounts for the position of this ligand as strong field ligand. Whereas ClTM cannot form л bonds by back bonding. Hence, it is a weak field ligand.
Question 29.
Write IUPAC name of the complex [Pt(en)2Cl2]. Draw structures of geometrical isomers for this complex.
Answer:
[Pt(en)2 Cl2]
IUPAC name: dichloridobis (ethylenediamine) platinum (II). Geometrical isomers of [Pt (en)2 Cl2] are
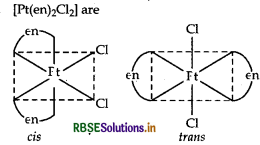
Question 30.
Using IUPAC names write the formulae for the following
(i) Hexaamminecobalt(III) sulphate
(ii) Potassium trioxalatochromate(III)
Answer:
(i) Hexaamminecobalt (III) sulphate
[CO (NH3)6]2 (SO4)3
(ii) Potassium trioxalatochromate (III) K3[Cr(Ox)3] or K3[Cr(C2O4)3]
Question 31.
Using IUPAC norms, write the formulae for the following:
(i) Potassium trioxalatoaluminate (III)
(ii) Dichlorido bis(ethane-1, 2-diamine) cobalt(III) ion.
Answer:
(i) Potassium trioxalatoaluminate (III)
K3[AI (C2O4)3]
(ii) Dichloridobis (ethane-1, 2-diamine) cobalt (III)
[COCl2 (en)2]+(iii)
Question 32.
Using IUPAC norms write the formulae for the following:
(i) Sodium dicyanidoaurate (I).
(ii) Tetraamminechloridonitrito-N-platinum (IV) sulphate.
Answer:
(i) Sodium dicyanidoaurate (1) Na[Au (CN)2]
(ii) Tetraamminechlorido nitrito-N-platinum (IV) sulphate [Pt(NH3)4 Cl(NO2)] SO4
Question 33.
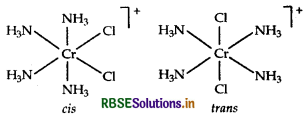
Write the IUPAC name of the complex [Cr (NH3)4Cl2]+. What type of isomerism does it exhibit?
Answer:
IUPAC name is tetraamminedichlorido chromium (III) ion.
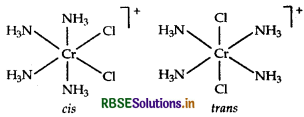
Isomerism It shows geometical isomerism and has two isomers cis and trans that can be represented as:
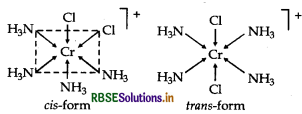
Question 34.
(i) Write the IUPAC name of the following complex:
[CO (NH3)5 Cl]2+
(ii) Write the formula for the following complex: Potassium tetrachloridonickelate (II)
Answer:
(i) The IUPAC name of the complex,
[CO(NH3)5Cl]2+ is pentaamminechloridocobalt (III) ion.
(ii) The formula for the complex, potassium tetrachloridonickelate (II) is K2[NiCl4].

Question 35.
(i) Write down the IUPAC name of the following complex:
[Cr (NH3)2Cl2(en)]Cl
(where en = ethylene diamine)
(ii) Write the formula for the following complex: Pentaamminenitrito-O-cobalt (III) ion.
Answer:
(i) The IUPAC name of [Cr(NH3)2Cl2(en)] Cl is diamminedichlorido (ethane-1,2-diamine) chromium (III) chloride.
(ii) The formula for the complex,
pentaamminenitrito-O-cobalt ion is [CO(NH3)5 (ONO)]2+.
Question 36.
(i) Write down the IUPAC name of the following complex: [Cr (en)3]Cl3
(ii) Write the formula for the following complex: Potassiumtrioxalato chromate (III)
Answer:
(i) The IUPAC name of the complex, [Cr(en)3] Cl3 is tris (ethane-1, 2 diamine) chromium (III) chloride.
(ii) The formula for the complex, potassiumtrioxalatochromate (III) is K3[Cr(C2O4)3].
Question 37.
Name the following coordination compounds and draw their structures.
(i) [COCl2(en)2]Cl
(ii) [Pt(NH3)2 Cl (NO2)]
(Atomic no. of CO = 27, Pt = 78)
Answer:
(i) [COCl2(en)2]Cl
IUPAC name Dichlorido bis-(ethane-1, 2-diamine) cobalt (III) chloride.
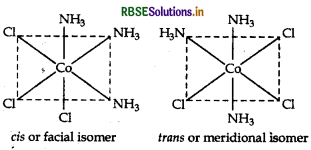
Structures There are two possible structures of [COCl2(en)2] Cl, one is cis and other is trans that can be represented as:
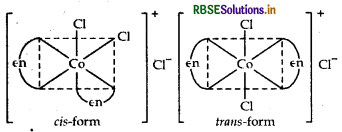
(ii) [Pt(NH3)2 Cl (NO)2]
IUPAC name
Diamminechloridonitrito-N-platinate (II).
Structures
There are two structures of [Pt(NH3)2 Cl (NO2)], one is cis form and other is transform that can be represented as:
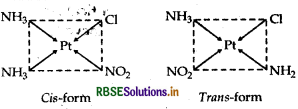
Question 38.
Draw the structures of isomers, if any and write the names of the following complex.
(i) [Cr(NH3)4Cl2]+
(ii) [CO(en)3]3+
(Atomic no. of Cr = 24, CO = 27)
Answer:
(i) IUPAC name of [Cr (NH3)4 Cl2]+ is tetraammine dichloride chormium (III)
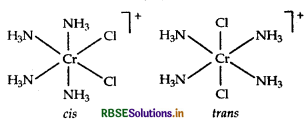
(ii) [CO(en)3]3+
IUPAC name
Tris-(ethane-1,2-diamine) cobalt (III) ion.
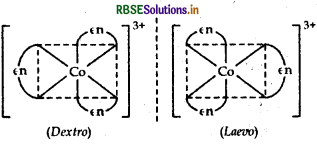
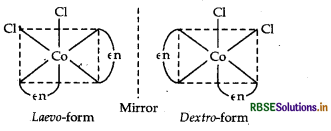
Structure There are two optical isomers of [CO(en)3]3+, one is dextro and other is laevo whose structures are:
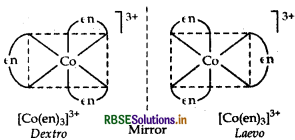
Question 39.
Name the following coordination, compounds according to IUPAC system of nomenelature.
(i) [CO(NH3)4 (H2O)CI] Cl2
(ii) [CrCl, (en)] C
(where, en = ethane-1, 2-diamine)
Answer:
IUPAC name of the following coordination compounds are:
(i) [CO(NH3)4 (H2O) Cl] Cl2
Tetraammineaquachloridocobalt (III) chloride salamat
(ii) [CrCl(en)]CH:
Dichlorido bis-(ethane-1, 2-diamine) chromium (III) chloride.

Question 40.
Indicate the types of isomerism exhibited by the following complexes.
(i) [CO(NH3)5 (NO2)]2+
(ii) [CO(en)3] Cl3 [where en = ethylene diamine]
(iii) [Pt(NH3)2Cl2]
Answer:
(i) [CO(NH3)5 (NO2)]2+: It exhibits linkage isomerism.

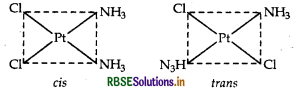
(ii) [CO(en)3] Cl3: It exhibits optical isomerism and exists in two forms: dextro and laevo that can be represented as:
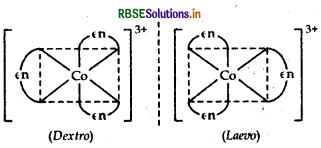
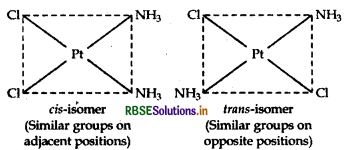
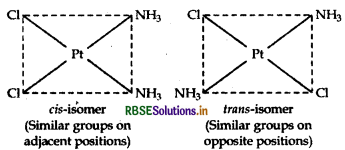
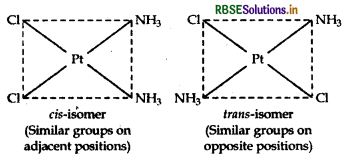
(iii) [Pt(NH3)2 Cl2]: It exhibits geometrical isomerism and exists in two geometrical forms, cis and trans. Their structures are:
Question 41.
Draw the structures of optical isomers of each of the following complex ions.
[Cr(C2O4)3]3, [Pt Cl2 (en)2]2+
[Cr(NH3)2Cl2(en)]+
(where, en = ethylene diamine)
Answer:
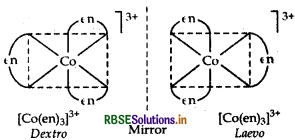
(i)[Cr(C2O4)3]3-
It exhibits two optical forms dextro and laevo, whose structure are:
(ii) [PtCl2 (en)2]2+ It has two geometrical isomers, cis and trans isomer shows optical isomerism. Their structures are:
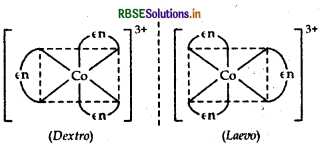
Note: The structure of trans [Pt Cl2(en)2]2+ isomer is optically inactive due to superimposable mirror images.
Question 42.
Write the IUPAC name of the following coordination compounds:
(i) [Cr(NH3)3Cl3]
(iii) [COBr2(en)2]+
(iii) K3[Fe(CN)6]
(where, en = ethylene diamine)
Answer:
The IUPAC name of the following given compounds is:
(i) [Cr(NH3)3Cl3]
Triamminetrichloridochromium (III)
(ii) K3[Fe(CN)6]
The formula for the complex, potassium tetrachloridonickelate (II) is K2[NiCl4].
(iii) [COBr2 (en)2]+
Dibromido bis-(ethane-1, 2 diamine) cobalt (III) ion.
Question 43.
Write the types of isomerism exhibited by the following complexes:
(i) [CO(NH3)5CI] SO,
(ii) [CO(en)3]3+
(iii) [CO(NH3)6] [Cr(CN)6]
Answer:
(i) [CO(NH3)5 CI] SO4: It exhibits ionisation isomer. The complex, [CO(NH3)5 SO4] Cl exhibit ionisation isomerism as it gives two different ions when dissolve in water. Its another ionisation isomer is [CO(NH3)5]
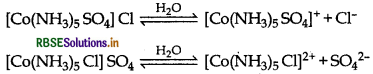
(ii) [CO(en)3]3+ : It shows optical isomerism. It exhibits optical isomerism and exists in two forms: dextro and laevo that can be represented as:

(iii) [CO(NH3)6] [Cr(CN)6]: It exhibit coordination isomerism The complex, [CO(NH3)6] [Cr(CN)6] shows coordination isomerism which is caused by the interchange of ligands between the two complex ions. Its another coordination isoner is [Cr(NH3)6] [CO(CN)6].
Question 44.
(i) How is double salt different from a complex?
(ii) Write IUPAC name of the following:
(a) K3[Fe(C2O4)3]
(b) [Pt (NH3)6] Cl4
(iii) Draw the structure of cis-isomer of [CO(NH3)4Cl2]+
Answer:
(i) Double salts dissociate completely into their constituent simple ions when dissolve in water, i.e. they lose their identity in aqueous solution.
e.g. KCl-MgCl2.6H2O → K+ + Mg2+ + 3Cl + 6H2O
While coordination compounds do not dissociate into simple ions, i.e. they retain their identity both in solid state and in aqueous solutions.
e.g. K4[Fe(CN)6] → 4K+ + [Fe(CN) 6]4-
(ii) The IUPAC name of the following given complexes
(a) K3[Fe(C2O4)3]:
Potassiumtrioxalatoferrate (III)
(b) [Pt(NH3)6] Cl4: Hexaammineplatinum (IV) chloride
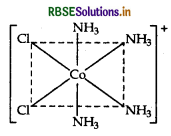
(iii) The structure of cis-isomer of [CO(NH3)4 Cl2]+ ion is :
Question 45.
Name the following coordination entities and draw the structures of their stereoisomers.
(i) [CO(en)2Cl2]+
(where, en = ethane-1,2-diamine)
(ii) [Cr(C2O4)3]3
(iii) [CO(NH3), Cl3]
(Atomic no. of Cr = 24, CO = 27)
Answer:
(i) IUPAC name Dichlorido bis-(ethane-1, 2 diamine) cobalt (III) ion
Possible isomers Geometrical isomers (cis and trans) Structure
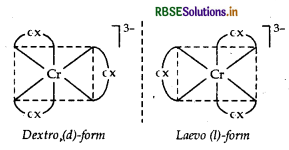
cis-[CO(en)2Cl2]+ is optically active and exits in dextro (d) and laevo (l) forms.
(ii) [Cr(C2O4)3]3
IUPAC name Trioxalatochromate (III) ion Possible isomers Optical isomers (d and l)
Structure
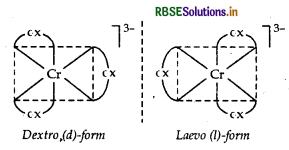
(iii) [CO(NH3)3Cl3]
IUPAC name Triamminetrichloridocobalt (III) Possible isomers Geometrical isomers
[i.e. shows cis and trans isomers]

Question 46.
Write the structures and names of all the stereoismers of the following compounds:
(i) [CO(en)3] Cl3
(ii) [Pt(NH3)2 Cl2]
(iii) [Fe(NH3)4 Cl2]
Answer:
(i) [CO(en)]Cl3:
IUPAC name: Tris-(ethane-1,2-diamine) cobalt(III) chloride.
fomers optical isomers (dextro and laevo forms)
Structure
It exhibits optical isomerism and exists in two forms: dextro and laevo that can be represented
(ii) [Pt(NH3)2Cl2]:
It exhibits geometrical isomerism and exists in two geometrical forms, cis and trans. Their structures are :
(iii) [Fe(NH3)4 Cl2] C
IUPAC name
Tetramminedichloridoiron (III) chloride
Isomers Geometrical (cis and trans)
Structure
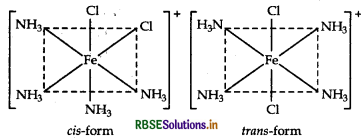
Question 47.
When a coordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) structural formula of the complex.
(ii) IUPAC name of the complex.
Answer:
(i) when one mole of CrCl3.6H2O is mixed with AgNO3 two molesof AgCl are precipated which indicates the two ionisable chloride ions in the complex are present. Hence, its structural formula is [Cr(H2O)5Cl] Cl2.H2O.
(ii) IUPAC name of the complex
[Cr(H2O)5 Cl] Cl2 H2O is pentaaquachloridochromium
(III) chloride.
Question 48.
When a coordination compound COCl3.6NH3 is mixed with AgNO3, 3 moles of AgCl are precipitated per mole of the compound. Write
(i) structural formula of the complex.
(ii) IUPAC name of the complex.
Answer:
(i) When one mole of COCl3.6NH3 is mixed with AgNO3, two moles of AgCl are precipitated which indicates two moles of AgCl are precipitated which indicates that two moles ionisable chloride ions in the complex are present. Hence, its structural formula is [CO(NH3)6]Cl3.
(ii) IUPAC name of the complex [CO(NH3)6] Cl3 is hexaamminecobalt (III) chloride.
Question 49.
Explain the following observation:
(i) CO2+ is easily oxidised to CO3+ in the presence of a strong ligand.
(ii) CO is a stronger complexing reagent than NH3.
Answer:
(i) First find the electronic configuration of CO3+ and CO2+. Then do pairing.
The electronic configuration of CO3+ is 3d 45°. So, pairing occurs in the presence of a strong ligand. Thus, there are no unpaired electrons and it is highly stable.
CO3+ = 3d6
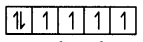
In the presence of a strong ligand.
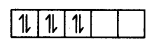
However, in CO2+, electronic configuration is 3d, there is one unpaired electron even after paring occurs in the presence of a strong ligand.
CO2+ = 3ď2,

In the presence of a strong ligand.
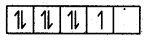
This unpaired electron can be easily lost and shows an oxidation state of +3.
Hence, CO2+ is oxidised to more stable CO3+.
(ii) CO is a stronger Complexing reagent than NH3 because of back bonding. In case of CO, It is a good Sigma donor and a pi acceptor. There exists a back bonding in CO complexes which are a donation of electrons from the filled d orbital of metals to a pi molecular orbital of CO.
Question 50.
What is meant by crystal field splitting energy? How does the magnitude of splitting decide the actual configuration of d-orbitals in an octahedral field for a coordination entily?
Answer:
When ligands approach a transition metal in a definite geometry, the degenerate d-orbitals splits into two sets, one with lower energy and the other with higher energy. The difference of energy between the two sets of d-orbitals is called crystal field splitting energy. It is denoted by ∆o (for octahedral complexes) and ∆o (for tetrahedral complexes).
The magnitude of splitting decide the actual configuration of d-orbital in an octahedral field for a coordination entity as follows. If ∆o < P (P is the energy required for pairing of electrons) then fourth electron enters one of the e orbitals giving the configuration tg eg thereby forming low spin complexes. Such ligands for which ∆ > P are called strong filed ligands.

Question 51.
What do you understand by stepwise stability constant and overall stability constant of a coordination compound? How are these two constants related?
Answer:
The equilibrium constant of each step of a complex reaction is called stepwise stability constant. Overall stability constant is the equilibrium constant for net reaction. The stepwise stability constant can be given as
\(\begin{aligned} &\mathrm{M}+\mathrm{L} \rightleftharpoons \mathrm{ML}: k_1=[\mathrm{ML}] /[\mathrm{M}][\mathrm{L}] \\ &\mathrm{ML}+\mathrm{L} \rightleftharpoons \mathrm{ML}_2 ; k_2=\left[\mathrm{ML}_2\right] /[\mathrm{ML}][\mathrm{L}] \\ &\mathrm{ML}_2+\mathrm{L} \rightleftharpoons \mathrm{ML}_3 ; k_3=\left[\mathrm{ML}_3\right] /\left[\mathrm{ML}_2\right][\mathrm{L}] \\ &\mathrm{ML}_3+\mathrm{L} \rightleftharpoons \mathrm{ML}_4 ; k_4=\left[\mathrm{ML}_4\right] /\left[\mathrm{ML}_3\right][\mathrm{L}] \end{aligned}\)
where, k1, k2 etc. are referred as stepwise stability constants.
\(\mathrm{M}+4 \mathrm{~L} \rightleftharpoons \mathrm{ML}_4 ; \beta_4=\left[\mathrm{ML}_4\right] /[\mathrm{M}][\mathrm{L}]^4\)
where, β4 is the overall stability constant.
Thus, stepwise stability constants and overall constants are related as
β4 = k1 × k2 × k3 × k4 or more generally.
βn = k1 × k2 × k3 × K4 .... kn
Question 52.
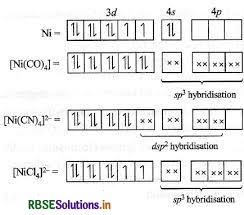
Describe the shape and magnetic behaviour of the following complex. [Ni(CN)4]2-
Answer:
(ii) In nickel is in +2 oxidation state and has the electronic configuration 3d8 4s0.
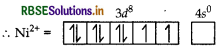
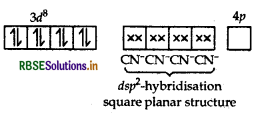
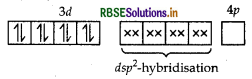
As CN- is a strong field ligand, pairing of electrons takes place and resulting in empty one 3d orbital, one 4s orbital and two 4p orbitals which undergoes hybridisation to form four d sp2-hybrid orbitals. These hybrid orbitals occupied by electron pairs of four CN- ions.
Ni2+
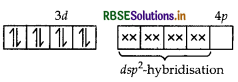
Magnetic behaviour As all the electrons are paired, so it is diamagnetic in nature.
Question 53.
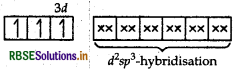
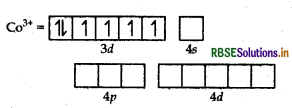
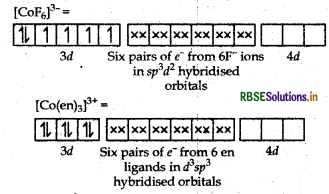
Using the valence bond theory predict the geometry and magnetic behaviour of [COF6]3-. (Atomic number of CO = 27)
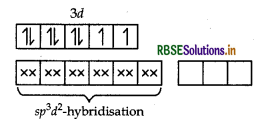
Answer:
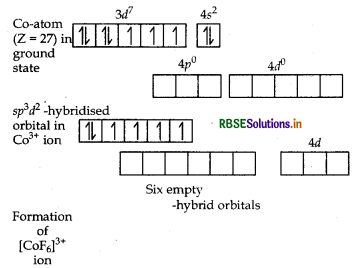
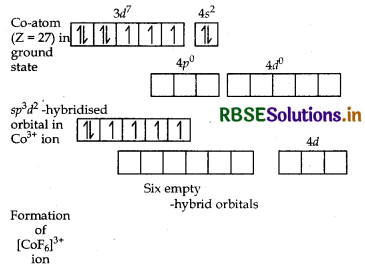
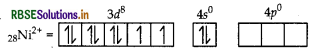
(i) In [COF6]3-, cobalt is in +3 oxidation state. F- is a weak field ligand. It does not cause pairing. Therefore, cobalt undergoes sp3d2 -hybridisation and have octahedral geometry.
CO-atom (Z = 27) in ground state
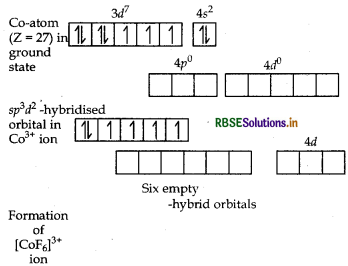
Magnetic behaviour As there are four unpaired electrons, so it is paramagnetic in nature.
Question 54.
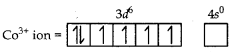
Using valence bond approach, deduce the magnetic character of [CO(NH3)6]3+ ion.
(Atomic no. of CO = 27)
Answer:
Orbitals of CO3+ ion
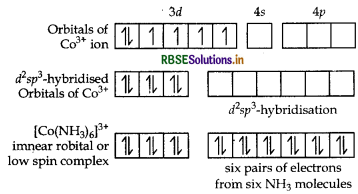
Magnetic behaviour Because of the absence of unpaired electrons it is diamagnetic.
Question 55.
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d+ in terms of t2 and eg in an octahedral field when
(i) Do > P
(ii) Do < P
Answer:
Crystal field splitting energy
Crystal Field Splitting Energy: Crystal field theory was given to explain the structure and stability of the coordination complexes. This theory has some assumption like the metal ion is considered to be a point positive charge and the ligands are negative charge. The complexes are formed mainly by the d-block elements due to their variable oxidation states and variable coordination number. The d - subshell has 5 degenerate orbitals. when the ligand approach to the metal ion, the energy of the degenerate orbitals is increased and further splitting of degenerate orbital takes place into t2 g and eg orbital. The difference between the t2g and eg orbitals is called as the crystal field splitting energy
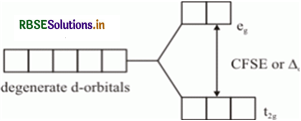
(i) ∆ > P, (pairing energy), the 4th electron pair up in one of the t2 orbitals giving the configuration t4g eg
(ii) If ∆ > P the 4th electron enters one of the eg orbitals giving the configuration t32g e1g.
Question 56.
Explain the following cases giving appropriate reasons:
(i) Nickel does not form low spin octahedral complexes.
(ii) The л-complexes are known for the transition metals only.
(iii) CO2+ is easily oxidised to CO3+ in the presence of a strong ligand.
Answer:
(i) In case of Ni, electrons configuration of Ni = 3d 4s2 and when it forms Ni2+ ion its electrons configuration becomes 3d84s0
Ni2+ =

For low spin complexes, electrons gets pair up. Thus, will produce only one empty d-orbital. Hence, d2sp3- hybridisation is not possible in Ni to form octahedral complexes.
(ii) The transition metals/ions have empty d-orbitals into which the electron pairs can be donated by ligands containing л-electrons (e.g. CH2 = CH2 and C6H6, etc.) while, the other metals do not have empty d-orbitals, hence, complexes are formed only by transition metal atom/ion.
(iii) Although the 3 rd ionization energy for CO is high, but the higher amount of crystal field stabilization energy (CFSE) released in the presence of strong field ligand overcome this 3rd ionization energy to form CO(III). So in presence of strong field ligand CO(II) is easily oxidised to CO(III)

Question 57.
Write the hybridisation, shape and IUPAC name, of the complex [COF6]3- (Atomic no. of CO = 27).
Answer:
[COF6]3-
For hybridisation and shape,
IUPAC name is: Hexafluorocobaltate (III) ion.
Question 58.
(i) Write the formula of the following coordination compound:
Iron (III) hexacyanoferrate(II)
(ii) What type of isomerism is exhibited by the complex [CO(NH3)5 Cl] SO4?
(iii) Write the hybridisation and number of unpaired electrons in the complex [COF6]-3. (Atomic number of CO = 27)
Answer:
(a) Iron (III) hexacyanoferrate (II) has formula- Fe4[Fe(CN)6]3
(b) [CO(NH3)5 CI] SO4 exhibit ionisation isomers. Its ionisation isomer is [CO(NH3)5 SO4] Cl
(c) [COF6]3: Oxidation state of cobalt is +3.
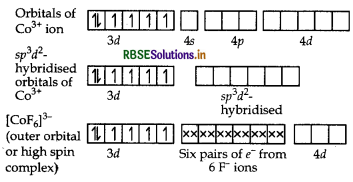
Question 59.
(i) What type of isomerism is shown by the complex [CO(en)3]Cl3?
(ii) Write the hybridisation and magnetic character of [CO(C2O4)3]3-
(Atomic number CO = 27)
(iii) Write IUPAC name of the following complex [Cr(NH3)3Cl3].
Answer:
(i) The formula for the complex, potassiumtrioxalatochromate (III) is K3[Cr(C2O4)3].
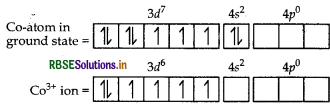
(ii) In [CO(C2O4)3]3-, cobalt is in + 3 oxidation state
CO = [Ar] 3d7 4s2
CO3+ = 3d6
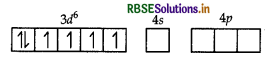
As C2O4 is a strong field ligand pairing of electrons will occur
[CO(C2O4)3]3-
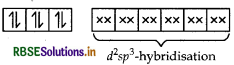
d2sp3 hybridisation
Question 60.
(i) What type of isomerism is shown by the complex
[Cr(H2O)6] Cl3?
(ii) On the basis of crystal field theory, write the electronic configuration for d1ion if Aq > P.
(iii) Write the hybridisation and shape of [COF6]3-. (Atomic number of CO = 27)
Answer:
(i) The isomerism shown by the complex
[Cr(H2O)6] Cl3 is hydrate or solvate isomerism. The hydrate isomers of the given complex are
(a) [Cr(H2O)5 Cl] Cl2- H2O
(b) [Cr(H2O)4 Cl1⁄2] Cl – H2O
Dark green (one ionisable chlorine)
Both isomers differ in the number of molecules of wa- ter in the coordination sphere.
(ii) ∆q > P electronic configuration becomes t2g4 eg0
(iii) [COF6]3-
For hybridisation and shape,

IUPAC name is: Hexafluorocobaltate (III) ion.
Quesytion 61.
(i) Draw the geometrical isomers of complex
[Pt(NH3)2 Cl2]
(ii) On the basis of crystal field theory, write the electronic configuration for d1 ion if ∆ < P.
(iii) Write the hybridisation and magnetic behaviour of the complex [Ni(CO)4].
(Atomic number of Ni = 28)
Answer:
(i) [Pt(NH3)2 Cl2]: It exhibits geometrical isomerism and exists in two geometrical forms, cis and trans. Their structures are:

(ii) On the basis of crystal field theory, for a d4 ion, if 0 < P, then the complex is a high spin complex formed by the association of weak field ligands with the metal ion. As a result, the fourth electron enters one of the eg orbitals, thereby, exhibiting the electronic configuration t2g3 eg1.
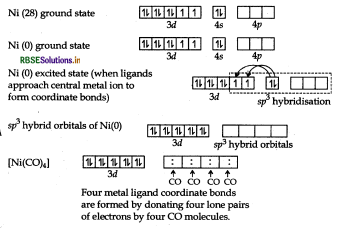
(iii) For shape and hybridisation [Ni(CO)4:
The atomic number of Ni is 28 and its electronic configuration is 3d8 4s2. The oxidation state of Ni in this complex is 0.
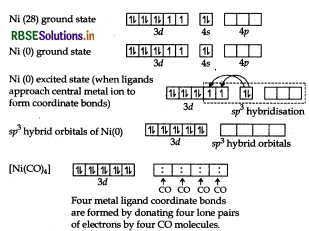
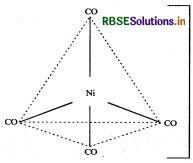
The complex is diamagnetic as all the electrons are paired.
Question 62.
(i) Draw the geometrical field theory, write the electronic configuration for d1ion, if ∆ > P.
(ii) On the basis of crystal field theory, write the electronic configuration for dion, if ∆o > P.
(iii) Write the hybridisation type and magnetic behaviour of the complex [Ni(CN)4]2. (Atomic number of Ni = 28)
Answer:
(i) The complex [Pt(en)2 Cl2]2+ has two geometrical isomers that are cis and trans which can be shown as
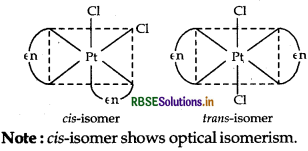
(ii)
Question 63.
(i) Write the IUPAC name of the complex [Cr(NH3)4Cl2]Cl.
(ii) What type of isomerism is exhibited by the complex [CO(en)3]3+?
(iii) Why is [NiCl4]2- paramagnetic but [Ni(CO)4] is diamagnetic?
(Atomic no. of Cr = 24, CO = 27, Ni = 28)
Answer:
(i) [Cr(NH3)4 Cl2] Cl
IUPAC name
Tetraamminedichlorido chromium (III) chloride,
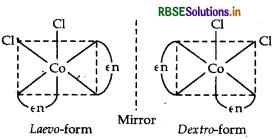
(ii) The complex shows optical isomerism.
Dextro (d) and laevo (l) refer to the directions in which the two forms rotate the plane of polarised light in a polarimeter (d rotates to the right, l rotates to the left).
Optical isomerism is common in octahedral complexes involving bidentate ligands as shown in the diagram given below:
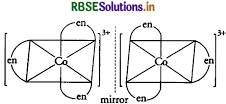
(iii) In the complex [NiCl4]2-, Ni is in + 2 oxidation state and has the configuration 3d8 4so. The Cl- ion being a weak field ligand cannot pair up the two unpaired electrons present in 3d-orbitals. That mean, 3d-orbitals are not involved in hybridisation. Thus, the complex is sp3-hybridised (tetrahedral) and is paramagnetic in nature.
In the complex [Ni(CO)4] the oxidation state of nickel is zero and outer electronic configuration is 3d 4s2. In the presence of strong field ligand CO, the 4s-electrons shifts to the two half-filled 3d-orbitals and make all the electrons paired. The valence 4s and 4p-orbitals are involved in hybridisation. Thus, the complex is tetrahedral but diamagnetic in nature.

Question 64.
For the complex [NiCl]2 ̄, write,
(i) the IUPAC name
(ii) the hybridisation type
(iii) the shape of the complex
(Atomic no. of Ni = 28)
Answer:
(i) The IUPAC name of [NiCl4]2 is tetrachlorido-nickelate (II) ion.
(ii) sp3 hybridisation
(iii) Shape Due to sp3-hybridisation, its shape is tetrahedral.
Question 65.
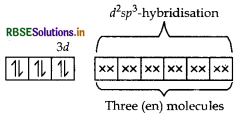
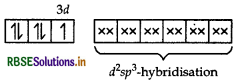
(i) Using valence bond theory, explain the geometry and magnetic behaviour of [Cr(NH3)6]3+. (Atomic no. of Cr = 24)
(ii) Write the IUPAC name of ionisation isomer of [Ni(NH3)3NO3]Cl.
Answer:
Orbitals of Cr3+ ion d2 sp3 hybridised orbitals of Cr3+.
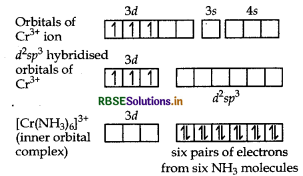
Geometry Octahedral geometry due to d2sp3-hybridisation.
Magnetic behaviour paramagnetic
(ii) The ionisation isomer of [Ni(NH3)3 NO3] Cl is [Ni(NH3)3 Cl] NO3. Its IUPAC name is triammine- chloridonickel (II) nitrate.]
Question 66.
Name the following coordination entities and describe their structures:
(Atomic no. of Fe = 26, Cr = 24, Ni = 28)
(i) [Fe(CN)6]4-
(iii) [Ni(CN),]2-
Answer:
(i) [Fe(CN)6]4-
(ii) [Cr(NH3)4 Cl2]+
IUPAC name Hexacyanoferrate (II) ion
Structure Octahedral complex due to d2sp3- hybridisation.
(ii) [Cr(NH3)4 Cl2]+
IUPAC name
Tetraamminedichloridochromium (III) ion
Structure
Octahedral complex due to d2sp3 hybridisation. Inner orbital complex (as inner d-orbital is involve in hybridisation)
(iii) [Ni(CN)4]2-
IUPAC name Tetracyanonickelate (II) ion
Structure Square planar complex due to dsp2 hybridisation.
Question 67.
Write the name, stereochemistry and magnetic behaviour of the following:
(i) K4 [Mn(CN)6]
(ii) [CO(NH3)5 CI] Cl2
(iii) K2 [Ni(CN)4]
Answer:
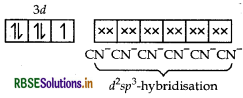
(i) K4 [Mn(CN)6]
IUPAC name
Potassium hexacyano manganate (II)
[1 × 4 + x + (-1) 6 - 0
⇒ x = +2 ]
Stereochemistry In this complex Mn is present as Mn2+.
Mn2+ = [Ar] 3d5
[CN- being strong field ligand, causes pairing]
K4[Mn(CN)6] =
2 sp3-hybridisation
dsp2-hybridisation
⇒low spin, inner orbital complex
Thus, the structure of this complex is octahedral.
Magnetic behaviour Paramagnetic (as one unpaired electron is present in the above orbital formula).
(ii) The IUPAC Name of the Complex [CO(NH3)5Cl]Cl2 is Pentaamminechloridocobalt(III) chloride.
Oxidation state of CO = +3
Coordination number = 6
Shape: octahedral.
Electronic configuration: d6 : t2g6.
(iii) Potassium tetracyanonickelate is the inorganic compound with the formula K2Ni(CN)4.
Oxidation state of K2Ni(CN) = +2
Oxidation number = -1
Shape: Square plannar
Electronic configuration:
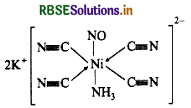
Question 68.
For the complex [Fe(en)2 Cl2] Cl, identify the following:
(i) Oxidation number of iron.
(ii) Hybrid orbitals and shape of the complex.
(iii) Magnetic behaviour of the complex.
(iv) Number of its geometrical isomers.
(v) Whether there may be optical isomer also.
(vi) Name of the complex.
Answer:
(i) [Fe(en)2 Cl2] Cl
x + 2 x 0 + (-1)2 = +1 x + 3
Thus, the oxidation number of iron = +3
(ii) In this complex, Fe is present as Fe3+ and en being a strong field ligand, causes pairing.
[Fe(en)Cl2] Cl = 3d
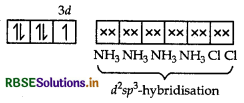
d2sp3-octahedral geometry.
(iii) Paramagnetic (as it contains one unpaired electron)
(iv) Two geometrical isomers, cis and trans.
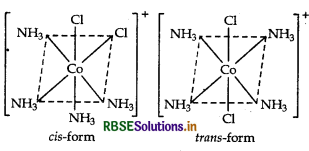
(v) Yes, cis isomer will have optical isomers, due to the non-superimposable mirror images.
(vi) Dichlorido bis-(ethane 1, 2-diamine) iron (III) chloride.
Long Type Answer Questions:
Question 1.
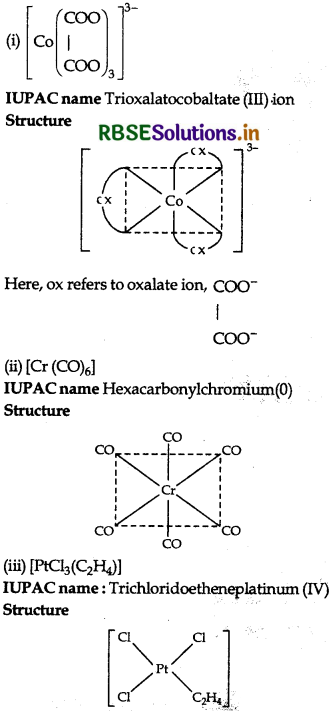
Write the IUPAC name and draw the structure of each of the following complex entities:
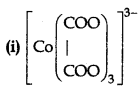
(ii) [Cr(CO)6]
(iii) [PtCl3(C2H4)]
(Atomic no. of Cr = 25, CO = 27, Pt = 78)
Answer:
Question 2.
Name the following complexes and draw the structure of one possible isomer of each:
(i) [Cr(C2O4)3]3-
(ii) [Pt (NH3)2 Cl2]
(iii) [CO(en)2Cl2]+
(where en = ethane-1, 2-diamine or ethylene diamine).
Answer:
Geometrical isomers are cis-and trans forms, optical isomers are d and f-forms (mirror images), [M(AA)3] type complex does not exhibit geometirical isomerism, they show optical isomerism.
(i) [Cr(C2O4)3]3-
IUPAC name Trioxalatochromate (III) ion Possible isomers Optical isomers (d and I)
Structure
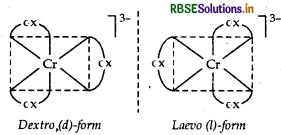
(ii) [Pt(NH3)2 Cl2]
IUPAC name
Diamminedichloridoplatinum(II)
Possible isomers Geometrical isomers (cis and trans)
Structure
(iii) [CO(en)2 Cl2]+
IUPAC name Dichlorido bis-(ethane-1, 2 diamine) cobalt (III) ion
Possible isomers Geometrical isomers (cis and trans)
Structure
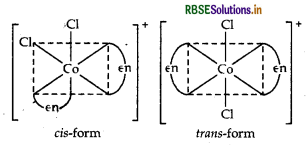
cis-[CO(en)2 Cl2]+ is optically active and exist in dextro (d) and laevo (1) forms.
Question 3.
Write the hybridisation and shape of the following complexes.
(i) [COF6]3-
(ii) [Ni(CN)]2-
Answer:
(i) In [COF6]3- , cobalt is in + 3 oxidation state. F is a weak field ligand. It does not cause pairing. Therefore, cobalt undergoes sp3 d2-hybridisation and have octahedral geometry.
Co-atom (Z = 27) in ground state
(ii) In [Ni(CN)4]2-, nickel is in + 2 oxidation state and has the electronic configuration 3d 4s.
Ni2+
As CN- is a strong field ligand, pairing of electrons takes place and resulting in empty one 3d orbital, one 4s orbital and two 4p orbitals which undergoes hybridisation to form four dsp2-hybrid orbitals. These hybrid orbitals occupied by electron pairs of four CN- ions.
Ni2+
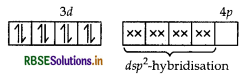
Thus, the hybridisation involved is dsp2 and therefore, complex is square planar.

Question 4.
State reason for each of the following:
(i) CO is stronger complexing reagent than NH3.
(ii) The molecular shape of Ni(CO)4 is not the same as that of [Ni(CN)4]2-.
Answer:
(i) As CO is a good o-sigma donor and a good л-accep- tor ligand, there exists a back bonding in CO complexes in which CO accepts an appreciable amount of electron density from the filled d-orbitals of metal atom into their empty л or л* orbitals. There л interactions increase the value of Ap. Whereas NH3 can form only o-bonds and no o-bonds with metals, therefore CO is a better complexing reagent than NH3.
(ii) In [Ni(CO)4], Ni has oxidation state equal to zero and involves sp3-hybridisation hence possesses a tetrahedral shape.

As CO is a strong field ligand, pairing of electrons occurs

sp3-hybridisation (4 electrons pair donated by 4CO) In [Ni(CN)4]2-, the oxidation state of Ni is +2 and it involves dsp2-hybridisation hence, possesses a square planat structure.

As CN- is also a strong field ligand, pairing of electrons occurs
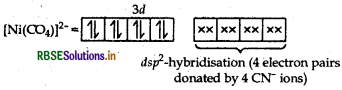
dsp2-hybridisation (4 electron pairs donated by 4 CN- ions)
Question 5.
Describe the state of hybridisation, shape and the magnetic behaviour of the following complexes.
(i) [Cr(H2O)2 (C2O4)2]-
(ii) [CO(NH3)2 (en)2]3+
(Atomic no of Cr = 24, CO = 27)
Answer:
(i) [Cr(H2O)2(C2O4)2]-
In this complex, Cr is present as Cr3+
Cr3+ = [Ar] 3d3 4s0
[Cr(H2O)2 (C2O4)2] =
d2 sp3-hybridisation
[∵ Oxalate is a bidentate ligand, one oxalate occupies two orbitals].
Thus, hybridisation-d2sp3
Structure Octahedral
Magnetic behaviour Paramagnetic
[as three unpaired electrons are present].
(ii) [CO(NH3)2 (en)2]3+
Here, CO is present as CO3+ (3d).
(en and NH3 are strong field ligands and thus, pair up the electrons of 3d-orbitals). Thus, [CO(NH3)2 (en)2]3+ =
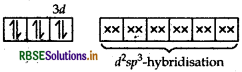
d-sp3-hybridisation
Hybridisation-sp3
Structure- Octahedral
Magnetic nature Diamagnetic ligand.
Question 6.
Explain the following terms:
(i) Crystal field splitting in an octahedral field.
(ii) Spectrochemical series.
Answer:
(i) Crystal field splitting In a free transition metal ion, all the five d-orbitals are degenerate (having equal energies.) But when ligand approaches a metal ion, this degeneracy splits due to repulsion of electrons of ligands and electrons of metal ions.
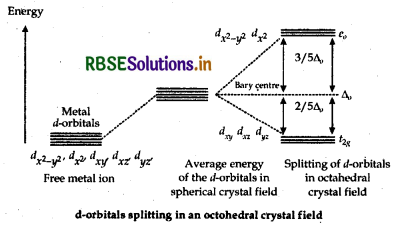
Free metal ion
Average energy of the d-orbitals in spherical crystal field. Splitting of d-orbitals in octahederal crystal field d-orbitals splitting in on octahedral crystal field Thus, the splitting of degenerate levels (or orbitals) due to the presence of ligands in a definite geometry is known as crystal field splitting in an octahedral complex.
(ii) Spectrochemical series The arrangement of ligands in the order of increasing field strengths, i.e. increasing crystal field splitting energy values is known as spectrochemical series.
Br < Cl < F < H2O < NH3 < en < CN- < CO
Question 7.
Write the hybridisation and magnetic character of the following complexes:
(i) [Fe(H2O)]2+
(ii) [Ni(CN)4]2-
[Atomic number: Fe = 26, Ni = 28]
Answer:
(i) [Fe(H2O)6]2+: outer configuration of 26Fe atom = [Ar] 3d6 4s2
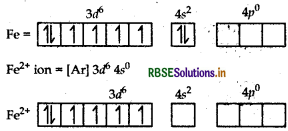
(H2O being weak field ligands, they do not cause electron pairing).
[Fe(H2O)6]2+ ion
The complex is paramagnetic in nature as it has four unpaired electrons.
(ii) [Ni(CN)4]2-: Outer configuration of 26Ni atom = [Ar] 3d8 4s2
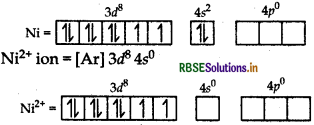
(CN-being strong field ligand pair up the d-electrons of metal atom.)
[Ni (CN)4]2- ion =
The complex is diamagnetic in nature as it has no unpaired electrons.

Question 8.
Out of [COF6]3- and [CO(en)3]3+, which one complex
Answer:
(i) paramagnetic
(ii) more stable
(iii) inner orbital complex and
(iv) high spin complex
(Atomic numbers of CO = 27)
Answer:
[COF6]3-
(i) [COF6]3- is paramagnetic since it contains 4 unpaired electrons.
(ii) [CO(en)3]3+ is more stable since ethylenediamine (en) is a chelating ligand and thus forms more stable complex.
(iii) [CO(en)3]3+ forms inner orbital complex since eth-ylenediamine (en) is a strong ligand which pairs up the electrons of CO3+.
(iv) [COF6]3- forms high spin complex as F could not pair up the electrons of CO3 being a weak ligand.
Question 9.
Write the state of hybridisation, the shape and the magnetic behaviour of the following complex entities.
(i) [Cr(NH3)4Cl2] Cl
(ii) [CO(en)3] Cl3
(iii) K2[Ni(CN)4]
Answer:
(i) In [Cr(NH3)4Cl2]Cl, Cr is present as Cr3+. Its formulation can be depicted as
Cr-atom in ground state =
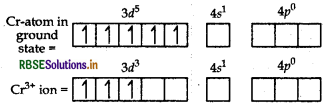
As Cl- is a weak field so no pairing of electrons takes place. Thus, two 3d, one 4s and three 4p-orbitals undergo hybridisation to form d2sp3 hybrid orbitals. Among six d2sp3-hybrid orbitals four orbitals occupied by electron pairs of four NH3 molecules, and two are occupied by electron pairs of two Cl ions.
Thus, the shape of the complex is octahedral. Because of the presence of three unpaired electrons, it is paramagnetic in nature.
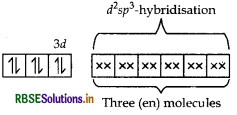
(ii) In [CO(en)3]Cl3 CO is present as CO3+.
en being a strong field ligand, pair up the electrons of 3d-orbital. Thus,
[CO(en)] Cl3 =
Shape Octahedral
Magnetic nature Diamagnetic (as all the electrons are paired).
(iii) K2[Ni(CN)4]
In K2[Ni(CN)4] Ni is present in + 2 oxidation state.
(CN- is a strong field ligand. It causes pairing of electrons in d-orbitals.)
[Ni(CN)4)]2- =
Shape Square planar
Magnetic nature Diamagnetic.
Question 10.
Write the state of hybridisation, shape and IUPAC name of the complex [CO(NH3)6]3 (Atomic no. of CO = 27).
Answer:
In [CO(NH3)6]3+ Co exists as CO3+ ion. The formation of [CO(NH3)6]3+ can be explained as
NH3 being a strong field ligand causes pairing of 3d electrons resulting in empty two 3d, one 4s and three 4p-orbitals which undergoes hybridisation to give six d'sp3-hybrid orbitals. These six empty dsp3-hybrid orbitals would be accommodated by electron pairs of six NH3 molescules resulting in the formation of complex [CO(NH3)6]3+ ion.
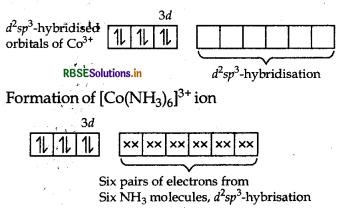
It is inner orbital, low spin complex due to d2sp3- hybridisation.
Thus, its shape is octahedral
(as it is d2sp3 hybridised).
IUPAC name is: Hexaamminecobalt (III) ion.
Question 11.
Give the name, the stereochemistry and the magnetic behaviour of the following complexes.
(i) [CO(NH3)5Cl] Cl2
(ii) K2[Ni(CN)4]
Answer:
(i) [CO(NH3)5Cl] Cl2
IUPAC name Pentaamminechloridocobalt(III) chloride.
Stereochemistry and magnetic behaviour
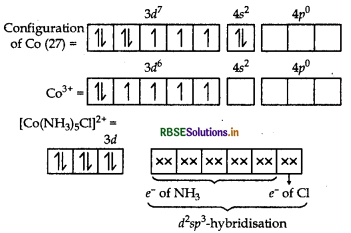
In [CO(NH3)5Cl]2+, NH, being a strong field ligand cause pairing of electrons in d-orbitals of CO3+ ion. Hence, the compound has dsp3-hybridisation and octahedral shape. Since, all the electrons are paired, the complex [CO(NH3)5Cl] Cl2 is diamagnetic.
(ii) K2[Ni(CN)4]
IUPAC Name
Potassium tetracyanonickelate (III)
Stereochemistry and magnetic behaviour [COF6]3-
K2[Ni(CN)4]
In K2[Ni(CN)4] Ni is present in + 2 oxidation state.

(CN- is a strong field ligand. It causes pairing of electrons in d-orbitals.)
[Ni(CN)4)]2- =

Shape Square planar
Magnetic nature Diamagnetic.

Question 12.
(i) What type of isomerism is shown by the complex [CO(NH3)5(SCN)]2+?
(ii) Why [NiCl]2 is paramagnetic while [Ni(CN)4]2- is diamagnetic?
(Atomic number of Ni = 28)
(iii) Why are low spin tetrahedral complexes rarely observed?
Answer:
Linkage isomerism arises in a coordination compound containing ambidentate ligand. Hence,
(i) [CO(NH3)3(SCN)]2+ exhibit linkage isomerism due to presence of SCN which is an ambidentate ligand and can linked with metal either through N or S. [CO(NH3),SCN)]2+ and [CO(NH3), NCS]2+
(ii) The complex in which one or more unpaired electrons are present is paramagnetic while, those which does not contain any unpaired electron is diamagnetic.
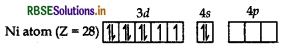
Oxidation state of Ni is + 2 in both the complexes i.e. [NiCl4]2- and [Ni(CN)4]2-.

In case of [NiCl4]2-, Cl- is a weak field ligand so pairing of electrons in 3d-orbital does not occur, hence compound is paramagnetic with two unpaired electrons. In [Ni(CN)4]2-, CN- is a strong field ligand, hence pairing occurs and [Ni(CN)4]2- is diamagnetic.
(iii) For tetrahedral complexes, the crystal field stabilisation energy is lower than pairing energy, so they are rarely formed in low spin state.
Question 13.
(i) For the complex, [Fe(CN)6]3, write the hybridisation type, magnetic character and spin nature of the complex. (Atomic number of Fe = 26)
(ii) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
Answer:
(i) In [Fe(CN)6]3 complex, Fe is present as Fe3+.
Configuration of Fe = [Ar] 3d6 4s2 4p0
Outer configuration of Fe3+ = 3d5

CN-being strong field ligand, pair up the unpaired d- electrons. Thus, two 3d-orbitals are now available for CN-ions.
[Fe(CN)6]3-
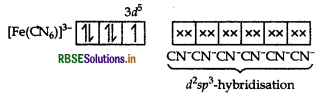
Since, one electron remains unpaired, the complex is paramagnetic.
Moreover, (n - 1) d-orbitals are involved in bonding. So, it is an inner orbital or low spin complex.
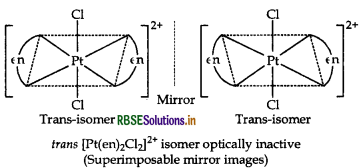
(ii) The complex, [Pt(en)2Cl2]2+ contains two symmetrical didentate ligands, ethylenediamine (en) and exists in two geometrical isomers, cis and trans. Trans isomer being symmetrical does not show optical isomer- ism and hence, this isomer is optically inactive. While cis being unsymmetrical shows optical isomerism.
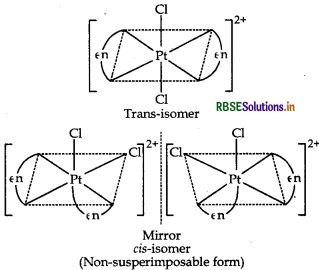
Hence, the structure of geometrical isomer (i.e. trans isomer) of the complex [Pt(en)2Cl2]2+ is optically inactive is as follows:
Question 14.
(i) For the complex [Fe(H2O)6]3, write the hybridisation, magnetic character and spin nature of the complex. (Atomic number of Fe = 26)
(ii) Draw one of the geometircal isomers of the complex [Pt(en),Cl2]2+ which is optically inactive.
Answer:
(i) In [Fe(H2O)6]3+, Fe exists in +3 oxidation state having a valence shell electronic configuration of 3d5 4s0.
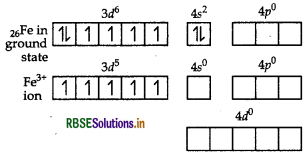
As H2O is a weak field ligand, electrons in 3d-subshell of Fe (III) ion will not pair up. Fe (III) ion uses its one 4s, three 4p and two 4d vacant orbitals for hybridisation and forms six sp3d2-hybrid orbitals which accommodates electrons from six H2O molecules, thus, forming
[Fe(H2O)6]3+ complex.
The complex, [Fe(H2O)]3+ involves
sp3d2 - hybridisation and thus, it is octahedral in shape. Since, the complex possesses five unpaired electrons in 3d-orbitals of Fe, therefore, the complex [Fe(H2O)6]3+ is paramagnetic in nature. Further, Fe forms an outer orbital complex by using its outer. 4d-orbitals for sp3d2- hybridisation. As H2O is weak field ligand, pairing of electrons will not occur which means ∆ < P, hence, it is a high spin complex.
Thus, for the complex, [Fe(H2O)6]3+ Hybridisation sp3d2
Magnetic character Paramagnetic Spin High spin complex
(ii) The complex, [Pt(en)2Cl2]2+ contains two symmetrical didentate ligands, ethylenediamine (en) and exists in two geometrical isomers, cis and trans. Trans isomer being symmetrical does not show optical isomerism and hence, this isomer is optically inactive. While cis being unsymmetrical shows optical isomerism.

Hence, the structure of geometrical isomer (i.e. trans isomer) of the complex [Pt(en)2Cl2]2+ is optically inactive is as follows:

Question 15.
Write the name, structure and the magnetic behaviour of each one of the following complexes:
(i) [Pt(NH3)2 CI(NO2)]
(ii) [CO(NH3), Cl2] Cl
(iii) Ni(CO), (Atomic no. of CO = 27, Ni = 28, Pt = 78)
Answer:
(i) [Pt(NH3)2 Cl(NO2)]
IUPAC name
Diamminechloridonitrito-N-platinum (II)
[Pt(NH3)2Cl(NO2)]
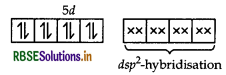
Structure Square planar.
Magnetic behaviour Diamagnetic (as all the electrons are paired.)
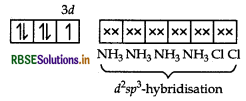
(ii) [CO(NH3),Cl2] Cl
IUPAC name
Tetraamminedichloridocobalt(III) chloride
In [CO(NH3)4Cl2]+, Co exits as CO3 ion. The electronic configuration of CO3+ ion is

Since, NH3 is a strong field ligand, so pairing of electrons takes place and the empty two 3d-orbitals, one 4s and three 4p-orbitals undergo dsp3-hybridisation. Among the 6d-sp3-hybrid orbitals, four are occupied by NH, molecules and two chlorine ions.
[CO(NH3),Cl2]+

Structure Octahedral
Magnetic behaviour Diamagnetic.
(iii) [Ni(CO)4]
The atomic number of Ni is 28 and its electronic configuration is 3d8 4s2. The oxidation state of Ni in this
Characteristics of complex
Geometry: Tetrahedral
Colour: Colourless because all the electrons are paired.
Magnetic character: Diamagnetic because all the electrons are paired.

Question 16.
Give the formula of each of the following coordination entities:
(i) CO3+ ion is bound to one CI-, one NH3 and two bidenate ethylene diamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions.
Write the name and magnetic behaviour of each of the above coordination entities.
(Atomic no. of CO = 27, Ni = 28)
Answer:
(i) The formula of the cationic complex is written as [metal symbol + ligand (with their number as sub-script)] counter ion satisfying the valency of metal or oxidation state of complex.
[CONH3Cl(en)2]+
IUPAC name Ammine bis (ethane-1, 2-diamine) chloridocobalt (III) ion
CO3+ = 3d6 4s0
Number of unpaired electrons = 0
So, the complex is diamagnetic.
Note: The formula of the anionic complex is written as counter ion satisfying valency of metal [metal symbol + ligand (with their number as subscript)].
(ii) [Ni(H2O)2(C2O4)2]2-
IUPAC name
Diaquadioxalationickelate (II) ion
Ni2+ = 3d8 4s0
Number of unpaired electrons = 2
So, the complex is paramagnetic.
Question 17.
Write the name, state of hybridisation, shape and magnetic behaviour of the following complexes: [COCl2], [Ni(CN)]2, [Cr(H2O)2 (C2O4)2]
(Atomic no. of CO = 27, Ni = 28, Cr = 24)
Answer:
(i) [COCl4]2-
IUPAC name Tetrachloridocobalt (II) ion In [COCl4]2-, CO is in + 2 state and has an outer electronic configuration of 3d7CI- is a weak field ligand so, pairing of electrons will not occur.
Four pairs of electrons one from each Cl-ion occupy the four sp3-hybrid orbitals. Therefore, the complex has tetrahedral geometry and paramagnetic nature as it contains three unpaired electrons. Thus,
Hybridisation: sp3
Shape: Tetrahedral
Magnetic nature: Paramagnetic
(ii) [Ni(CN)4]2-
IUPAC name Tetracyanonickelate (II) ion
Hybridisation dsp2
Shape Square planar
Magnetic nature Diamagnetic
(iii) [Cr(H2O)2(C2O)]2
IUPAC name
Diaquadioxalatochromate III ion Hybridisation d2sp3
Shape Octahedral
Magnetic behaviour Paramagnetic
COMPETITION CORNER:
Question 1.
Which one of the following is expected to exhibitoptical isomerism?
(en-ethylenediamine)
(a) cis-[Pt(NH3)2 Cl2]
(b) trans-[Pt(NH3)2Cl2]
(c) cis-[CO(en)2Cl2]
(d) trans-[CO(en)2 Cl2]
Answer:
(c) cis-[CO(en)2Cl2]

Question 2.
[CO(NH3)4 (NO2)2] Cl exhibits:
(a) Linkage isomerism, ionization isomerism and geometrical isomerism
(b) Ionization isomerism, geometrical isomerism and optical isomerism
(c) Linkage isomerism, geometrical isomerism andoptical isomerism
(d) Linkage isomerism, ionization isomerism and optical isomerism
Answer:
(a) Linkage isomerism, ionization isomerism and geometrical isomerism
Question 3.
[Cr(H2O)6] Cl3 (At. no. of Cr = 24) has a magnetic moment of 3.83 B.M. The correct distribution of electrons in the chromium of the complex is ?
(a) 3d1xy, 3d1x2-y2, 3d1xy
(b) 3d1xy, 3d1xy, 3d1xx
(c) 3d1xy, 3d1xy, 3d1x2
(d) 3d1x1-y2, 3d1x2, 3d1xz
Answer:
(b) 3d1xy, 3d1xy, 3d1xx
Question 4.
The d-electron configuration of Cr2+, Mn2+, Fe2 and Ni2+ are 3d, 3d and 3d respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
(a) [Fe(H2O)]2+
(b) [Ni(H2O)6]2+
(c) [Cr(H2O)6]2+
(d) [Mn(H2O)]2+
Answer:
(b) [Ni(H2O)6]2+
Question 5.
In which of the following coordination entities, the magnitude of A, (CFSE in octahedral field) will bemaximum?
(a) [CO(NH3)6]3+
(b) [CO(CN)6]
(c) [CO(C2O4)]3-
(d) [CO(H2O)6]3+
Answer:
(b) [CO(CN)6]
Question 6.
Which of the following complexes exhibits the highest paramagnetic behaviour?
(a) [Fe(en) (bpy) (NH3)2]2+
(b) [CO(OX)2 (OH)2]
(c) [Ti(NH3)6]3+
(d) [V(gly)2 (OH)2 (NH3)2]
Where gly = glycine, en = enthylenediamine and bpy = bipyridyl)
(At. nos: Ti = 22, V = 23, Fe = 26, CO = 27).
Answer:
(b) [CO(OX)2 (OH)2]

Question 7.
Which of the following complex ions is expected to absorb visible light? [At. no. Zn = 30, Sc = 21, Ti = 22, Cr = 24]
(a) [Ti(en)2 (NH3)2]+
(b) [Cr(NH3)6]2+
(c) [Zn(NH3)6]2+
(d) [Sc(H2O)3 (NH3)3]3+
Answer:
(b) [Cr(NH3)6]2+
Question 8.
Which of the following does not show optical isomerism?
(a) [CO(NH3)3 Cl]
(b) [CO(en) Cl2 (NH3)2]+
(c) [CO(en)3]3+
(d) [CO(en)2 Cl2]+
Answer:
(a) [CO(NH3)3 Cl]
Question 9.
Out of the TiF62-, COF63-, Cu2Cl2 and NiCl42-, thecolourless species are:
(a) Cu2Cl2 and NiCl2
(b) TiF2 and Cu2Cl2
(c) COF, and NiCl2
(d) TiF2-and COF63-
Answer:
(b) TiF2 and Cu2Cl2
Question 10.
The existence of two different coloured complexes with the composition of [CO(NH3)4 Cl2] is due to:
(a) coordination isomerism
(b) ionisation isomerism
(c) linkage isomerism
(d) geometrical isomerism
Answer:
(d) geometrical isomerism
Question 11.
Crystal field stabilisation energy for high spin doctahedral complex is:
(a) -1.2∆0
(b) -0.6 ∆0
(c) -1.8 ∆0
(d) -1.6 ∆0
Answer:
(b) -0.6 ∆0
Question 12.
The complexes [CO(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [CO(CN)6] are the examples of which type of isomerism ?
(a) Linkage isomerism
(b) Ionization isomerism
(c) Coordination isomerism
(d) Geometrical isomerism
Answer:
(c) Coordination isomerism
Question 13.
The complex, [Pt(Py)(NH3)BrCl] will have how many geometrical isomers?
(a) 3
(b) 4
(c) 0
(d) 2
Answer:
(a) 3

Question 14.
The d-electron configurations of Cr2+, Mn2+, Fe2+ and CO2+ are d1, do, do and d respectively. Which one of the following will exhibit minimum paramagnetic
behaviour?
(a) [Mn(H2O)6]2+
(b) [Fe(H2O)6]2+
(c) [CO(H2O)6]2+
(d) [Cr(H2O)6]2+
(At. nos: Cr = 24, Mn = 25)
Answer:
(c) [CO(H2O)6]2+
Question 15.
Of the following complex ions, which is diamagnetic in nature?
(a) [NiCl4]2-
(b) [Ni(CN)4]2-
(c) [CuCl]2-
(d) [COF6]3-
Answer:
(b) [Ni(CN)4]2-
Question 16.
Which one of the following is an outer orbital complex and exhibits paramangentic behaviour?
(a) [Ni(NH3)6]2+
(b) [Zn(NH3)]2+
(c) [Cr(NH3)6]3+
(d) [CO(NH3)6]3+
Answer:
(a) [Ni(NH3)6]2+
Question 17.
A magnetic moment of 1.73 B.M. will be shown by one among of the following:
(a) TiCl4
(b) [COCl6]4-
(c) [Cu(NH3)4]2+
(d) [Ni(CN)4]2-
Answer:
(c) [Cu(NH3)4]2+
Question 18.
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichloridotetraaquachromium (III) chloride. The number of moles of AgCl precipitated
would be:
(a) 0.003
(b) 0.01
(c) 0.001
(d) 0.002
Answer:
(c) 0.001
Question 19.
Among the following complexes, the one which shows zero crystal field stabilization energy is:
(a) [Mn(H2O)6]3+
(b) [Fe(H2O)6]3+
(c) [CO(H2O)6]2+
(d) [CO(H2O)6]3+
Answer:
(b) [Fe(H2O)6]3+
Question 20.
Which of the following complexes is used as ananticancer agent?
(a) mer-[CO(NH3)3 Cl3]
(b) cis-[PtCl (NH3)2]
(c) cis-K2 [PtCl2Br2]
(d) Na2COCl
Answer:
(b) cis-[PtCl (NH3)2]

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम