RBSE Class 12 Chemistry Important Questions Chapter 8 The d-and f-Block Elements
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 8 The d-and f-Block Elements Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 8 Important Questions The d-and f-Block Elements
Multi Choice Questions (MCQ):
Question 1.
A transition element containing only one electron in d-orbital is:
(a) 21Sc
(b) 25Mn
(c) 26Fe
(d) 29Cu
Answer:
(d) 29Cu
Question 2.
The basic character of the transition metal monoxide follows the order:
(a) CrO > VO > FeO > TiO
(b) TiO > FeO > VO > CrO
(c) TiO > VO > CrO > FeO
(d) VO> CrO > TiO > FeO
Answer:
(c) TiO > VO > CrO > FeO

Question 3.
Find the species/elemental atom has minimum number of unpaired electrons :
(a) Kr+
(b) Mn2+
(c) Fe3+
(d) Fe1+
Answer:
(a) Kr+
Question 4.
Which of the following species will be the strongest Lewis acid?
(a) Fe0
(b) Fe3+
(c) Fe2+
(d) Fe3+
Answer:
(b) Fe3+
Question 5.
Which is not a transition element?
(a) Cu
(b) AC
(c) Zn
(d) Pd
Answer:
(c) Zn
Question 6.
The maximum oxidation state of osmium is :
(a) +6
(b) +7
(c) +8
(d) +5
Answer:
(c) +8
Question 7.
Which forms interstitial compounds?
(a) Fe
(b) CO
(c) Ni
(d) All
Answer:
(d) All
Question 8.
The highest magnetic moment is shows by the transition metal with the configuration?
(a) 3d2
(b) 3d5
(c) 3d7
(d) 3d9
Answer:
(b) 3d5
Question 9.
K2Cr2O7 reacts with NHCl in presence of H2SO4 The product formed is:
(a) chromyl chloride with green vapour.
(b) chromous chloride with white vapour.
(c) chromous chloride with blue vapour.
(d) chromyl chloride with deep red colour.
Answer:
(d) chromyl chloride with deep red colour.

Question 10.
Which of the ions will give colourless aqueous solution?
(a) Ni2+
(b) Fe2+
(c) Cu2+
(d) Cu+
Answer:
(d) Cu+
Question 11.
In which of the following pairs, both the ions are coloured in aqueous solution?
(a) Sc3+, CO2+
(b) Ni2+, Cu+
(c) Ni2+, Ti3+
(d) Sc3+, Ti3+
Answer:
(c) Ni2+, Ti3+
Question 12.
The "spin-only” magnetic moment of Ni2 in aqueous solution would be (At No. Ni = 28)?
(a) 0
(b) 1.73
(c) 2.84
(d) 4.90
Answer:
(c) 2.84
Question 13.
The equivalent weight of KMnO, (formula weight: M) when it is used as an oxidant in neutral medium is:
(a) M
(b) M/2
(c) M/3
(d) M/5
Answer:
(c) M/3
Question 14.
Which of the following has the maximum number of unpaired electrons?
(a) Mg2+
(b) Ti3+
(c) V3+
(d) Fe2+
Answer:
(d) Fe2+
Question 15.
In C12O2, every Cr is linked to:
(a) two O-atoms
(b) three O-atoms
(c) four O-atoms
(d) five O-atoms
Answer:
(c) four O-atoms
Very Short Answer Type Questions:
Question 1.
Write the formula of an oxo-anion of manganese (Mn) in which it shows the oxidation state equal to its group number.
Answer:
The formula of an oxo-anion of manganese (Mn) in which it shows the oxidation state equal to its group number is MnO-4.
Here, oxidation state of Mn is + 7 which is same as is its group number.
Question 2.
Zn salts are white while Cu2+ salts are coloured. Why?
Answer:
Cu2+ salts are coloured because Cu2+ ion has 3d4s0 valence shell configuration with one unpaired electron and therefore, it is paramagnetic in nature. Hence, Cu2+ ion undergoes d-d transition and forms coloured salts. In contrast to Cu2+, Zn2+ has all paired electrons due to 3d10 4s0 valence shell configuration and therefore, it does not undergo d-d transition. Hence, Zn2+ salts are white.
Question 3.
Why do transition elements show variable oxidation states?
Answer:
ns and (n - 1) d electrons of transition metal haye very little difference in the energies and hence both can participate in bonding, which results in variable oxi- dation states. When ns electrons take part in bonding, they exhibit lower oxidation states whereas when (n - 1)d electrons alongwith ns electrons participate in bonding, they exhibit variable oxidation states.

Question 4.
Transition metals are much harder than the alkali metals. Why?
Answer:
Transition metals have more number of unpaired electrons in their valence shells. As a result they are able to form very strong metallic bonds and hence, they are much harder than alkali metals.
Question 5.
Which of following cations are coloured in aqueous solutions and why?
Sc3+, V3+, Ti4+, Mn2+ (At. no. Sc = 21, V=23, Ti = 22, Mn = 25)
Answer:
Electronic configuration of the ions are
Sc3+ = [Ar]3d04s0, V3+ = [Ar]3d24s0,
Ti4+ = [Ar]3d04s0 and Mn2+ = [Ar]3d54s0.
Since, V3+, Mn2+ contains partially filled d-orbitals, electrons can undergo d-d transition, hence are coloured while Ti4+ and Sc3+ do not have any electron. Therefore, are colourless.
Question 6.
Sc(21), is a transition element but Ca(20) is not. Why?
Answer:
Sc(21) with electronic configuration [Ar]3d14s2 have incompletely filled d-orbitals whereas Ca(20) does not. Thus, Sc(21) is a transition element.
Question 7.
Explain the following observation: Most of the transition metal ions exhibit characteristic colours in aqueous solutions.
Answer:
Most of the complexes of transition elements an coloured. This is because of the absorption of radia tion from visible light region to promote an electro from one of the d-orbital to another. These are know as d-d transitions and are responsible for impartin colour to the solution. The ions of transition element absorb the radiation of a particular wavelength an the rest is reflected, imparting colour to the solution
Question 8.
How would you account for the following? Many the transition elements are known to form interst tial compounds.
Answer:
In the crystal lattice, transition elements have interst tial vacant spaces into which small sized non-met atoms such as H, B, C, or N are trapped. These com pounds are known as interstitial compounds. Thes are neither typically ionic nor covalent, e.g. TiC, M N, etc.
Question 9.
How would you account for the following? The E° M2+ /M for copper is positive (0.34 V). Copper the only metal in the first series of transition el ments showing this behaviour.
Answer:
E° value for any metal depends on three factors: h dration enthalpy, ionisation enthalpy and enthalp of atomisation. The enthalpy of atomisation is very high for Cu but i hydration energy is very low. The high energy to tran form Cu(s) to Cu2+(aq) is not balanced by its hydratio enthalpy. Thus, for this conversion, EO value is pos tive.
Question 10.
Assign reason for the following: Copper (I) ion is not known in aqueous solution.
Answer:
Copper (I) ions are unstable in aqueous solution and undergo disproportionation.
2Cu+ → Cu2+ + Cu
The stability of Cu2+ (aq) rather than Cu+ (aq) is due the much more negative Ahyd H of Cu2+ (aq) than Cu which compensates more for the second ionisatio enthalpy of Cu.
Question 11.
Transition metals and their compounds generally e hibit a paramagnetic behaviour. Give reason.
Answer:
Paramagnetism arises due to the presence of unpaire electrons. When transition metal ions have unpaire electrons in d-orbitals (d1 to d0). They exhibit par magnetic behaviour.

Question 12.
Cr2+ is a strong reducing agent whereas Mn3+ wi the same (d) configuration is an oxidising agen Give reason.
Answer:
First, find the electronic configuration of Cr2+, Cr Mn2+, Mn3+. Then compare them to find the stabilit Cr2+ is a reducing agent because it can lose an electr to form Cr3+ which has stable 3d3 configuration (as has half-filled tą level). On the other hand, Mn3+ can accept an electron to form Mn2+ resulting in the half- filled (d) configuration which has extra stability. Thus, it behaves as an oxidising agent.
Question 13.
Explain the following observation: The enthalpies of atomisation of transition metals are quite high.
Answer:
Transition metals have high enthalpies of atomisation due to strong metallic bonding and additional covalent bonding. Metallic bonding is due to their smaller size while covalent bonding is due to d-d overlapping.
Question 14.
Name a member of the lanthanoid series which is well known to exhibit + 2 oxidation state.
Answer:
Europium have half-filled f-orbital in + 2 oxidation state. Thus, in lanthanoid series, it exhibit + 2 oxidation state.
Question 15.
What are the different oxidation states exhibited by the lanthanoids?
Answer:
Most common oxidation state of lanthanoid is + 3. However, Ce shows + 4, Eu and Yb show + 2 oxida- tion state becuase they acquire stable configuration.
Question 16.
Zr(Z = 40) and Hf(Z = 72) have almost identical radii. Give reason.
Answer:
Due to lanthanide contraction, the atomic radii of 4d and 5d transition series elements are almost same. That's why, Zr (Z = 40) and Hƒ (Z = 72) have almost identical radii.
Question 17.
How would you account for the following? Lanthanoids form primarily + 3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even + 6 being typical.
Answer:
The wide range of oxidation states of actinoids is at- tributed to the fact that the 5f, 6d and 7s energy levels are of comparable energies. Therefore, all these three subshells can participate in bonding. But the most common oxidation state of actinoids is also + 3.
Question 18.
How would you account for the following? Among lanthanoids, Ln (III) compounds are predominant. However, occasionally in solutions or in solid compounds, +2 and + 4 ions are also obtained.
Answer:
+2 and + 4 oxidation states are also obtained due to high stabilities of fo, f7 and f14 configuration.

Question 19.
Explain the following observation: The members of the actinoid series exhibit a larger number of oxidation states than the corresponding members of the lanthanoid series.
Answer:
Actinoids have lower ionisation energy and less effective nuclear charge, hence more number of valence electrons can take part in bond formation. It is due to the fact that 5f, 6d and 7s levels are of comparable energies. Therefore, actinoids exhibit +3, +4, +5, +6 and +7 oxidation states due to the participation of 5f, 6d and 7s electrons in bond formation. Hence, actinoids exhibit greater range of oxidation states than lanthanoids.
Question 20.
The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group member of the second (4d) series. Give reason.
Answer:
Metallic radii of third (5d) series of transition metals are virtually same as those of second (4d) series be- cause of the lanthanoid contraction. This is associated with the intervention of the 4f-orbitals which are filled before the filling of 5d-series of elements starts. The filling of 4f-orbitals before 5d-orbitals result in a regular decrease in atomic radii, called lanthanoid contraction which compensates the expected increase in atomic size with increasing atomic numbers.
Question 21.
What is meant by ‘lanthanoid contraction'?
Answer:
Lanthanoid contraction: The overall decrease in atomic and ionic radii from lanthanum to lutetium, due to the imperfect shielding of one electron by another in the same subshell is known as lanthanoid contraction.
Question 22.
Answer:
Chemistry of the actinoids is more complex in the view of their ability to exist in different oxidation states. Further, many of the actinoid elements are radioactive which make the study of these elements rather difficult.
Question 23.
State reasons for the following: Unlike Cr+, Mn2+, Fe3+ and the subsequent other M2+ ions of the 3d- series of elements, the 4d and the 5d-series metals generally do not form stable cationic species.
Answer:
The energy required to remove electron that is to form cationic species is more in 4d and 5d series because of greater effective nuclear charge which is due to lanthanoid contraction. Thus, 4d and 5d series metals generally do not form stable cationic species.
Question 24.
Chemistry of the actinoids is much more complicated than that of lanthanoids. Give reason.
Answer:
Chemistry of the actinoids is more complex in the view of their ability to exist in different oxidation states. Further, many of the actinoid elements are radioactive which make the study of these elements rather difficult.
Question 25.
La3+(Z = 57) and Lu3+(Z = 71) do not show any colour in solutions. Give reason.
Or
Lanthanum and lutetium do not show colouration in solutions. Give reason.
Answer:
La3+ (lanthanum) have 4ƒ0 and Lu3+ (lutetium) have 4f14 configuration. Because of the absence of unpaired electrons, these ions impart no colour to the solution.

Question 26.
There is a close similarity in physical and chemical properties of the 4d and 5d-series of the transition elements, much more than expected on the basis of usual family relationship.
Answer:
Due to the lanthanoid contraction, the metallic radii of the third series (5d) of transition metals are virtually the same as those of the corresponding group members of the second series (4d). This results in close similarity in their physical and chemical properties.
Question 27.
Why is europium (II) more stable than cerium (II)?
Answer:
Electronic configuration of Eu2+ is [Xe] 4f75d0 and Ce2+ is [Xe]4f15d1. Since, Eu2+ have half-filled 4f7 configuration, therefore, it is more stable than Ce2+ in which neither 4f nor 5d-subshell are half-filled or completely filled.
Short Answer Type Questions:
Question 1.
Use the data to answer the following and also justify giving reason:
|
|
Cr |
Mn |
Fe |
Co |
|
Eo M2+/M |
- 0.91 |
- 118 |
- 0.44 |
- 0.28 |
|
Eo M3+/M2+ |
-0.41 |
+1.57 |
+0.77 |
+1.97 |
(a) Which is a stronger reducing agent in aqueous medium, Cr2+ or Fe2+ and why?
(b) Which is the most stable ion in + 2 oxidation and why?
Answer:
(a) Reactivity series is made on the basis of standard reduction potential (E°) and E° for Cr is more negative than that of Fe. Thus, Fe get reduced and Cr get oxidised, in other words Cr is a stronger reducing agent.
(b) Among the given ions, the ion with more negative value of E° (red.) will loose the electron more easily, thus is more stable in (+) 2 oxidation state. Hence Mn in (+) 2 oxidation state is the most stable species.
Question 2.
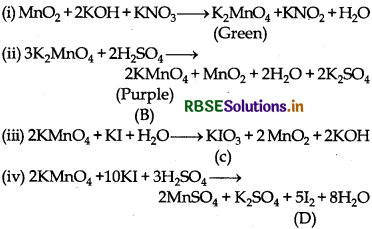
When MnO2 is fused with KOH in the presence of KNO3 as an oxidising agent, it gives a dark green compound (A).
Compound (A) disproportionates in acidic solution to give purple compound (B). An alkaline solution of compound (B) oxidises KI to compound (C) whereas an acidified solution of compound (B) oxidises KI to (D). Identify (A), (B), (C) and (D).
Answer:
When MnO2 is fused with KOH in presence of KNO3, green coloured compound K2MnO4 (A) is formed. Since (A) disproportionates in acidic medium to give purple compound (B), thus (B) is KMnO4. In alkaline medium, KMnO4 (B) oxidise KI to (C), thus compound (C) is KIO3; whereas in acidic medium, KI is oxidised to compound (D) which is I2.
The reactions involved are
Question 3.
Complete and balance the following chemical equations:
(a) Fe2+ + MnO4- → H+
(b) MnO4 + H2O + I- →
Answer:
(a) 5Fe2+ + MnO4 + 8H+ → Mn2+ + 5Fe3+ + 4H2O
(b) 2MnO4 + H2O + I- → IO3- + 2MnO2 + 2OH-

Question 4.
When chromite ore, FeCr2O4, is fused with NaOH ir the presence of air, a yellow-coloured compound (A] is obtained, which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KCl forms an orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A), (B) and (C).
(ii) Write one use of compound (C).
Answer:
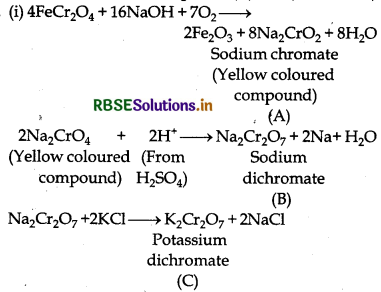
(ii) K2Cr2O7 (C) is a strong oxidising agent. It is used as a primary standard in volumetric analysis.
Question 5.
Complete the following chemical equation
(i) 8MnO4 + 3S2O32- + H2O →
(ii) Cr2O72- + 3Sn2+ + 14H+
Answer:
(i) 8MnO-4 + 3S2O32- + H2O → 8MnO2 + 6SO42- + 2OH-
(ii) Cr2O72- + 14H+ + 3Sn2+ → 3Sn4+ + 2Cr3+ + 7H2O
Question 6.
What are transition elements? Write two to characteristics of the transition elements.
Answer:
The elements which lie in between s-and p-block elements in the long form of periodic table belonging to groups 3-12 in which different electrons of d-orbitals are progressively filled in each of the four long periods are called transition elements. Their general elec- tronic configuration is (n - 1)d1-10 ns1-2. The general characteristics of transition elements are high melting and boiling points paramagnetic behaviour, variable oxidation states, catalytic properties, etc.
Question 7.
What is meant by "disproportionation"? Give an example of a disproportionation reaction in aqueous solution.
Answer:
The disproportionation reactions are those in which the same substance gets oxidised as well as reduced. When a particular oxidation state becomes less stable relative to other oxidation states (one lower and one higher), it undergoes disproportionation. e.g. Mn (VI) becomes unstable relative to Mn (VII) and Mn (IV) in acidic solution.
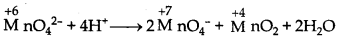
Question 8.
Suggest reasons for the following features of transition metal chemistry:
(i) The transition metals and their compounds are usually paramagnetic.
(ii) The transition metals exhibit variable oxidation states.
Answer:
(i) Paramagnetism arises due to the presence of unpaire electrons. When transition metal ions have unpaire electrons in d-orbitals (d1 to d0). They exhibit par magnetic behaviour.
(ii) ns and (n - 1) d electrons of transition metal haye very little difference in the energies and hence both can participate in bonding, which results in variable oxidation states. When ns electrons take part in bonding, they exhibit lower oxidation states whereas when (n - 1)d electrons alongwith ns electrons participate in bonding, they exhibit variable oxidation states.

Question 9.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with oxalic acid? Write the ionic equations for the reactions.
Answer:
Preparation of KMnO4 It is prepared by the fusion of pyrolusite ore (MnO2) with an alkali metal hydroxide and an oxidising agent like KNO3. Dark green KMnO4 is obtained which on disproportionation in neutral or acidic solution gives potassium permanganate.
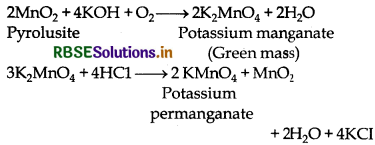
Reaction between acidified KMnO4 and oxalic acid: Oxalate ions or oxalic acid is oxidised.
5C2O42 + 2MnO4- + 16H+ → 2Mn2+ + 8H2O + 10CO2
Question 10.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reactions with (i) iodide and (ii) H2S.
Answer:
Potassium dichromate is a strong oxidising agent. In acidic solution, its oxidising action is represented as:
Cr2O2 + 14H + 6e- → 2Cr3+ + 7H2O
Ionic equations
(i) Reaction of K2Cr2O7 with I-
Cr2O72- + 14H+ + 6I- → 2Cr3+ + 3I2 + 7H2O
(ii) Reaction of K2Cr2O7 with H2S
Cr2O72- + 8H+ + 3H2S → 2Cr3+ + 3S + 7H2O
Question 11.
Why do transition elements show variable oxidation states? In 3d series (Sc to Zn), which element shows the maximum number of oxidation states and why?
Answer:
Due to lanthanide contraction, the atomic radii of 4d and 5d transition series elements are almost same. That's why, Zr (Z = 40) and Hƒ (Z = 72) have almost identical radii. Manganese exhibits all the oxidation states from + 2 to + 7. It shows largest number of oxidation states as it has the maximum number of unpaired electrons.
Question 12.
Assign a reason for each of the following observations:
(i) The transition metals (with the exception of Zn, Cd and Hg) are hard and have high melting and boiling points.
(ii) The ionisation enthalpies (first and second) in the first series of the transition elements are found to vary irregularly.
Answer:
(i) The transition metals (except Zn, Cd and Hg) are hard and have high melting and boiling points because transition elements display metallic properties. They show strong metallic bonding. Greater the number of valence electrons, stronger is the resultant bonding.
(ii) Ionisation enthalpy increases with increase in nuclear charge along each series. However, Cr has low first IE because loss of one electron gives stable electronic configuration (3d). Zn has very high IE because electron has to be removed from 4s orbital of the stable configuration (3d104s2). Similarly, Cr and Cu show much higher values for second IE because the second electron has to be removed from the stable configuration of Cr+ (3d) and Cu+(3d10).

Question 13.
Assign reason for each of the following:
1. Transition elements exhibit paramagnetic behaviour.
2. CO? is easily oxidised in the presence of a strong ligands.
Answer:
- Transition metals have in its ground state or ionised state have number of unpaired d-electrons which gives it a paramagnetic behaviour.
- In CO3+, electronic configuration is 3d. There is one unpaired electron even after pairing occurs in the preserce of a strong ligand. Hence, CO2+ is oxidised to more stable CO3+.
Question 14.
Account for the following:
(i) Mn2+ is more stable than Fe2+ towards oxidation to + 3 state.
(ii) The enthalpy of atomisation is lowest for Zn in first series (3d) of the transition elements.
Answer:
(i) Electronic configuration of Mn2+ is 3d which is half- filled and hence stable. Therefore, third ionisation enthalpy is very high, i.e. third electron cannot be lost easily. In case of Fe2+, electronic configuration is 3d Hence, it can lose one electron easily to give haif-filled stable configuration, i.e. 3ď3.
(ii) Enthalpy of atomisation depends upon the strength of bonding. In case of zinc, only metallic bonding occurs but no d-d overlapping takes place whereas in case of other metals of first transition series, both the metallic as well as covalent bonding are present. Thus, enthalpy of atomisation is lowest for Zinc.
Question 15.
Describe the general trends in the following properties of the first series (3d) of the transition elements:
(i) Number of oxidation states exhibited.
(ii) Formation of oxo metal ions.
Answer:
(i) In 3d-series all the elements show + 2 oxidation state except Sc (Sc+3). Oxidation states first in- creases from Sc to Mn due to increase in number of unpaired electrons and then decreases because pairing take place. Fe and Ni show zero oxidation state in metal carbonyls.
(ii) All the metals except scandium form MO oxides which are ionic. The highest oxidation number in the oxides, coincide with the group number and is at- tained in Sc2O3 to Mn2O7. Beyond group 7, no higher oxides of iron above Fe2O3 are known. Besides the oxides, the oxocations stabilise as VO2, VIV as VO2+ and Ti as TiO2+. As the oxidation number of a metal increases, ionic character decreases. In case of Mn, Mn2O, is a covalent green oil. Even CrO3 and V2O5 have low melting points. In their higher oxides, the acidic character is predominant. Thus, Mn2O7 gives HMnO and CrO3 gives H2CrO4 and H2Cr2O7. V2O5 is however, amphoteric though mainly acidic and it gives VO43- as well as to VO2+ salt.
Question 16.
Assign reasons for the following:
1. Copper (I) ion is not known to exist in aqueous solutions.
2. Both O2 and F2 stabilise high oxidation states of transition metals but the ability of oxygen to do so exceeds that of fluorine.
Answer:
1. This is because energy is required to remove one electron from Cu+ to Cu2+, high hydration energy of Cu2+ compensates for it. Therefore, Cu+ ion in an aqueous solution is unstable.
2. Both O2 and F2 stabilise high oxidation states but the ability of oxygen to stabilise these higher oxida- tion states exceeds that of fluorine due to ability of oxygen to form multiple bonds with the metal atoms. e.g. Mn form the highest fluoride as MnF. whereas, the highest oxide is Mn2O7. This is due to the tendency of oxygen to form multiple bonds.

Question 17.
Assign reasons for the following:
1. Transition metals and many of their compounds act as good catalysts.
2. Transition metals generally form coloured compounds.
Answer:
- Transition metals are good catalyst because of their ability to adopt multiple oxidation states and to form complexes. Transition metals because of their variable valencies and vacant d-orbitals form unstable intermediate compounds and provide a new path with lower activation énergy for the reaction.
- Transition metals form colored compounds due to the presence of vacant d-orbitals from the d-d transition of electrons which causes the color.
Question 18.
Complete the following equations:
(i) 2MnO4 + 5S2- + 16H+ →
(ii) Cr2O72- + 2OH →
Answer:
(i) 2MnO-4 + 5S2- + 16H+ → 2M2+ + 8H2O + 5S
(ii) Cr2O72- + 2OH- → 2CrO3- + H2O
Question 19.
How would you account for the following?
(i) The highest oxidation state of a transition metal is usually exhibited in its oxide.
(ii) The oxidising power of the following three oxo-ions in the series follows the order:
VO2+ < Cr2O72- < MnO-4
Answer:
(i) The highest oxidation state of a transition metal is usually exhibited in its oxides because of the ability of oxygen to form multiple bonds with metal.
(ii) It is because V in the lower oxidation state is less stable than Cr which in turn is less stable than Mn. Thus, MnO4 has a great tendency to get reduced and hence, behave as a good oxidising agent. Similarly, VO2+ has the least oxidising power.
Question 20.
How would you account for the following?
(i) Transition metals exhibit variable oxidation states.
(ii) Transition metals and their compounds act as catalysts.
Answer:
(i) They show variable oxidation state because transition metals have (n - 1)d orbitals empty that are closer to the outermost ns orbital in energy levels. These orbitals are never fully filled. So, they can always accommodate more electrons in (n - 1)d orbitals.
(ii) Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process.
Question 21.
Which metal in the first transition series (3d-series) exhibits +1 oxidation state most frequently and why?
Answer:
Copper (Cu) is the only metal in the first transition series (3d-series) which shows +1 oxidation state most frequently. It is because the electronic configuration of Cu is 3d10 4s1 which loses 1 electron and form Cu+ ion with stable 3d10 configuration.
Question 22.
Complete the following chemical reaction equations :
(i) Cr2O72- + I- + H+ →
(ii) MnO4 + NO-2 + H+ →
Answer:
(i) Cr2O72- + 6I- + 14H+ → 2Cr3+ + 7H2O + 3I2
(ii) 2MnO2 + 5NO-2 + 6H+ → 2Mn2+ + 5NO3 + 3H2O
Question 23.
Explain the following observations.
(i) Many of the transition elements are known to form interstitial compounds.
(ii) There is a general increase in density from tita- nium (Z = 22) to copper (Z = 29).
Answer:
(i) Lanthanoid contraction: The overall decrease in atomic and ionic radii from lanthanum to lutetium, due to the imperfect shielding of one electron by another in the same subshell is known as lanthanoid contraction.
(ii) As we move from left to right along a transition series (from Ti to Cu), the atomic radii decreases due to increase in nuclear charge. Therefore, atomic vol- ume decreases with increase in atomic mass. That's why density of transition metals increases from Ti to Cu.
Question 24.
Explain each of the following observations:
(i) With the same d-orbital configuration (d1), Cr2+ is a reducing agent while Mn3+ is an oxidising agent.
(ii) There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Answer:
(i) La3+ (lanthanum) have 4ƒ0 and Lu3+ (lutetium) have 4f14 configuration. Because of the absence of unpaired electrons, these ions impart no colour to the solution.
(ii) In general, atoms in a given series of transition metals show progressive decrease in radius with increasing atomic number. This is because as new elec- tron enters in a d-orbital, each time the nuclear charge increases by unity. Shielding effect of a d-electron is not effective and hence, effective nuclear charge increases and radius decreases. Both these effects counter each other, therefore change in atonic size is negligible.
Question 25.
Assign reasons for each of the following:
(i) Transition metals generally form coloured compounds.
(ii) Manganese exhibits the highest oxidation state of +7 among the 3d-series of transition elements.
Answer:
(i) Metallic radii of third (5d) series of transition metals are virtually same as those of second (4d) series be- cause of the lanthanoid contraction. This is associated with the intervention of the 4f-orbitals which are filled before the filling of 5d-series of elements starts. The filling of 4f-orbitals before 5d-orbitals result in a regular decrease in atomic radii, called lanthanoid contraction which compensates the expected increase in atomic size with increasing atomic numbers.
(ii) Mn has electronic configuration [Ar]3d 34s2. It has the maximum number of unpaired electrons in d orbitals and all the electrons in d orbital as well as in d orbital can take part in bond formation, therefore, it shows + 7 highest oxidation state.
Question 26.
Complete the following chemical equations:
(i) MnO-4(aq) + S2O32-(aq) + H2O (l) →
(ii) Cr2O72-(aq) + Fe2+(aq) + H+(aq) →
Answer:
(i) 8MnO4(aq) + 3S2O32-(aq) + H2O(l) → 8MnO2 + 6SO42- + 2OH-
(ii) Cr2O72-(aq) + 6Fe2+ (aq) + 14H+(aq) → 2Cr3+ + 6Fe3+ + 7H2O

Question 27.
Explain giving a suitable reason for each of the following:
(i) Transition metals and their compounds are generally found to be good catalysts.
(ii) Metal-metal bonding is more frequent for the 4d and 5d-series transition metals than that for the 3d-series.
Answer:
(i) Transition metals are good catalyst because of their ability to adopt multiple oxidation states and to form complexes. Transition metals because of their variable valencies and vacant d-orbitals form unstable intermediate compounds and provide a new path with lower activation énergy for the reaction.
(ii) Metal-metal bonding is more frequent for 4d and 5d series of transition metals than that for 3d series. It is due to poor shielding effect of 4f and 5f-orbitals, more unpaired electrons take part in metallic bond formation.
Question 28.
Complete the following reactions in the aqueous medium:
(i) MnO-4 + C2O42- + H+ →
(ii) Cr2O72- + H2S + H+ →
Answer:
(i) 2MnO2 + 5C2O42- + 16H+ → 2Mn2+ + 8H2O + 10CO2
(ii) Cr2O72-(aq) + 3H2S(g) + 8H+(aq) + 7H2O(l) → 2Cr3+(aq) + 3S
Question 29.
Complete the following chemical equations:
(i) Fe3+ + I- →
(ii) CrO42- + H+ →
Answer:
(i) 2Fe3+ + 2I- → 2Fe3+ + I2
(ii) 2CrO2 (aq) + 2H+ → + Cr2O72- + H2O
Question 30.
Write balance chemical equations of two reactions in which KMnO4 acts as an oxidising agent in the acidic medium.
Answer:
Oxidising reactions of KMnO4 in acidic solutions are as follows:
(i) Iodine is liberated from potassium iodide
10I- + 2MnO4 + 16H+ → 2Mn2+ + 8H2O + 5I2
(ii) Fe2+ ion (green) is converted into Fe3+ (yellow).
5 Fe2+ + MnO4 + 8H+ → Mn2+ + 4H2O + 5Fe3+
Question 31.
Explain the following observations:
(i) Generally, there is an increase in density of elements from titanium (Z = 22) to copper (Z = 29) in the first series of transition elements.
(ii) Transition elements and their compounds are generally found to be good catalysts in chemical reactions.
Answer:
(i) Lanthanoid contraction: The overall decrease in atomic and ionic radii from lanthanum to lutetium, due to the imperfect shielding of one electron by another in the same subshell is known as lanthanoid contraction.
(ii) Transition metals are good catalyst because of their ability to adopt multiple oxidation states and to form complexes. Transition metals because of their variable valencies and vacant d-orbitals form unstable intermediate compounds and provide a new path with lower activation énergy for the reaction.
Question 32.
Explain the following observations:
(i) Transition elements generally form coloured compounds.
(ii) Zinc is not regarded as a transition element.
Answer:
(i) Metallic radii of third (5d) series of transition metals are virtually same as those of second (4d) series be- cause of the lanthanoid contraction. This is associated with the intervention of the 4f-orbitals which are filled before the filling of 5d-series of elements starts. The filling of 4f-orbitals before 5d-orbitals result in a regular decrease in atomic radii, called lanthanoid contraction which compensates the expected increase in atomic size with increasing atomic numbers.
(ii) Zn (3d10 4s2) has completely filled d-orbitals in its atomic as well as in its common oxidation state (Zn2+ state). Therefore, it is not regarded as transition element.
Question 33.
Explain the following observations:
(i) Among the divalent cations in the first series of transition elements, manganese exhibits the maximum paramagnetism.
(ii) Cu+ion is not known in aqueous solutions.
Answer:
(i) 1. First, find the electronic configuration of Mn2+, then pair the electrons.
2. Find out the number of unpaired electrons. Mn2+ has maximum number of unpaired electrons. As paramagnetic nature is directly proportional to the number of unpaired electrons. Thus, it exhibits maximum paramagnetism.
(ii) The energy required to remove electron that is to form cationic species is more in 4d and 5d series because of greater effective nuclear charge which is due to lanthanoid contraction. Thus, 4d and 5d series metals generally do not form stable cationic species.
Question 34.
Account for the following:
1. Cu+ ions are not stable in aqueous solution.
2. Most of the transition metal ions exhibit paramagnetic behaviour.
Answer:
- The energy required to remove electron that is to form cationic species is more in 4d and 5d series because of greater effective nuclear charge which is due to lanthanoid contraction. Thus, 4d and 5d series metals generally do not form stable cationic specieś.
- Chemistry of the actinoids is more complex in the view of their ability to exist in different oxidation states. Further, many of the actinoid elements are radioactive which make the study of these elements rather diffi-
Question 35.
Account for the following:
(i) In the series Sc to Zn, the enthalpy of atomisation of zinc is the lowest.
(ii) E° value for the Mn3+/ Mn2+ couple is much more positive than that for Cr3+/Cr2+.
Answer:
(i) Electronic configuration of Mn2+ is 3d which is half- filled and hence stable. Therefore, third ionisation enthalpy is very high, i.e. third electron cannot be lost easily. In case of Fe2+, electronic configuration is 3d Hence, it can lose one electron easily to give haif-filled stable configuration, i.e. 3ď3.
(ii) The comparatively high E° value for Mn3+/Mn2+ is due to the fact that Mn2+ (d3) is quite stable whereas comparatively low value for Cr3+/Cr2+ is because of the extra stability of Cr3+. Therefore, Cr3+ cannot be reduced to Cr2+.

Question 36.
(i) potassium dichromate from sodium chromate and potassium chloride.
(ii) KMnO4 from K2MnO4.
Answer:
(i) Sodium chromate solution is acidified with H2SO4 to obtain orange coloured sodium dichromate.
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Potassium dichromate crystals are obtained by the treatment of sodium dichromate solution with potassium chloride.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
(ii) In neutral or acidic solution, K2MnO4 disproportionates to yield permanganate.
3MnO42- + 4H+ → 2MnO4 + MnO2 + 2H2O
NOTE: Commercially, KMnO4 is obtained by the electrolytic oxidation of manganate (VI) ion.
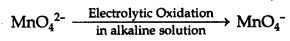
Question 37.
Explain the method of preparation of sodium dichromate from chromite ore. Give the equation representing oxidation of ferrous salts by dichromate ion.
Answer:
Sodium dichromate is prepared by chromite ore
(FeCr2O4).
(i) When chromite ore is fused with sodium carbonate in free access of air.
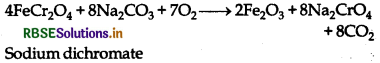
(ii) Yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give orange sodium dichromate solution.
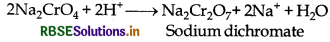
The equation representing oxidation of ferrous salts by dichromate ion is as follows
Cr2O72- + 14H+ + 6Fe2+ → 6Fe3+ + 2Cr3+ + 7H2O
Question 38.
Complete the following reactions:
(a) MnO2 + KOH + O2
(b) I- + MnO4 + H+ →
(c) Cr2O72- + Sn2+ + H+ →
Answer:
(a) 2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
(b) 10I- + 2MnO4- + 16H+ → 2Mn2+ + 5I2 + 8H2O
(c) Cr2O72- + 3Sn2+ + 14H+ → 3Sn4+ + 2Cr3+ + 7H2O
Question 39.
Give reasons:
(i) E° value for Mn3+/Mn2+ couple is much more positive than that for Fe3+/Fe2+.
(ii) Iron has higher enthalpy of atomisation than that of copper.
(iii) Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
Answer:
(i) Mn2+ compounds are more stable due to half-filled d-orbitals. Fe2+ compounds are comparatively less stable as they have six electrons in their orbitals. So, they tend to lose one electron from Fe2+ and get stable 3d-configuration in Fe3+. Therefore, comparatively high positive value of E° for Mn3+/Mn2+ indicates the stability of Mn2+ (d) whereas comparatively low value for Fe3+/Fe2+ indicates the extra stability of Fe3+ (d3).
(ii) Energy required to convert metallic crystal into individual atom is enthalpy of atomisation. In transition row elements it first increases and reaches to maximum upto middle element and then decreases. This is because of strong interatomic interaction due to unpaired electron. Greater the number of unpaired electron, stronger will be bonding and thus enthalpy of atomisation will also be more. Since iron has more unpaired electron than copper therefore its enthalpy of atomisation is more.
(iii) The metal ions with partially or incomplete filled d-orbitals will be coloured in aqueous solution. While the metal ions having either empty or completely filled d-orbitals are colourless. The colour will be due to d-d transition of electrons. Thus, the outer electronic configuration of metal ions are Sc3+: 3d°, Ti3+ : 3d1 Hence, among the given ions, Ti3+ will exhibit colour in aque- ous solution while Sc3+ will be colourless.
Question 40.
(i) Complete the following equations:
(a) 2MnO4- + 5SO32- + 6H+ →
(b) Cr2O72- + 6Fe2+ + 14H+ →
(ii) Bastion the data, arrange Fe2+, Mn2+ and Cr2+ in the increasing order of stability of +2 oxidation state.
E° Cr3+/Cr2+ = -0.4 V
E° Mn3+/Mn2+ = +1.5 V
E° Fe3+/Fe2+ = +0.8 V
Answer:
(i) (a) 5SO32 + 2MnO4 + 6H+ → 2Mn2+ + 3H2O + 5SO42-
(b) Energy required to convert metallic crystal into individual atom is enthalpy of atomisation. In transition row elements it first increases and reaches to maximum upto middle element and then decreases. This is because of strong interatomic interaction due to unpaired electron. Greater the number of unpaired electron, stronger will be bonding and thus enthalpy of atomisation will also be more. Since iron has more unpaired electron than copper therefore its enthalpy of atomisation is more.
(ii) Negative value of E° for Cr3+/Cr2+ shows that Cr2+ is least stable. Greater positive value for Mn3+/Mn2+ than that of Fe3+/Fe2+ shows that Mn2+ is more stable than Fe2+. Hence, stability of +2 oxidation state is in the order:
Cr2+ < Fe2+ < Mn2+

Question 41.
Write the preparation of following:
(i) KMnO4 from K2MnO4
(ii) Na2CrO4 from FeCr2O4
(iii) Cr2O72 from CrO2
Answer:
(i) (ii) In neutral or acidic solution, K2MnO4 disproportionates to yield permanganate.
3MnO42- + 4H+ → 2MnO4 + MnO2 + 2H2O
NOTE: Commercially, KMnO4 is obtained by the electrolytic oxidation of manganate (VI) ion.
(ii) 4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
(iii) 2CrO42- + 2H+ → Cr2O72- + H2O
Question 42.
(i) Account for the following:
(a) Cu+ is unstable in an aqueous solution.
(b) Transition metals form complex compounds.
(ii) Complete the following equation:
Cr2O22- + 8H+ +3NO2- →
Answer:
(i) ((i) Lanthanoid contraction: The overall decrease in atomic and ionic radii from lanthanum to lutetium, due to the imperfect shielding of one electron by another in the same subshell is known as lanthanoid contraction.
(ii) As we move from left to right along a transition series (from Ti to Cu), the atomic radii decreases due to increase in nuclear charge. Therefore, atomic vol- ume decreases with increase in atomic mass. That's why density of transition metals increases from Ti to Cu.
(b) Due to the comparatively smaller size of the metal ions, high ionic charges and the availability of vacant d-orbitals for bond formation, transition metals form a large number of complex compounds.
(ii) Cr2O72- oxidises nitrites, NO2 to nitrates, NO3-
Cr2O72- + 8H+ + 3NO2- → 2Cr3+ + 3NO3- + 4H2O
Question 43.
(i) How would you account for the following?
(a) Highest fluoride of Mn is MnF, whereas the highest oxide is Mn2O7.
(b) Transition metals and their compounds show catalytic properties.
(ii) Complete the following equation:
3MnO42- + 4H+ →
Answer:
(i) (a) Highest oxide of Mn is Mn2O7 because oxygen can form multiple bonds whereas fluorine can only form single bonds with metals therefore, highest fluoride of Mn is MnF4.
(b) (i) Iodine is liberated from potassium iodide
10I- + 2MnO4 + 16H+ → 2Mn2+ + 8H2O + 5I2
(ii) 3MnO42- + 4H+ → 2MnO4- + MnO2 + 2H2O
Question 44.
From the given data of E° values, answer the following questions:
E° (M2+/M) Cr Mn Fe Co Ni Cu
-0.91 -118 - 0.44 -0.28 -0.25 +0.34
(i) Why is E° Cu2+/Cu value exceptionally positive?
(ii) Why is E° (Mn2+/Mn) value highly negative as compared to other elements?
(iii) Which is a stronger reducing agent Cr2+ or Fe2+? Give reason.
Answer:
(i) Cr2O72- + 6I- + 14H+ → 2Cr3+ + 7H2O + 3I2
(ii) 2MnO2 + 5NO-2 + 6H+ → 2Mn2+ + 5NO3 + 3H2O
(ii) Ionisation enthalpy of Mn is lower than its hydration enthalpy due to stable 3d5 configuration. Thus, EMn2+/Mn is more negative.
(iii) Cr2+ is stronger reducing agent than Fe2+ because d4 → d3 transition occurs in case of Cr2+ to Cr3+ while d6 → d5 transition occurs in case of Fe2+ to Fe3+. In a medium like water, d3 is more stable as compared to d5.
Question 45.
Assign suitable reasons for the following:
(i) The Mn2+ compounds are more stable than Fe2+ towards oxidation to their + 3 state.
(ii) Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
Answer:
(i) Electronic configuration of Mn2+ = [Ar]18 3d5
Electronic configuration of Fe2+ = [Ar]18 3ď.
It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in + 2 state has a stable d configuration. Due to this, Mn2+ is resistance towards oxidation to Mn3+. Also, Fe2+ has 3d configuration and by losing one electron from 3d, its configuration changes to a more stable 3d configuration. Therefore, Fe2+ easily gets oxidised to Fe3+ oxidation state.
(ii) Sc3+ is colourless because it has do orbitals con- figuration, here d-d transition is forbidden. But in Ti3+ due to the presence of one electron in d-orbital (d1), d- d transition is possible (allowed) and hence Ti3+ is coloured.

Question 46.
Complete the following chemical equations:
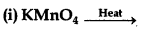
(ii) Cr2O72- + H2S + H+ →
Answer:
(i) On strong heating, KMnO4 decomposes to potassium manganate.
(ii) 4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Question 47.
Compare the stability of + 2 oxidation state for the elements of the first transition series. (Atomic number of Sc = 21 to Cu = 29)
Answer:
In first transition series, the stability of +2 oxidation state decreases from Sc to Cu. This is because of the increase in the sum of first and second ionisation en- ergies. However, the + 2 state of Mn, Ni and Zn is highly stable because of the presence of half-filled and fully-filled orbitals in Mn2+ and Zn2+ respectively. The stability in Ni2+ is due to its highly negative heat of hydration.
Question 48.
Describe the preparation of potassium permanganate from pyrolusite ore. Write the ionic equation for the reaction that takes place between acidified KMnO4 solution and iron (II) ions. The d-and f-Block Elements
Answer:
Preparation of KMnO4: Potassium permanganate is commercially prepared by mixing solution of KOH and powdered manganese oxide, with oxidizing agents like potassium chlorate. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
6KOH + 3MnO2 + 6KCIO3 → 3K2MnO4 + 6KCl + 3H2O
The potassium manganate (green) so formed is boiled with a large quantity of water and current of chlorine, CO2 and ozonized air is passed into the liquid until it is converted into permanganate. The MnO2 formed is removed continuously in order to prevent its breaking down the permanganate.
6K2MnO4 + 3Cl2 → 6KMnO4 (Potassium permanganate) + 6KCl
The solution of KMnO4 is drawn off from any precipitate of MnO2 concentrated and crystallized. The crystals are centrifuged and dried.
Reaction between acidified KMnO4 and iron (II) ions KMnO4 oxidises ferrous salts to ferric salt.
5Fe2+ + MnO4- + 8H+ → Mn2+ + 4H2O + 5Fe3+
Question 49.
Account for the following:
(i) The enthalpies of atomisation of the transition metals are high.
(ii) The lowest oxide of a transition metal is basic, the highest is amphoterie or acidic.
(iii) Cobalt (II) is stable in aqueous solution but in the presence of complexing agents, it is easily oxidised.
Answer:
(i) The enthalpies of atomization of a transition metal are high because they have a large number of unpaired electrons and hence have strong metallic bonding.
(ii) In lower oxidation states, transition metals behave like metals and metal oxides are basic in nature. Thus, in lower oxidation states, transition metal oxides are basic.
As the oxidation state increases, its metallic character decreases due to decrease in size, thus, it becomes less metallic or more non-metallic. Oxides of a non-metal may be acidic or neutral. Thus, in higher oxidation states, transition metal oxides are amphoteric or acidic.
(iii) In the presence of complexing agents, cobalt gets oxidised from + 2 to + 3 state because it provides energy to remove an electron from CO2+. Moreover, CO (III) is more stable than CO (II).

Question 50.
Identify the following:
(i) Transition metal of 3d series that exhibits the maximum number of oxidation states.
(ii) An alloy consisting of approximately 95% lanthanoid metal used to produce bullet, shell and lighter flint.
Answer:
(i) Manganese exhibit the largest number of oxidation states. It shows the oxidation states 2+,+3,+4,+5,+6 and +7. The reason is the maximum no. of unpaired respresent in the outermost shell. i.e. 3d94s2
(ii) Misch metal, a well known alloy, consists of lanthanoid metal (~95%), iron (~5%) and traces of S, C, Ca and Al, etc. and is used to produce bullets, shell and lighter flint.
Question 51.
Write one similarity and one difference between the chemistry of lanthanoids and that of actinoids.
Answer:
Comparison of lanthanoids and actinoids
Similarities:
- Both have mainly an oxidation state of +3.
- Both show magnetic and spectral properties.
Differences Lanthanoids:
- They have less tendency towards complex formation.
- Do not form oxocations.
Actinoids:
- They have greater tendency towards complex formation.
- Form oxocations, e.g. UO22+, PuO222+ and UO2+.
Question 52.
What is lanthanoid contraction? What are its two consequences?
Answer:
Consequences of lanthanoid contraction are
(i) With increase in atomic number, the basic strength of oxides and hydroxides of lanthanoids decreases.
(ii) Elements of second and third d-series exhibit simi- lar radii (e.g. Zr-160 pm, Hf-159 pm) and have very similar physical and chemical properties.
Question 53.
What is lanthanoid contraction? What is its effect on the chemistry of the elements which follow the lanthanoids?
Answer:
Lanthanoid contraction: lanthanide contraction, also called lanthanoid contraction, in chemistry, the steady decrease in the size of the atoms and ions of the rare-earth elements with increasing atomic number from lanthanum (atomic number 57) through lutetium (atomic number 71).
The cumulative effect of the contraction of the lanthanoid series causes the radii of the members of third transition series to be similar to those of corresponding members of second series. Zr (160 pm) and Hf (159 pm) have identical radii. Due to their similar radii, they have same physical and chemical properties.

Question 54.
Write the electronic configuration of Ce3+ ion and calculate the magnetic moment on the basis of spin-only formula. (Atomic number of Ce = 58)
Answer:
(i) Write the configuration of Ce and Ce3+.
(ii) Find the number of unpaired electrons.
(iii) Calculate magnetic momentu, using formula,
\(\mathrm{u}=\sqrt{n(n+2)}\)
58Ce = [Xe]4f15d16s1; Ce3+ = [Xe] 4f15d06s0
\(\mathrm{u}=\sqrt{n(n+2)}\)
(n = number of unpaired electrons)
\(\mathrm{u}=\sqrt{n(n+2)}\)
= 1.73 BM
Question 55.
Give reasons for the following:
(i) Transition elements and their compounds act as catalysts.
(ii) E° value for (Mn2+/Mn) is negative whereas for (Çu2+/Cu) is positive.
(iii) Actinoids show irregularities in their electronic configuration.
Answer:
(i) (i) Iodine is liberated from potassium iodide
10I- + 2MnO4 + 16H+ → 2Mn2+ + 8H2O + 5I2
(ii) E° value for (Mn33+/Mn) is negative whereas for (Cu2+/Cu) is positive because in case of copper, the enthalpy of atomisation is very high while - its hydration enthalpy is very low. The high energy to trans- form Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) The general electronic configuration of actinoids is 5f 1-14 6d0-1 7s2. The irregularities in the electronic configuration of actinoids is due to the stabilities of fo, f7 and f1 configuration of 5 f-orbitals.
Question 56.
Give reasons for the following:
(i) Transition metals form alloys.
(ii) Mn2O3 is basic whereas Mn2O7 is acidic.
(iii) Eu2+ is a strong reducing agent.
Answer:
(i) Atoms of transition metal can easily take place in the crystal lattice of another metal in the molten state and are miscible with each other and thus forms alloys.
(ii) With increase in the oxidation state of a given transition metal (i.e. Mn), the covalent character of its compound increases and thus acidic character also increases. Therefore, Mn2O3 (oxidation state = + 3) is basic while Mn2O7 (oxidation state = +7) is acidic.
(iii) Eu2+ having electronic configuration [Xe]4ƒ” is a strong reducing agent because in the aqueous solution, it reverts back to the most stable + 3 oxidation state.
Question 57.
(i) How would you account for the following?
(a) Actinoid contraction is greater than lanthanoid contraction.
(b) Transition metals form coloured compounds.
(ii) Complete the following equation:
2MnO-4 + 6H+ + 5NO-2 →
Answer:
(i) (a) In actinoids, 5f orbitals are filled which have more poor shielding effect than 4f orbitals in lanthanoids. Thus, the effective nuclear charge experienced by electrons in valence shells in case of actinoids is much more than that experienced by lanthanoids. Hence, the size contraction in actinoids is greater as compared to that of lanthanoids.
(b) Transition metals form colored compounds due to the presence of vacant d-orbitals from the d-d transition of electrons which causes the color.
(ii) The comparatively high E° value for Mn3+/Mn2+ is due to the fact that Mn2+ (d3) is quite stable whereas comparatively low value for Cr3+/Cr2+ is because of the extra stability of Cr3+. Therefore, Cr3+ cannot be reduced to Cr2+.

Question 58.
Give reasons for the following:
(i) Transition metals exhibit a wide range of oxidation states.
(ii) Cobalt (II) is very stable in aqueous solutions but gets easily oxidised in the presence of strong ligands.
(iii) Actinoids exhibit a greater range of oxidation states than lanthanoids.
Answer:
(i) Transition elements exhibit a wide variety of oxidation states in their compounds. For example: manganese shows all the oxidation states from +2 to +7 in its compounds. However, some elements exhibit few oxidation states, for example: Sc, Zn.
(ii) Cobalt (II) is stable in aqueous solution but in the presence of complexion agent, it undergoes change in oxidation state from +2 to +3 and is easily oxidised. This is because CO3+ has more tendency to form coordination complexes than CO2+.
(iii) Actinides exhibit larger oxidation states than lanthanides, because of the very small energy gap between 5f, 6d and 7s subshells. Thus, the outermost electrons get easily excited to the higher energy levels, giving variable oxidation states.
Question 59.
Compare the chemistry of the actinoids with that of lanthanoids with reference to the following:
(i) Electronic configuration
(ii) Oxidation states
(iii) Chemical reactivity
Answer:
(i) Electronic configuration
Lanthanoids = [Xe] 4f0-14 5d0-1 6s2
Actinoids = [Rn]5f0-14 6d0-17s2
(ii) Oxidation states: In lanthanoids, + 3 oxidation state is most common along with+2 and +4. While in actinoids, there is a greater range of oxidation states
because 5f, 6d and 7s levels are of comparable ener- gies. They show +2, +3, +4, +5, +6 and +7 oxidation states. Common oxidation state in actinoids is +3
(iii) Chemical reactivity: Lanthanoids are less reac- tive than actinoids. Actually, carlier members of lanthanoids are quite reactive similar to calcium but with increasing atomic number, they behave more like aluminium. Lanthanoids react with dilute acids to liberate H2 gas while actinoids react with boiling water and gives a mixture of oxide and hydrogen gas.
Long Answer Type Questions:
Question 1.
(i) Account for the following:
(a) Mn shows the highest oxidation state of +7 with oxygen but with fluorine, it shows the highest oxidation state of +4.
(b) Cr2+ is a strong reducing agent.
(c) Cu2+ salts are coloured while Zn2+ salts are white.
(ii) Complete the following equations:
(a) 2MnO2 + 4KOH + O2 →
(b) Cr2O72 + 14H+ + 6I- →
Ans.wer:
(a) With increase in the oxidation state of a given transition metal (i.e. Mn), the covalent character of its compound increases and thus acidic character also increases. Therefore, Mn2O3 (oxidation state = + 3) is basic while Mn2O7 (oxidation state = +7) is acidic.
(b) Cr2+ is a strong reducing agent because on oxida- tion, it forms a more stable Cr3+ ion with stable d3 (or t2g3) electronic configuration and hence, it can reduce others easily.
(c) Zn2+ has completely filled d-orbitals (3d10) while Cu2+ has incompletely filled d-orbitals (3d8). Due to this d-d transition take place and impart colour.
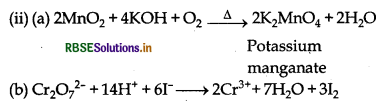
(b) Cr2O72- + 14H+ + 6I- → 2Cr3+ + 7H2O + 3I2
Question 2.
The elements of 3d transition series are given as:
Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn
Answer the following:
(i) Write the element which shows maximum number of oxidation states. Give reason.
(ii) Which element has the highest melting point?
(iii) Which element shows only +3 oxidation state?
(iv) Which element is a strong oxidising agent in +3 oxidation state and why?
Answer:
(i) Mn shows the highest number of oxidation state.
(ii) Chromium has highest melting point among all the given elements.
(iii) Scandium shows only + 3 oxidation state.
(iv) In the + 3 oxidation state, Mn is a strong oxidising agent because in Mn ion, Mn exists in 3d configuration which is less stable and it can reduce to Mn2+ giving a more stable 3d configuration. Hence, it acts as a strong oxidising agent.
Question 3.
(i) Complete the following equations:
(a) Cr2O72- + 2OH- →
(b) MnO4 + 4H+ + 3e- →
(ii) Account for the following:
(a) Zn is not considered as a transition element.
(b) Transition metals form a large number of complexes.
(c) The E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ couple.
Answer:
(i) (a) Cr2O72- + 2OH- → 2CrO42- + H2O
(b) MnO4- + 4H+ + 3e- → MnO
(ii) (a) As zinc atom has completely filled d-orbitals (3d10) in its ground state as well as oxidised state, therefore, it is not regarded as transition element.
(b) It forms complexes due to the presence of vacant d orbitals.
(c) The E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ couple or Fe3+/Fe2+ couple because Mn3+ ion receiving an electron gets d-subshell half-filled which is highly stable, while in case of Fe3+, d- subshell is already half-filled, so it does not receive electron easily.
Question 4.
(a) Mn3+ is a good oxidising agent.
(b) E°M2+/M values are not regular for first row transition metals (3d-series).
(c) Although F is more electronegative than O, the highest Mn fluoride is MnF4, whereas the highest oxide is Mn2O7.
(ii) Complete the following equations:
(a) 2CrO22- + 2H+ →
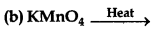
Answer:
(i) (a) Mn3+/Mn2+ has large positive E° value. Hence, Mn3+ can be easily reduced to Mn2+. Therefore, it is a good oxidising agent. Also, Mn2+ has half-filled electronic configuration, so it is more stable than Mn3+ state.
(b) There is decreasing negative electrode potentials of M2+/M in the first transition series due to increase in the sum of IE1 and IE2. It shows that in general, the stability of + 2 oxidation state decreases from left to right. Exceptions are Mn and Zn in which the greater stability of + 2 state for Mn is due to half-filled d- subshell (ď3) and that of Zn is due to completely filled d-subshell (d10).
(c) It is because oxygen can form multiple bonds, whereas fluorine can only form single bonds with metals.

Question 5.
(i) Complete and balance the following chemical equations:
(a) Cr2O72- + I- + H+ →
(b) MnO-4 + SO22- + H+ →
(ii) Explain the following observations:
(a) Transition elements and their compounds are known to act as catalysts.
(b) The higher oxidation states are usually exhibited by the members in the middle of a series of transition elements.
(c) The metal-metal bonding is more frequently found in the second and third series of transition elements.
Answer:
(i) (a) Cr2O72- + 6I- + 14H+ → 2Cr3+ + 7H2O + 3I2
(b) 5SO32 + 2MnO4 + 6H+ → 2Mn2+ + 3H2O + 5SO42-
(ii) (a) Transition metals are good catalyst because of their ability to adopt multiple oxidation states and to form complexes. Transition metals because of their variable valencies and vacant d-orbitals form unstable intermediate compounds and provide a new path with lower activation énergy for the reaction. Transition metals form colored compounds due to the presence of vacant d-orbitals from the d-d transition of electrons which causes the color.
(b) This is due to the presence of large number of un-paired electrons in d-orbitals in the middle of the series and involvement of all ns and (n-1)d electrons in the bonding. e.g. Mn has oxidation states from + 2 to +7.
(c) Metal-metal bonding is more frequent for 4d and 5d series of transition metals than that for 3d series. It is due to poor shielding effect of 4f and 5f-orbitals, more unpaired electrons take part in metallic bond formation.
Question 6.
(i) Calculate the number of unpaired electrons in the following gaseous state ions. Mn3+, Cr3+, V3+ and Fe2+ Which one of these is the most stable in aqueous solutions?
(Atomic number of V = 23, Cr = 24, Mn = 25, Fe = 26)
(ii) Explain the following observations:
(a) The transition metal ions are usually coloured in aqueous solutions.
(b) Cu(I) ion is not stable in an aqueous solution.
(c) The highest oxidation state of a transition metal is exhibited in its oxide or fluoride.
Answer:
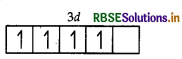
(i) Mn = 3d54s2
So, Mn3+ = 3d4
Number of unpaired electrons = 4
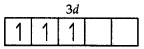
Cr = 3d54s1
Cr3+ = 3d3
Number of unpaired electrons = 3
V = 3d34s2
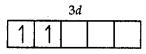
Number of unpaired electrons = 2
Fe = 3d 4s2
Fe2+ = 3d6
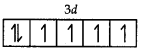
Number of unpaired electrons = 4
Mn3+ will be most stable because being smallest in size, it has maximum hydration energy and hence, more stability.
(ii) (a) Transition metal ions can be identified by their colour. Colour arises when some of the wavelengths of visible light are absorbed and the remaining wavelengths of light are transmitted or reflected. d electrons move from the ground state to an excited state when light is absorbed.
(b) This is because although energy is required to remove one electron from Cu+ to Cu2+, high hydration energy of Cu2+ compensates for it. Therefore, Cu+ ion in an aqueous solution is unstable. It disproportionates to give Cu2+ and Cu.
(c) Both fluorine and oxygen have small size, high electronegativity and also high negative electron gain enthalpy. They can oxidise a metal to the highest oxidation state. Thus, the highest oxidation states are shown in the oxides and fluorides of transition metals.
7. (i) Account for the following:
(a) The transition metals and their compounds act as good catalysts.
(b) The lowest oxide of transition metal is basic, whereas the highest is amphotericoracidic.
(c) A transition metal exhibits higher oxidation states in oxides and fluorides.
(ii) Describe the reactions involved in the preparation of K2Cr2O7 from chromite ore.
Answer:
(i) (a) (i) Iodine is liberated from potassium iodide
10I- + 2MnO4 + 16H+ → 2Mn2+ + 8H2O + 5I2
(b) The lower oxide of transition metal is basic because the metal atom has low oxidation state and still has electrons to donate whereas highest is acidic due to highest oxidation state and not left with free electrons.
(c) A transition metal exhibits higher oxidation state in oxides an fluorides because oxygen and fluorine are highly electro negative elements, small in size (and strongest oxidising agents).
(ii) Preparation of K2Cr2O7 from chrom ore: Chromite ore (FeCr2O4) is fused with sodium or potassium carbonate in free excess of air.

Yellow solution of sodium chromate is filtered, acidified with H2SO4 to obtain sodium dichromate.
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Sodium dichromate is treated with the solution of potassium chloride.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
(Orange crystals)
Question 8.
(i) Complete the following chemical equations:
(a) Cr2O72-(aq) + H2S(g) + H+(aq) →
(b) Cu2+(aq) + I-(aq) →
(ii) How would you account for the following?
(a) The oxidising power of oxoanions are in the order
VO2+ < Cr2O72- < MnO4-
(b) The third ionisation enthalpy of manganese (Z = 25) is exceptionally high.
(c) Cr2+ is a stronger reducing agent than Fe2+.
Answer:
(i) (a) (ii) 4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
(b) 2Cu2+ (aq) + 4I-(aq) → Cu2I2 + I2(g)
(ii) (a) Zn (3d10 4s2) has completely filled d-orbitals in its atomic as well as in its common oxidation state (Zn2+ state). Therefore, it is not regarded as transition element.
(b) The third ionisation enthalpy of Mn is very high because the third electron has to be removed from stable half-filled 3d configuration
(c) This can be explained on the basis of the standard electrode potential values E°(Cr3+/Cr2+ = –0.41 V) and E° (Fe3+/Fe2+ = + 0.77 V). Thus Cr2+ is easily oxidised to Cr3+ but Fe2+ cannot be as readily oxidised to Fe3+.
Question 9.
(i) How does the acidified potassium permanganate solution react with (a) iron (II) ions and (b) oxalic acid?
Write the ionic equations for the reactions.
(ii) Name the oxo metal anion of one of the transition metals in which the metal exhibits the oxidation state equal to the group number.
(iii) Account for the following:
(a) Scandium (Z = 21) is regarded as a transition element but zinc (Z = 30) is not.
(b) E°(M2+/M) value for copper is positive.
Answer:
(i) MnO-4 + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O
(ii) In MnO4 ion, the oxidation state of Mn is +7. It is equal to its group number 7.
In CrO2 ion, the oxidation state of Cr is + 6. It is equal to its group number 6.
(iii) (a) For an element to be a transition element, it should have incomplete filled d-subshell in ground state or in most common oxidation state. Sc in its ground state has incompletely filled d-orbitals (3d1, 4s2). The orbitals in the zinc (3d10 4s2) are completely filled in the ground state as well as in their common oxidation states. Therefore, Sc is regarded as transition element but Zn is not regarded as transition element.
(b) Copper has high atomisation ΔaH° and low hydration energy Δhyd H°. Due to which the E° value is positive.

Question 10.
(i) Account for the following:
(a) Transition metals show variable oxidation states.
(b) Zn, Cd and Hg are soft metals.
(c) E° value for the Mn3+ /Mn2+ couple is highly positive (+ 1.57 V) as compared to Cr3/Cr2+.
(ii) Write one similarity and one difference between the chemistry of lanthanoid and actinoid elements.
Answer:
(i) (a) One of the properties of transition metals is that they have variable oxidation states. In a transition metal atom, once the 3d subshell is occupied with electrons, the electrons in the 4s subshell increases to a higher energy.
(b) Zn, Cd and Hg have completely filled d1o electronic configuration. Hence, the metallic bonds present in them are weak. That's why they are soft metals and have low melting and boiling points.
(c) E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compared to Cr3+/Cr2+. Cr+3 exist in 3d3 half-filled d orbital (3 e; electrons in t2g ) extra stability is attained by Cr+3 than Cr+2. Hence Cr+3 → Cr+2 is less feasible.
(ii) lanthanoid elements are non-radioactive in nature, whereas all actinoid series members are radioactive. Lanthanoids are less reactive as compared to actinoids. The magnetic property of Lanthanoids is less complex as compared to actinoids.
Question 11.
(i) (a) How is the variability in oxidation states of transition metals different from that of the p-block elements?
(b) Out of Cu2+ and Cu2+, which ion is unstable in aqueous solution and why?
(c) Orange colour of Cr2O72- ion changes to vellow when treated with an alkali. Why?
(ii) Chemistry of actinoids is complicated as compared to lanthanoids. Give two reasons.
Answer:
(i) (a) p-block elements show variable oxidation state. It increases as we move from left to right in the periodic table. The maximum oxidation state shown by p- block element is equal to the total number of valence electrons. Whereas, d-block elements show different oxidation states because of incomplete d-subshell. The variable oxidation state is due to the participation of both ns and (n-1) d electrons in bonding.
(b) In aqueous solution, Cu+ ion undergoes disproportionation reaction.
2Cu+(aq) → Cu2+ + Cu(s)
The stability of Cu2+ ion in aqueous solution is due to negative enthalpy of hydration which compensates more for the IE2 of Cu.
(c) When orange solution containing Cr2O72 (dichromate ion) is treated with an alkali, a yellow solution of CrO42 (Chromate ion) is obtained.
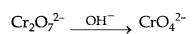
(ii) Chemistry of actinoids is complicated as compared to the lanthanoids. The two reasons are
(a) 5-forbital present in actinoids is more exposed to. the outer environment while 4-f orbital present in lanthanoid are deeply buried.
(b) Lanthanoids show limited number of oxidation states as + 2, + 3 and + 4 (out of which + 3 is most common). This is due to the large energy gap between 4f and 5d subshells. The dominant oxidation state of actinoids is also + 3 but they show a number of other oxidation states also like uranium (Z = 92) and plutonium (Z = 94) show + 3, +4, +5 and +6, neptunium shows + 3, + 4, + 5 and + 7. This is due to small energy difference between 5ƒ, 6d and 7s subshells.
Question 12.
(i) With reference to structural variability, chemical reactivity, write the differences between lanthanoids and actinoids.
(ii) Name a member of the lanthanoid series which is well known to exhibit + 4 oxidation state.
(iii) Complete the following equation:
MnO4- + 8H+ + 5e- →
(iv) Out of Mn3+ and Cr3+, which is more paramag- netic and why? (Atomic number of Mn = 25, Cr = 24)
Answer:
(i) Differences between lanthanoids and actinoids
Structural variability
|
Lanthanoids |
Actinoids |
|
There is small regular decrease in atomic and ionic radii of lanthanoids, known as lanthanoid contraction. |
There is also regular decrease in atomic and ionic radii of actinoids, known as actinoid contraction, but this contraction vary from element to element due to poor shielding effect of 5f electrons. They show great structural variability. |
Chemical reactivity
|
Lanthanoids |
Actinoids |
|
They have less tendency towards complex formation. Except promethium, they are nonradioactive. They do not form oxocations. Their oxides and hydroxides are less basic. |
They have stronger tendency towards complex formation. They are radioactive. 'They form oxocations Their oxides and hydroxides are more basic. |
(ii) Cerium (Ce) is well known to exhibit + 4 oxidation state.
(iii) MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
(iv) Outer shell electronic configuration of
Mn = 3d4s2 so, Mn3+ = 3d4

Number of unpaired electrons in Mn3+ = 4
Similarly, outer shell electronic configuration of Cr = 3d54s1 so, Cr3+ = 3ď3
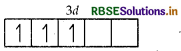
Number of unpaired electrons in Cr3+ = 3
As, Mn3+ has 4 unpaired electrons, whereas Cr3+ has 3 unpaired electrons, therefore Mn3+ exhibits more paramagnetism.

Question 13.
(i) How do you prepare:
(a) K2MnO4 from MnO2?
(b) Na2Cr2O7from Na2CrO4?
(ii) Account for the following:
(a) Mn2+ is more stable than Fe2+ towards oxidation to + 3 state.
(b) The enthalpy of atomisation is lowest for Zn in 3d-series of the transition elements.
(c) Actinoid elements show wide range of oxidation states.
Answer:
(i) (a) 2 MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
(b) 2NaCrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
(ii) (a) E.C. of Mn2+ [18Ar] 3d5 (Stable configuration) E.C. of Fe2+; [18Ar] 3d6. Since Mn2+ has stable half filled electronic configuration, therefore Mn2+ compounds are more stable than Fe2+ towards oxidation to their +3 state.
(b) Due to the absence of these unpaired electrons in Zinc, the interatomic bonding is weak in Zinc. Thus, the enthalpy of atomization of zinc is lowest as the result of this weak metallic bonding.
(c) Actinoid elements show wide range of oxidation states due to comparable energies of 5f, 6d and 7s orbitals.
Question 14.
(i) Name the element of 3d-transition series which shows maximum number of oxidation states. Why does it show so?
(ii) Which transition metal of 3d-series has positive E° M2+/M value and why?
(iii) Out of Cr3+ and Mn3+, which is a stronger oxidising agent and why?
(iv) Name a member of the lanthanoid series which is well known to exhibit + 2 oxidation state.
(v) Complete the following equation, MnO-2 + 8H+ + 5e-
Answer:
(i) Manganese exhibits all the oxidation states from + 2 to +7 as it has maximum number of unpaired electrons.
(ii) Copper has E°(M2+/M) value, as high energy (ΔaH; ΔiH) to transform Cu(s) to Cu(aq)2+ is not balanced by hydration enthalpy.
(iii) Mn3+ is stronger oxidising agent because the change from Mn3+ to Mn2+ results in half-filled (d) configuration which has extra stability. Thus, it behaves as a strong oxidising agent. On the other hand, Cr3+ has d3 configuration, i.e. half-filled t2, level, so it is already stable and does not reduce to less stable Cr2+. In the other words, it does not behave as an oxidising agent.
(iv) Europium (Eu) and Ytterbium (Yb) is well known to exhibit + 2 oxidation state.
(v) MnO-4 + 8H+ + 5e- → Mn2+ + 4H2O
COMPETITION CORNER:
Question 1.
Which of the following statement is wrong for lanthanon?
(a) All lanthanon are more reactive than aluminium.
(b) In volumetric analysis Ce(+4) solution is widely used as oxidant.
(c) Europium show (+2) oxidation state.
(d) As size decreases from Pr to Lu, basicity also decreases.
Answer:
(a) All lanthanon are more reactive than aluminium.
Question 2.
In which of the following pair of d-orbitals the electron density is parallal to the axis?
(a) dxy, dx2-y2
(b) dx2, dxy
(c) dxz, dyz
(d) dz2, dx2-y2
Answer:
(d) dz2, dx2-y2
Question 3.
What is the general molecular formula of product formed when sulphur is heated with lanthanon?
(a) Lns
(b) Lns2
(c) Ln3S2
(d) Ln2S3
Answer:
(d) Ln2S3

Question 4.
Which of the following statement is wrong in case of lanthanoid?
(a) All the lanthanoids are solid at room temperature.
(b) Their general oxidation state is +3.
(c) They can be separated by ion exchange method.
(d) The ionic radius of trivalent ion increases with increase in atomic number.
Answer:
(d) The ionic radius of trivalent ion increases with increase in atomic number.
Question 5.
Match the following:
Atomic number: Ti = 22, V = 23, Fe = 26
|
Transition element |
Magnetic moment (BM) |
|
(a)Titanium (III) |
4.9 |
|
(b) Vanadium (II) |
1.73 |
|
(c) Iron |
3.87 |
(a) A-2; B-3; C-1
(b) A-2; B-1; C-3
(c) A-1; B-2; C-3
(d) A-1; B-3; C-2
(e) A-3; B-2; C-1
Answer:
(a) A-2; B-3; C-1
Question 6.
Which is the correct order for the energy of orbitals for titanium atom?
(a) 3s 4s 3p 3d
(b) 4s 3s 3p 3d
(c) 3s 3p 3d 4s
(d) 3s 3p 4s 3d
Answer:
(c) 3s 3p 3d 4s
Question 7.
Which ion has the maximum magnetic moment?
(a) V3+
(b) Mn3+
(c) Fe3+
(d) Cu2+
Answer:
(c) Fe3+
Question 8.
Which of the following oxide has maximum basisity?
(a) La2O3
(b) Pr2O3
(c) Sm2O3
(d) Gd2O3
Answer:
(a) La2O3

Question 9.
Which of the following pair has approximate equal atomic radius because of lanthanoid contraction [atomic number is given is bracket]?
(a) Zr (40) and Nb (41)
(b) Zr (40) and Hf (72)
(c) Zr (40) and Ta (73)
(d) Ti (22) and Zn (30)
Answer:
(b) Zr (40) and Hf (72)
Question 10.
Which of the following lanthanoid is radioactive?
(a) Gadolinium
(b) Holmium
(c) Promethium
(d) Neodimium
Answer:
(c) Promethium
Question 11.
Which is chemically twin:
(a) Zr-Ta
(b) Nb-Tc
(c) Hf-Re
(d) Nb-Ta
Answer:
(d) Nb-Ta
Question 12.
Which of the following ion has magnetic moment equal to 2.83 B.M.? [Atomic number Ti = 22, Cr = 24,
Mn = 25, Ni = 28] ?
(a) Cr3+
(b) Mn2+
(c) Ti3+
(d) Ni2+
Answer:
(d) Ni2+
Question 13.
Gas lighter is made up of:
(a) alloy
(b) alkali metal
(c) noble metal
(d) none of these
Answer:
(a) alloy
Question 14.
Cause of lanthanoid contraction is
(a) decrease in nuclear charge.
(b) decrease in shielding effect.
(c) negligible shielding effect of f-orbitals.
(d) increase in nuclear charge.
Answer:
(c) negligible shielding effect of f-orbitals.
Question 15.
Pair of compound which exist simultaneously:
(a) FeCl2, SnCl2
(b) FeCl3, KI
(c) FeCl3, SnCl2
(d) HgCl2, SnCl2
Answer:
(a) FeCl2, SnCl2

Question 16.
Which of the following is a transition metal according to its ground state configuration?
(a) Au
(b) Hg
(c) Cd
(d) Zn
Answer:
(a) Au
Question 17.
The general electronic configuration of transition metals is :
(a) (n - 1)d1-5
(b) (n - 1)d1-10 ns1
(c) (n-1)d1-10 ns1-2
(d) ns2 (n - 1)d10
Answer:
Question 18.
Which of the following has the maximum number of unpaired d-electrons ?
(a) Zn
(b) Fe2+
(c) Ni3+
(d) Cu+
Answer:
(b) Fe2+
Question 19.
Which of the following does not show variable oxidation state?
(a) Cu
(b) Fe
(c) Ni
(d) Sc
Answer:
(d) Sc
Question 20.
Scandium [Z = 21] is a transition element but Zinc [Z = 30] is not because:
(a) both Sc3+ and Zn2+ ions are colourless and form compounds.
(b) in Sc, d-orbital is partially filled while in case of Zn, it is completely filled.
(c) in Zn, last electron enters in orbital.
(d) both Sc and Zn do not show variable oxidation state
Answer:
(b) in Sc, d-orbital is partially filled while in case of Zn, it is completely filled.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम