RBSE Class 12 Chemistry Important Questions Chapter 6 General Principles and Processes of Isolation of Elements
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 6 General Principles and Processes of Isolation of Elements Important Questions and Answers.
RBSE Class 12 Chemistry Chapter 6 Important Questions General Principles and Processes of Isolation of Elements
Multi Choice Questions (MCQ):
Question 1.
The formula of Haematite is :
(a) Fe3O4
(b) Fe2O3
(c) FeS4
(d) FeO
Answer:
(b) Fe2O3
Question 2.
Which metal is extracted from its ore by electrolytic method?
(a) Pb
(b) Cu
(c) Al
(d) Ag
Answer:
(c) Al

Question 3.
Galena is an ore of :
(a) Ag metal
(b) Pb metal
(c) Cu metal
(d) Fe metal
Answer:
(b) Pb metal
Question 4.
The most abundant metal in the earth's crust is:
(a) Fe
(b) Al
(c) Ca
(d) Na
Answer:
(b) Al
Question 5.
Fool's gold is:
(a) As2O3
(b) Sb2S5
(c) FeS2
(d) Alloy of Cu-Zn
Answer:
(c) FeS2
Question 6.
Cryolite is an ore of:
(a) Fe
(b) Al
(c) Cu
(d) Ag
Answer:
(b) Al
Question 7.
Which of the following is not an ore of Aluminium?
(a) Bauxite
(c) Diaspore
(b) Corrundum
(d) Azurite
Answer:
(d) Azurite
Question 8.
Silver metal is extracted from its argentite ore by the method of:
(a) self reduction
(b) carbon-reduction
(c) formation of complex salt
(d) by electrolytic method
Answer:
(c) formation of complex salt
Question 9.
Magnetite is an ore of:
(a) Mg
(b) Ag
(c) Mn
(d) Fe
Answer:
(d) Fe
Question 10.
Froth floatation process is used to concentrate which type of ores?
(a) Sulphide ores
(c) Silicate ores
(b) Phosphate ores
(d) Oxide ores
Answer:
(a) Sulphide ores
Question 11.
Gravity separation method is used to concentrate which type of ores?
(a) Sulphide ores
(b) Phosphate ores
(c) Silicate ores
(d) Oxide ores
Answer:
(d) Oxide ores

Question 12.
Magnetic separation method is used for:
(a) separating magnetic ores or gangue.
(b) separating coloured ores or gangue.
(c) separating colourless ores or gangue.
(d) none of the above.
Answer:
(a) separating magnetic ores or gangue.
Question 13.
Iron can be extracted from its oxide ores by which method?
(a) Carbon reduction method
(b) Auto reduction method
(c) Carbon mono oxide reduction method
(d) Complex formation method
Answer:
(c) Carbon mono oxide reduction method
Question 14.
Copper can be extracted from its sulphide ore by:
(a) electrolytic reductions
(b) formation of complex
(c) auto reduction
(d) carbon reduction
Answer:
(c) auto reduction
Question 15.
Those natural occurring components from which el- ement can be extracted profitably and conveniently known as:
(a) minerals
(b) gangue
(c) ores
(d) flux
Answer:
(c) ores
Very Short Answer Questions:
Question 1.
What is the role of zinc metal in the extraction of silver?
Answer:
In the extraction of silver zinc metal acts as a reducing agent.
Question 2.
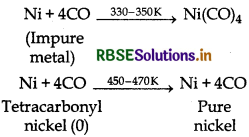
Name the method that is used for refining of nickel.
Answer:
Mond's process is used for refining of nickel metal. It involves the following reactions:
Question 3.
Name the method used for refining of copper metal.
Answer:
Electrolysis is the method used for refining the copper metal. In this method, block of impure metal is used as anode and a thin strip of pure metal as cathode. A solution of copper sulphate acts as an electrolyte.
Question 4.
Which reducing agent is employed to get copper from the leached low grade copper ore?
Answer:
Scrap iron or H2 is used as a reducing agent to get copper from the leached low grade copper ore.
Question 5.
On what principle is the method of zone-refining of metals based?
Answer:
Zone-refining: It is based on the fact that the impurities are more soluble in the molten state than in the solid state of the metal.
Question 6.
Name the depressant which is used to separate ZnS and PbS ores in froth floatation process.
Answer:
Sodium cyanide is the depressant that is used to separate ZnS and PbS ores in the froth flotation process.
Question 7.
What is the function of collectors in the froth floatation process for the concentration of ores?
Or
What is the role of collectors in froth floatation process?
Answer:
Collectors are reagents that are used to selectively adsorb onto the surface of particles. A monolayer is formed by the collectors on the particles surface that essentially makes a thin film of non-polar hydrophobic hydrocarons.
Question 8.
Name the chief ores of aluminium and zinc.
Answer:
Chief ore of aluminium is bauxite (AlO, (OH)3 – 2x) where, 0 < x < 1 and of zinc is zinc blende or sphalerite (Zns).

Question 9.
What is the role of CO2, in the extractive metallurgy of aluminium from its ore?
Or
What role is played by CO2 in getting pure alumina (Al2O3) in the extraction of aluminium?
Answer:
The aluminate in solution is neutralised by passing CO2 and hydrated Al2O3 is precipitated.
2Na[Al(OH)4](aq) + CO2(g) → Al2O3.XH2O(s) + 2NaHCO3(aq)
Question 10.
What are the collectors used in froth floatation process? Name a substance that can be used as such.
Answer:
Collectors are reagents that are used to selectively adsorb onto the surface of particles. A monolayer is formed by the collectors on the particles surface that essentially makes a thin film of non-polar hydrophobic hydrocarons.
Question 11.
What is the role of NaOH in the metallurgy of aluminium?
Answer:
Role of NaOH in the metallurgy of aluminium: The ore of aluminium (bauxite) usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. Concentration of bauxite ore is carried out by digesting the powdered ore with a concentrated solution of NaOH at 473 - 523 K and 35 - 36 bar pressure. This way, Al2O3 is leached out as sodium aluminate (and SiO2 too as sodium silicate) leaving behind the impurities.
Al2O3(s) + 2 NaOH(aq) + 3H2O(l) → 2Na [AI(OH)4](aq)
Question 12.
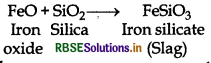
What is the function of SiO2 in the metallurgy of copper?
Answer:
Silica (SiO2) is added in the reverberatory furnace during the extraction of Cu to remove impurities of iron oxide (FeO) present in the ore. Silica here acts as flux and reacts with iron oxide gangue to remove it as slag, iron silicate.
Question 13.
What is the composition of copper matte?
Answer:
Copper matte contains Cu2S and FeS.
Question 14.
Name the methods used for the vapour phase refining of impure titanium and nickel metals.
Answer:
Van-Arkel method for titanium and Mond's process for nickel.
Question 15.
Write the reaction involved in the extraction of silver after the silver ore has been leached with NaCN.
Answer:
During leaching, Ag is oxidised to Ag+ which then combines with CN ions to form soluble complex, [Ag(CN)2]-.
Silver is then recovered as follows
2 [Ag(CN)2 (aq) + Zn(s) → + 2Ag (s) + [Zn(CN)4]2- (aq)
Question 16.
What is the role of graphite in the electrometallurgy of aluminium?
Answer:
In the metallurgy of aluminium, steel cathode and graphite anode are used. The graphite anode is useful for the reduction of Al2O3 into Al in Hall-Heroult process.
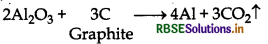
Question 17.
How is copper extracted from a low grade ore of it?
Answer:
Copper is extracted by hydrometallurgy from grade ores: It is leached out using acid or bacteria. The solution containing Cu2+ is treated with iron or H2.
Cu2+ (aq) + H2(g) → Cu(s) + 2H+ (aq)
Cu2+ (aq) + Fe(s) → Cu(s) + Fe2+ (aq)
Question 18.
Although thermodynamically feasibile, in practice, magnesium metal is not used for reduction of alumina in the metallurgy of aluminium. Why?
Answer:
In the Ellingham diagram, Al2O3 and MgO curve intersect to a point at the temperature below 1623K. Hence, Mg can reduce alumina but Mg is a costlier metal than Al2O3. So, the process will not be economical.
Question 19.
Differentiate between a mineral and an ore.
Answer:
Naturally occurring chemical substances obtained from earth crust obtained by mining are known as minerals. The minerals from which metal can be extracted chiefly, easily and profitably are known as ores.
Question 20.
What types of ores can be concentrated by magnetic separation method?
Answer:
Those ores which are magnetic in nature and associated impurities are non-magnetic in nature or vice-versa, are concentrated by magnetic separation method.

Question 21.
Copper matte is charged into a silica lined converter in extraction of copper. What is in the role of silica lining here?
Answer:
In copper matte, iron sulphide, FeS is present as impurity which in the presence of oxygen converts into iron oxide, a basic impurity. Silica (SiO2) is an acidic flux.
General Prin Copper matte containing Cu2S and FeS (impurity) is charged into silica lined converter. Some silica is also added to it. Here, silica acts as a flux. A hot air blast is blown to convert FeS to FeO.
FeO is removed by silica as slag.
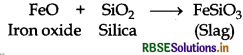
Short Answer Questions:
Question 1.
Describe the role of the following:
(i) Sil, in the extraction of copper from copper matte.
(ii) NaCN in froth floatation process.
Answer:
(i) Silica in the extraction of copper:
Silica (SiO2) is added in the reverberatory furnace during the extraction of Cu to remove impurities of iron oxide (FeO) present in the ore. Silica here acts as flux and reacts with iron oxide gangue to remove it as slag, iron silicate.

(ii) NaCN acts as depressant, e.g. it prevents ZnS from coming in froth but allows Pbs to come with the froth.
Question 2.
Explain the principle of the method of electrolytic refining of metals. Give one example.
Answer:
Electrolytic refining: In this method, the impure metals is made anode and a strip of pure form of same metal is made cathode. Aqueous solution of salt of same metal is taken as electrolyte. On passing electric current, metal ions from the electrolyte are deposited at the cathode in the form of pure metal while an equivalent amount of metal dissolves from the anode and goes into the electrolyte solution as metal ions.
Electrolytic refining of copper: Here, anodes are of impure copper and pure copper strips are taken as cathode. The electrolyte is acidified solution of copper sulphate. Due to electrolysis, pure copper from anode is transferred to cathode.
At anode, Cu → Cu2+ + 2e-
At cathode, Cu2+ + 2e- → Cu
Impurities from the blister copper deposit as anode mud which contains impurities like antimony, selenium, tellurium, silver, gold and platinum. Write the role of the following:
Question 3.
(i) CO in the purification of nickel.
(ii) Graphite rod in the electrometallurgy of aluminium.
Answer:
(i) Impure nickel reacts with CO to form volatile tetracarbonyl nickel (O) which on heating forms pure nickel.
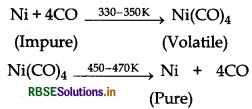
(ii) In the metallurgy of aluminium, steel cathode and graphite anode are used. The graphite anode is useful for the reduction of Al2O3 into Al in Hall-Heroult process.
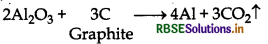
Question 4.
Write the role of the following:
(i) Iodine in the refining of zirconium.
(ii) Silica in the extraction of copper from copper matte.
Answer:
(i) Crude zirconium is heated in an evacuated vessel with iodine, volatile zirconium iodide is formed. It is decomposed on a tungsten filament, electrically heated to about 1800 K. Pure zirconium is deposited on tungsten filament.
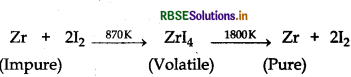
(ii) Silica (SiO2) is added in the reverberatory furnace during the extraction of Cu to remove impurities of iron oxide (FeO) present in the ore. Silica here acts as flux and reacts with iron oxide gangue to remove it as slag, iron silicate.

Question 5.
Write the chemical reactions involved in the extraction of silver from silver ore.
Answer:
Extraction of silver: The chief ore of silver is argentite or silver glance (Ag2S). The following reactions are used in the extraction of silver.
2AgS(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Ag(CN)2-(aq) + 4OH-(aq) + 2s
2[Ag(CN)2]-(aq) + Zn(s) → 2Ag(s) + [Zn(CN)4]2-(aq)
4Ag + 8NaCN + O2 + 2H2O → 4Na[Ag(CN)2] + 4NaOH
Or
Ag2S + 4NaCN → 2Na[Ag(CN)2] + Na2S
Sodium dicyanoargentate (I) (Soluble complex)
2Na[Ag(CN)2] + Zn → Na2[Zn(CN)4] + 2Ag↓
Question 6.
Describe the underlying principle of each of the following processes:
(i) Recovery of silver from the solution obtained by leaching silver ore with a solution of NaCN.
(ii) Electrolytic refining of a crude metal.
Answer:
(i) When silver ore is treated with NaCN solution, silver ore is dissolved and impurities remain insoluble. Recovery of silver from the solution is done by displacement method.
4Ag(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Ag(CN)2]-(aq) + 4OH-(aq)
2[Ag(CN)2]-(aq) + Zn(s) → 2Ag(s) + [Zn(CN)4]2-(aq)
In this reaction, zinc acts as a reducing agent.
(ii) Refining is a method of removing impurities in order to obtain metals of high purity. The impurities are removed from crude metal by various methods based on the properties of the metal and the properties of impurities. Some methods involved in the purification of crude metal are: Distillation. Liquation
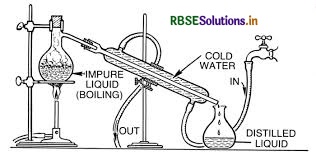
Question 7.
Describe underlying principles of the following processes:
1. Froth floatation process of concentration of ores.
2. Vapour phase refining of metals.
Answer:
- Froth floatation process: It is based on the fact that sulphide ores are preferentia wetted by pine oil whereas, the gangue particles are wetted by water.
- Vapour phase refining is when the impure form of metal is changed to a volatile compound that is collected. After that, it will go through the process of decomposition and then be used again to produce a pure metal.

Question 8.
Define the following terms:
1. Roasting
2. Calcination
Answer:
- Roasting: The process of heating of metal ore below its melting point in the presence of air is called roasting.
- Calcination: The process of heating of metal ore in the absence of air is called calcination.
Question 9.
Give reasons for the following:
(i) Alumina is dissolved in cryolite for electrolysis instead of being electrolysed directly.
(ii) ZnO can be reduced to metal by heating with carbon lout Cr2O3 cannot be reduced by heating with carbon.
Answer:
(i) Cryolite is added to alumina to lower its melting point and to make it more conducting.
(ii) Consider Ellingham diagram and find the ∆G° for the formation of ZnO and Cr2O3. For the process to be spontaneous, ∆G° must be negative. ∆G° for the formation of Cr2O3 is more negative than that of ZnO. Thus, the sum of ∆G° in the two combined redox process is positive in case of Cr2O3/C couple and negative in case of ZnO/C couple. That's the reason that carbon cannot reduce Cr2O3 but can reduce zinc oxide.
Question 10.
(i) Which solution is used for the leaching of silver metal in the presence of air in the metallurgy of silver?
(ii) Out of C and CO, which is a better reducing agent in the lower temperature range in the blast furnace to extract iron from its oxide ore?
Answer:
(i) In the metallurgy of silver and that of gold, the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air (for O2).
(ii) Consider Ellingham diagram for the value of ∆G of C and CO at different temperatures. On the basis of ∆G, give answer.
At lower temperature (<1073 K), ∆G° for the formation of CO from C is less negative than ∆G° for the formation of Fe2O3. Thus, ∆G° for the reduction of iron oxide with C will be positive and hence, the reaction is not possible. However, ∆G° for the formation of CO2 from CO is more negative than ∆G° for the formation of Fe2O3. Thus, for Fe2O3: CO redox couple, ∆Go° is negative, i.e. the reduction is possible. Thus, CO is a better reducing agent than C at lower temperature range for reducing iron oxide.
Question 11.
(i) Give an example of zone refining of all metals.
(ii) What is the role of cryolite in the metallurgy of aluminium?
Answer:
(i) Zone-refining method is used for the production of semiconductors and various other metals of high purity. e.g. silicon, boron, germanium, gallium and indium.
(ii) Cryolite is used as a solvent for bauxite in the electrolytic production of aluminum and has various other metallurgical applications, and it is used in the glass and enamel industries, in bonded abrasives as a filler, and in the manufacture of insecticides. A large amount of synthetic cryolite is made from fluorite.
Question 12.
(i) Which of the following ores can be concentrated by froth floatation method and why?
Fe2O3, ZnS, Al2O3
(ii) What is role of silica in the metallurgy of copper?
Answer:
(i) Froth floatation method is used to concentrate only sulphide ores because of their preferential wettability by pine oil. Thus, ZnS being a sulphide ore will be concentrated by this method.
(ii) The role of silica in the metallurgy of copper is to remove the iron oxide obtained during the process of extraction of pure copper from copper pyrite, SiO2 acts as acidic flux which combines with iron oxide(FeO) to form iron silicate (FeSiO3).
Question 13.
1. Name the method used for removing gangue from sulphide ores.
2. How is wrought iron different from steel?
Answer:
- Froth floatation method is used to remove gangue from sulphide ores because of the high wettability of ore in pine oil as compared to gangue.
- Wrought iron is almost pure iron with around 0.02 - 0.03% carbon whereas steel contains 0.15% - 0.25% of carbon. Wrought iron is ductile and resistant to corrosion whereas steel has more strength due to more percent of carbon in it.

Question 14.
Name the principal ore of aluminium. Explain the significance of leaching in the extraction of aluminium.
Answer:
The principal ore of aluminum iş bauxite, usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. In leaching of alumina, concentration is carried out by digesting the powdered ore with a concentrated solution of NaOH at 473-523 K and 35 - 36 bar pressure. This way, Al2O3 is leached out as sodium aluminate leaving impurity behind
Al2O3(s) + 2NaOH(aq) + 3H2O(l) → 2Na[Al(OH)4](aq)
Question 15.
Name one chief ore each of copper and aluminium. Name the method used for the concentration of these two ores.
Answer:
One chief ore of copper is copper pyrites (CuFeS2) and of aluminium is bauxite (Al2O3. xH2O) or AlOx(OH)3-2x Froth floatation process is used for the concentration of copper pyrite and leaching is used for the concentration of bauxite.
Question 16.
Which methods are usually employed for purifying the following metals?
(i) Nickel
(ii) Germanium
Mention the principle behind each one of them.
Answer:
(i) Purification of nickel:
- The Mond process, sometimes known as the carbonyl process, is a technique created by Ludwig Mond in 1890, to extract and purify nickel.
- This process involves the fact that carbon monoxide combines with nickel readily and reversibly to give nickel carbonyl.It is purified by Mond's process.
(ii) Purification of germanium: Zone-refining is used to obtain metal of very high purity. The principle on which it is based is that an impure molten metal on gradual cooling will deposit its crystals of pure metal, while the impurities will be left in the remaining part of molten metal. Germanium metal can be purified by this method.
Question 17.
The extraction of gold by leaching with NaCN, involves both oxidation and reduction. Justify giving chemical equations.
Answer:
The extraction of Au involves leaching the metal with CN- which is an oxidation reaction because during the leaching process, Au is first oxidise to Au+ by air (O2) and then combined with CN to form a soluble complex.
4Au(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2-](aq) + 4OH-(aq)
In the above process Zn acts as a reducing agent.
2[Au(CN)2]-(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2-(aq)
Thus, extraction of Au by leaching with NaCN involves both oxidation and reduction.
Question 18.
Describe the principle controlling each of the
following processes.
(i) Vapour phase refining of titanium metal.
(ii) Froth floatation method of on concentration of a sulphide ore.
Answer:
(i) van-Arkel method is used for refining titanium. Crude titanium is heated in an evacuated vessel with iodine, titanium iodide is formed which is more covalent, volatilises.

Titanium iodide is decomposed on a tungsten filament electrically heated to about 1800 K. Pure titanium is deposited on the filament.
(ii) The principle of froth flotation process is that sulphide ores are preferentially wetted by the pine oil, whereas the gangue particles are wetted by the water. Hence the method used for the concentration of sulphide ores is froth flotation.
Question 19.
Describe the principle controlling each of the
following processes:
(i) Preparation of cast iron from pig iron.
(ii) Preparation of pure alumina (Al2O3) from bauxite ore.
Answer:
(i) Cast iron is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (in pig iron, C is 4%) and in cast iron, C is about 3% and is extremely hard and brittle.
(ii) Leaching of alumina from bauxite ore: Bauxite usually contains SiO2, iron oxide and titanium oxide (TiO2) as impurities. Powdered ore is digested with conc. NaOH solution at 473 - 523 K and 35 - 36 bar pressure. Al2O3 and SiO2 are dissolved in the solution while impurities do not.
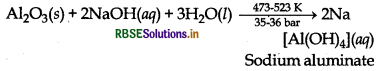
Filtrate is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. Some freshly prepared hydrated Al2O3 is also added to solution to induce precipitation.
2Na[Al(OH)4](aq) + 2CO2(g) → Al2O3.xH2O(s) + 2NaHCO3(aq)
Precipitate is filtered, washed, dried and heated to give back pure Al2O3.
Question 20.
How is chemical reduction different from electrolytic reduction? Name a metal of each which is obtained by
(i) electrolytic reduction.
(ii) chemical reduction.
Answer:
In chemical reduction, reduction is done by heating with reducing agent while in electrolytic reduction, reduction is done by electrolysis. Chemical reduction is the process involving electron gain. Usually, reduction of the metal oxide involves heating with reducing agent. The reducing agent combines with oxygen of the metal oxide.
M2Oy + yC → xM + yCO
Some metal oxides get reduced easily while others are very difficult to be reduced.
In electrolytic reduction, metal in solution or montent state is reduced by etectrolysis. In simple electrolysis, Mn+ ions are discharged at negative electrode (cathode) and deposited there.
- Sodium is extracted from fused NaCl by electrolytic reduction.
- Iron is obtained by chemical reduction.
Question 21.
(i) Name the method of refining which is
(a) used to obtain semiconductor of high purity.
(b) used to obtain low boiling metal.
(ii) Write chemical reactions taking place in the extraction copper from Cu2S.
Answer:
(i) (a) Zone refining method is used for producing semiconductor and other metals of very high purity. e.g. Si, Ge, Ga, B, In etc.
(b) Distillation method is used to refine low boiling metals which are volatile in nature.
(ii) Extraction of copper from Cu2S involves the following reactions:
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2S + 2Cu2O → 6Cu + SO2

Question 22.
(i) Write the role of 'CO' in the purification of nickel.
(ii) What is the role of silica in the extraction of copper?
(iii) What type of metals are generally extracted by electrolytic method?
Answer:
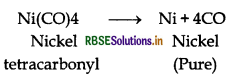
(i) In the purification of nickel; impure nickel is heated in a steam of carbon monoxide forming a volatile complex nickel tetracarbonyl which is decomposed when heated at 450 K to give pure nickel.
(ii) In the extraction of copper, silica is added to remove iron impurity in the form of fusible slag.
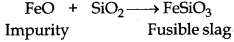
(iii) Electrolytic method is used for the extract of highly electropositive metals of group 1 and group 2 in the periodic table, e.g. Na, K, Mg etc.
Question 23.
What is the role of
(i) depressants in froth floatation?
(ii) carbon monoxide in Mond's process?
(iii) concentrated sodium hydroxide in leaching of alumina from bauxite?
Answer:
(a) Role of depressants in froth-floatation: Sometimes it is possible to separate two sulphide ores by adjusting proportion of oil to water or by using 'depressants' For example, in case of an ore containing ZnS and PbS, the depressant used is NaCN.
Thus, the substances which selectively prevent certain type of particles from forming the froth with the bubbles are called depressants.
(b) Role of carbon mono-oxide in Mond's process Carbon mono-oxide is used for refining. Ni is purified by this method. Impure nickel is heated in a stream of carbon monoxide forming a volatile complex nickeltetracarbonyl which is decomposed when heated at 450 K to give nickel metal.

(c) Role of concentrated sodium hydroxide in leaching of alumina from bauxite: Pure aluminium is obtained from the bauxite ore by treating the powdered ore with a concentrated solution of NaOH, resulting in dissolution of Al2O3, leaving the impurities behind as,
Al2O3(s) + 2OH- (aq) + 3H2O → 2AI(OH)-4(aq)
Question 24.
Write the chemical reactions involved in the process of extraction of gold. Explain the role of dilute NaCN and Zn in this process.
Answer:
In extraction of gold, NaCN is used for leaching o metal. The chemical reactions involved in the process are given below
4Au(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2]-(aq) + 4OH-(aq)
2[Au(CN)2]-(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2-(aq)
or
4Au + 8NaCN + O2 + 2H2O → 4Na[Au(CN)2] + 4NaOH
2Na[Au(CN)2] + Zn → Na2[Zn(CN)4] + 2Au↓
In this process, Zn acts as a reducing agent.
Question 25.
(i) Why does copper obtained in the extraction from copper pyrites have a blistered appearance?
(ii) What is the role of depressants in the Froth floatation process?
Answer:
(i) Copper obtained in the extraction from copper pyrites have a blistered appearance due to evolution of SO2. Thus, it is also called blistered copper.
- 2Cu2S + 3O2 → 2Cu2O + 2SO2↑
- 2Cu2O + Cu2S → 6Cu + SO2↑
(ii) The substances which selectively prevent certain type of particles from forming the froth with the bubbles are called depressants. e.g. in case of an ore containing ZnS and PbS, NaCN is used as depressant.
Question 26.
Out of PbS and PbCO3 (ores of lead), which one is concentrated by froth floatation process preferably?
Answer:
Out of Pbs and PbCO3 Pbs is concentrated by froth floatation process. It is generally used for removing gangue from sulphide ores. This method is used for the extraction of those metals in which the ore particles are preferentially wetted by oil and gangue by water.
Question 27.
1. Name the method of refining of nickel.
2. What is the role of limestone in the extraction of iron from its oxides?
Answer:
- Mond's process is used for refining of nickel.
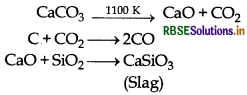
- Limestone decomposes to form CaO which acts as a flux and combines with silica to form fusible slag, calcium silicate. Thus, CaO removes silicate impurity of the ore as slag. The molten slag being lighter separates out from iron.

Question 28.
(i) Name the method of refining of metals such as germanium.
(ii) In the extraction of Al, impure Al2O3 is dissolved in conc. NaOH to form sodium aluminate and leaving behind impurities. What is the name of this process?
(iii) What is the role of coke in the extraction of iron from its oxides?
Answer:
(i) Zone-refining.
(ii) Leaching.
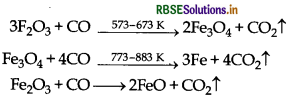
(iii) Coke reduces CO2 to CO which helps in the reduction of iron oxides as follows.
- CO2(g) + C(s) → 2CO(g)
- 3Fe2O3 + CO → 2Fe3O4 + CO2
- Fe2O3 + CO → 2FeO + CO2
- FeO + CO → Fe + CO2
Question 29.
(i) Indicate the principle behind the method used for the refining of zinc.
(ii) Which form of the iron is the purest form of commercial iron?
Answer:
Electrolytic refining method is used for the refining of zinc. In this method, anode is made up of impure metal and cathode is made up of the same pure metal. Anode and cathode are put in a suitable electrolytic solution of soluble salt of the same metal. The more basic metal ions remain in the solution and the less basic metal ions go to the anode mud. On passing, current, following reactions take place:
At Anode: M → Mn+ + ne-
At Cathode: Mn+ + ne- → M
Therefore, in electrolysis, there is the transfer of metal in pure form from the anode to the cathode.
Question 30.
Outline the principles of refining of metais by the following methods.
1. Distillation
2. Electrolysis
Answer:
- Distillation: This is a very useful method for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
- Electrolysis is a process of decomposition of an electrolyte by the passage of electricity through its aqueous solution or molten state. As a result, cation move toward cathode and anion moves towards anode.
Question 31.
(i) What is the role of dilute NaCN in the extraction of gold?
(ii) What is 'copper matte'?
Answer:
(i) Role of NaCN in the extraction of gold: In this process, leaching of gold ore with a dilute solution of NaCN occurs in the presence of air from which the gold is obtained later by displacement method.
4Au(s) + 8CN-(aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2]-(aq) + 4OH-(aq)
2[Au(CN)2]-(aq) + Zn(s) → [Zn(CN)4]2-(aq) + 2Au(s)
(ii) Copper matte (containing Cu2S and Fes) is obtained by the fusion of roasted copper sulphide concentrated ores. It is done to separate the copper sulphide from the gangue and other metals that floats on the surface of the melt.
Question 32.
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron. How is pig iron different from cast iron?
Or
Write the reactions taking place in different zones of the blast furnace to obtain iron.
Answer:
Reactions at the lower temperature range (500 - 800 K)
(Reduction zone)
Combustion zone : Reactions at higher temperature range (900 - 1500 K)
C + CO2 → 2CO(g)
FeO + CO → Fe + CO2
Cast iron contains about 3% carbon whereas pig iron contains about 4% carbon. Pig iron is converted into cast iron by heating molten pig iron with scrap iron and coke in especially designed furnace.
Question 33.
Write down the reactions which occur in upper, middle and lower zones in the blast furnace during the extraction of iron from iron ore.
Answer:
Reduction of iron oxide in blast furnace
(i) Lower zone of the blast furnace
C + O2 → CO2 + Heat
C + CO2 → 2CO
Coke is burnt to give temperature upto 2200 K at lower part of the blast furnace.
(ii) Middle zone of the blast furnace
CO and heat move up in the furnace. The temperature range in the middle zone of the blast furnace is 900 - 1500 K.
FeO + CO → Fe + CO2
Limestone is also decomposed to CaO which removes silicate impurity of the ore as slag.
(iii) Upper zone of the blast furnace
Temperature range in this zone is 500 - 800 K. Following reactions take place in this zone:
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
The iron obtained from blast furnace is known as pig iron. It contains about 4% carbon.
Question 34.
Describe how the following changes are brought about?
(i) Pig iron into steel.
(ii) Zinc oxide into metallic zinc.
Answer:
Pig iron contains 4% C and small amount of S, P, Si and Mn. When it is heated strongly in bessemer converter in the presence of oxygen, C gets oxidised to CO2, P to P2O5S to SO, which are removed as gases. Si is converted into SiO2 and Mn into Mno. Both react with each other and form MnSiO2(slag) which is removed. This results into the formation of wrought iron. Then, wrought iron is heated by adding 0.5% of carbon to get steel.
(ii) Reduction of zinc oxide is done using coke. The temperature in this case is higher than that in case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.
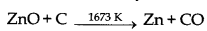
The metal is distilled off and collected by rapid chilling.

Question 35.
Describe how the following changes can be brought about?
Impure copper into pure copper.
Answer:
Impure copper into pure copper: Copper is refined using an electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. Acidified aqueous solution of copper sulphate acts as an electrolyte. The net result of electrolysis is the transfer of copper in pure form from the anode to cathode.
At anode Cu → Cu2+ + 2e
At cathode Cu2+ + 2e → Cu
Impurities deposit as anode mud below anode which contains Sb, Se, Te, Ag, Au, Pt, etc.
Question 36.
Give reasons for the following:
(i) Alumina is dissolved in cryolite instead of being electrolysed directly.
(ii) Extraction of copper directly from sulphide ores is less favourable than that from its oxide ore through reduction.
Answer:
(i) Melting point of alumina is very high and it is a bad conductor of electricity. Cryolite is added to alumina to lower its melting point and to make it conductor of electricity.
(ii) Consider the standard free energy of formation (∆G°) of reactants and products. More negative ∆G° means higher is the stability.
The standard free energy for the formation of CS2 is less negative than that of CO2 and CO. Moreover, ∆G°/ T line for CO has a negative slope but there is no compound analogous to CO with a steep negative ∆G°/T line.
Therefore, in case of CS2, the overall ∆G° value is positivè, i.e. the reaction is non-feasible.
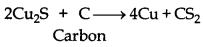
Thus, carbon is a good reducing agent for oxides but not for sulphides. Hence, it is essential to convert the sulphide ores into oxide by roasting before subjecting to reduction with C.
Alternate answer:
The standard free energy (∆fG) of formation of Cu2S is greater than those of CS2 and H2S. Neither carbon nor hydrogen can reduce Cu2S to Cu metal.
Cu2S + H2 → 2Cu + H2S
2Cu2S + C → Cu + CS2
On the other hand, ∆fG° of Cu2O is much lower than that of CO2. So, carbon can easily reduce Cu2O to Cu as follows:
Cu2O(s) + C(s) → 2Cu(s) + CO(g)
Therefore, the extraction of copper directly from sulphide ores is less favourable than that from its oxide ore through reduction.
Long Answer Questions:
Question 1.
Describe the principle involved in each of the following processes:
(i) Mond's process for refining of nickel
(ii) Column chromatography for purification of rare elements.
Answer:
(i) Mond's process for refining of nickel (vapour phase refining) is based upon the principle that nickel is heated in the presence of carbon monoxide to form nickel tetracarbonyl, which is a volatile complex.

Then, the obtained nickel tetracarbonyl is decomposed by subjecting it to a higher temperature (450 - 470 K) to obtain pure nickel metal.
(ii) Column chromatography is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. In it, there are two phases, mobile phase and stationary phase. The stationary phase is immobile and immiscible. Al2O3 column is usually used as the stationary phase in column chromatography.
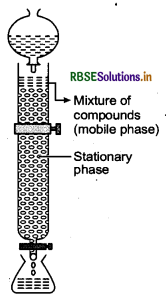
The mobile phase may be a gas, liquid or supercritical fluid in which the sample extract is dissolved. Then, the mobile phase is forced to move through the stationary phase. The component that is more strongly adsorbed on the column takes a longer time to travel through it than the component that is weakly adsorbed. The adsorbed components are then removed (eluted) by using a suitable solvent (eluant).
Question 2.
Write chemical reactions taking place in the extraction of aluminium from bauxite ore.
Answer:
Chemical reaction taking place in extraction of aluminium from bauxite ore: Aluminium is mainly isolated from bauxite ore (impurities are SiO2, FeO, TiO2) which involves two steps
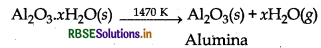
Step I Concentration of ore (Leaching of alumina) Concentration of bauxite ore is carried out by leaching (Baeyer's process). In this process, pure alumina (Al2O3) is obtained. The principal ore of aluminium, i.e. bauxite, usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. Concentration is carried out by digesting the powdered ore with a concentrated solution of NaOH at 473 - 523 K and 35 - 36 bar pressure. Al2O3 is leached out as sodium aluminate (and SiO2 as sodium silicate) leaving the impurities behind.
Al2O3(s) + 2NaOH(aq) + 3H2O → 2Na[Al(OH)4](aq)
The aluminate in solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. Here, the solution is seeded with freshly prepared samples of hydrated Al2O3 which induces precipitation.
2Na [Al(OH)4](aq) + CO2(g) → Al2O2.xH2O(s) + 2NaHCO3(aq)
The sodium silicate (impurities of silica) remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3.
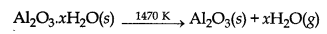
Step II Electrolytic reduction (Hall's process) It is also known as the Hall-Hernult process. In this process, purified alumina is mixed with Na3AlF6 or CaF2 (cryolite or fluorspar), which lowers the melting point of alumina and brings conductivity. In the electrolytic cell, steet cathode and series of graphite anode are used. The overall reaction is
2Al2O3 + 3C → 4AI + 3CO2↑
The electrolytic reactions are as follows
Al2O3 → 2Al3+ + 3O2-
At cathode
Al3+ + 3e- → Al(l)
At anode
C(s) + O2-(melt) → CO(g) + 2e-
C(s) + 2O2- (melt) → CO2(g) + 4e-
COMPETITION CORNER:
Question 1.
Which of the following ore is concentrated by froth floatation method?
(a) Galena
(b) Cassetterite
(c) Magnetite
(d) Cuprite
Answer:
(a) Galena

Question 2.
In Van-Arkel method for the purification of zirconium:
(a) all the impurities of oxygen and nitrogen are removed.
(b) impurities of CO is removed.
(c) impurities of hydrogen is removed.
(d) impurities of silica is removed.
Answer:
(a) all the impurities of oxygen and nitrogen are removed.
Question 3.
Composition of copper matte is:
(a) Cu2S + FeS
(b) Cu2S+ Cu2O
(c) Cu2S+ FeO
(d) Cu2O + FeS
Answer:
(a) Cu2S + FeS
Question 4.
Match the column I with column II:
Column-I
A. Cyanide process
B. Froth floatation process
C. Electrolytic reduction
D. Zone refining
Column-II
(i) Extra pure Ge
(ii) Concentration of ZnS
(iii) Extraction of Al
(iv) Extraction of Au
(v) Purification of N
Code
A B C D
(a) (iv) (ii) (iii) (i)
(b) (ii) (iii) (i) (iv)
(c) (i) (ii) (iii) (iv)
(d) (iii) (iv) (v)
Answer:
(a) (iv) (ii) (iii) (i)
Question 5.
Which is related to the extraction of copper from copper pyrite?
(a) concentration of ore by froth floatation process then combustion.
(b) extraction of Fe as slag.
(c) the process of self reduction for the formation of blister copper after the removal of gas.
(d) purification of blister copper by carbon reduction.
Answer:
(a) concentration of ore by froth floatation process then combustion.
Question 6.
The element found in excess amount in earth crust is:
(a) hydrogen
(b) calcination
(c) silicon
(d) use of wilfley table
Answer:
(b) calcination
Question 7.
How aluminium ore is concentrated?
(a) roasting
(b) oxygen
(c) froth floatation
(d) carbon
Answer:
(b) oxygen
Question 8.
Which of the following statement is wrong about extraction of aluminium from Hall-Heroult's process?
(a) In this process CO and CO2 are formed.
(b) When CaF2 is added to Al2O3, the melting point of mixture decreases and conductivity increases.
(c) Al3+ on reduction produce Al at cathode.
(d) Na3AlF6 works as electrolyte.
Answer:
(d) Na3AlF6 works as electrolyte.

Question 9.
Copper is extracted from copper pyrite on heating in Bessemer converter. The method is based on which principle?
(a) the affinity of copper at high temperature is very low for oxygen in comparison to sulphur.
(b) the affinity of copper at high temperature is very high for oxygen in comparison to sulphur.
(c) the affinity of sulphur is very low for oxygen at high temperature.
(d) the affinity of iron at high temperture is very low for oxygen in comparison to sulphur.
Answer:
(b) the affinity of copper at high temperature is very high for oxygen in comparison to sulphur.
Question 10.
In extraction of copper from sulphide ores, cuprous oxide is reduced with which compound to obtain metal:
(a) iron (II) sulphide
(b) carbon monoxide
(c) copper (I) sulphide
(d) sulphur dioxide
Answer:
(c) copper (I) sulphide
Question 11.
Roasted copper pyrite form on smelting with lime:
(a) Malleable slag FeSiO3 and Cu2S matte.
(b) Non-malleable slag CaSiO3 and Cu2O matte.
(c) Malleable slag Ca3(PO4)2 and Cu2S matte.
(d) Non-malleable slag Fe3 (PO4)2 and Cu2S matte.
Answer:
(a) Malleable slag FeSiO3 and Cu2S matte.
Question 12.
Which method is used to obtain very pure germanium which is used as semiconductor?
(a) Electrolytic reduction.
(b) Vapour phase purification.
(c) Liquification.
(d) Zone refining.
Answer:
(d) Zone refining.
Question 13.
The name of metal is which is purified by transfering its impure form in reverberatory furnace and heated at its boiling point in the absence of air.
(a) Mercury
(b) Gallium
(c) Zirconium
(d) Copper
Answer:
(a) Mercury
