RBSE Class 12 Chemistry Important Questions Chapter 5 Surface Chemistry
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 5 Surface Chemistry Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 5 Important Questions Surface Chemistry
Question 1.
Adsorption is a/an:
(a) Colligative
(b) oxidation process
(c) reduction process
(d) surface phenomenon
Answer:
(d) surface phenomenon
Question 2.
Physical adsorption is due to:
(a) fromation of van der Waal's interaction
(b) formation of chemical bonds
(c) formation of hydrogen bonds
(d) formation of metallic bonds
Answer:
(a) fromation of van der Waal's interaction

Question 3.
Physical adsorption is:
(a) reversible
(b) exothermic
(c) increase with decrease in temperature
(d) all of the above
Answer:
(d) all of the above
Question 4.
Chemical adsorption is due to:
(a) formation of van der Waal's interaction
(b) formation of chemical bonds
(c) formation of metallic bonds
(d) none of the above
Answer:
(b) formation of chemical bonds
Question 5.
Chemical adsorption is:
(a) irreversible
(b) exothermic
(c) (1) and (2) both
(d) none of these
Answer:
(c) (1) and (2) both
Question 6.
A straight line is obtained when a plot between log X/m vs logp is drawn. In this plot, the value of slope
(a) k
(b) n
(c) log k
(d) 1/n
Answer:
(d) 1/n
Question 7.
Which relation shows Freundlich adsorption isotherm:

Answer:
\(\text { (d) } \frac{m}{x}=k p^{1 / n}\)
Question 8.
The components of adsorption are:
(a) dispersed phase and dispersion medium
(b) solute and solvent
(c) adsorbate and adsorbent
(d) none of the above
Answer:
(c) adsorbate and adsorbent

Question 9.
The value of enthalpy of adsorption of chemisorption is:
(a) 20-40 kJ/mol
(b) 1-20 kJ/mol
(c) 30-40 kJ/mol
(d) none of these
Answer:
(c) 30-40 kJ/mol
Question 10.
On increasing surface area, the process of adsorption:
(a) decreases
(b) increases
(c) either increases or decreases
(d) neither decreases nor increases
Answer:
(b) increases
Question 11.
How many phases are there in colloidal system?
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(b) 2
Question 12.
Which colloidal system is not possible?
(a) solid-solid
(b) gas-gas
(c) solid-liquid
(d) gas-liquid
Answer:
(a) solid-solid
Question 13.
The size of colloidal particles is:
(a) 10-5 cm to 10-7 cm
(b) 10-3 cm to 10-4 cm
(c) 10-7 cm to 10-9 cm
(d) 10-2 cm to 10-4cm
Answer:
(a) 10-5 cm to 10-7 cm
Question 14.
Which of the following species forms lyophilic colloid?
(a) Sulphur
(b) Strach
(c) Silver
(d) Carbon
Answer:
(b) Strach

Question 15.
Milk is a colloid, which have:
(a) liquid is dispersed in liquid
(b) solid is dispersed in liquid
(c) gas is dispersed in liquid
(d) solid is dispersed in gas
Answer:
(a) liquid is dispersed in liquid
Question 16.
Solid aerosol is a colloid system, which has:
(a) liquid dispersed in solid
(b) liquid dispersed in gas
(c) gas dispersed in liquid
(d) solid dispersed in gas
Answer:
(d) solid dispersed in gas
Question 17.
Which colloidal system has as example of fog?
(a) liquid dispersed in gas
(b) solid particles dispersed in gas
(c) gas dispersed in liquid
(d) solid dispersed in liquid
Answer:
(a) liquid dispersed in gas
Question 18.
The reason of Brownian movement is:
(a) variation of temperature in liquid state
(b) attractive and repulsive forces of charge on colloidal particles
(c) collison of colloidal particles with the particles of dispersion medium
(d) size of particle
Answer:
(c) collison of colloidal particles with the particles of dispersion medium
Question 19.
Milk is an example of:
(a) foam
(b) emulsion
(c) gel
(d) aerosol
Answer:
(b) emulsion
Question 20.
The method of purification of colloid is:
(a) peptization
(b) Coagulation
(c) dialysis
(a) electrical nature
Answer:
(c) dialysis

Question 21.
Crystalloids are different from colloid in:
(a) electrical nature
(b) particle nature
(c) size of particles
(d) solubility
Answer:
(c) size of particles
Question 22.
Sulphur sol has:
(a) discrete sulphur molecules
(b) discrete sulphur atoms
(c) associated group of sulphur molecules
(d) water dispersed in solid sulphur
Answer:
(c) associated group of sulphur molecules
Question 23.
Which of the following form positive sol?
(a) As2S
(b) Blood
(c) Fe(OH)3
(d) Starch
Answer:
(c) Fe(OH)3
Question 24.
Smoke is an aerosol. It has the particles of dispersed
phase having:
(a) negative charge
(b) positive charge
(c) neutral
(d) none of these
Answer:
(a) negative charge
Question 25.
Peptization is a process of:
(a) precipitation of colloidal particles
(b) purification of colloidal particles
(c) conversion of precipitate into colloidal solution
(d) none of these
Answer:
(c) conversion of precipitate into colloidal solution

Question 26.
In butter:
(a) fat is dispersed in solid casein.
(b) particles of fat are dispersed in water.
(c) water is dispersed in fat.
(d) casein is precipitated in water.
Answer:
(c) water is dispersed in fat.
Question 27.
The example of dispersion of liquid in gas is:
(a) milk
(b) vegetable oil
(c) foam
(d) mist
Answer:
(d) mist
Question 28.
In any reaction, the catalyst:
(a) decreases the rate
(b) increases the rate
(c) alters the rate
(d) initiates the reaction
Answer:
(c) alters the rate
Question 29.
Which metals act as catalsty:
(a) Alkali metals
(b) Alkaline earth metals
(c) Radioactive metals
(d) Transition metals
Answer:
(d) Transition metals

Question 30.
The function of catalyst is:
(a) to decrease the rate of reaction
(b) to alter the rate of reaction
(c) to increase the time of reaction
(d) to increase the area of reaction
Answer:
(b) to alter the rate of reaction
Question 31.
A catalyst increases the rate of a chemical reaction:
(a) by increasing activation energy
(b) by decreasing activation energy
(c) by reacting with reactants
(d) by reacting with products
Answer:
(b) by decreasing activation energy
Question 32.
Adsorption theory is favourable:
(a) for liquid catalysts
(b) for gaseous catalysts
(c) for solid catalysts
(d) for all types of catalysts
Answer:
(c) for solid catalysts
Question 33.
A catalyst is a substance which:
(a) compensates the energy of reaction.
(b) alters the equilibrium constant.
(c) decreases the time of altaining the equilibrium.
(d) increases the concentration of reaction.
Answer:
(c) decreases the time of altaining the equilibrium.
Question 34.
Whcih statement is true for catalyst?
(a) It can change the heat of reaction.
(b) It can increase the activation energy of reaction.
(c) It increases the rate of reaction in both directions.
(d) All the statements are true.
Answer:
(c) It increases the rate of reaction in both directions.
Question 35.
The term Catalyst is first given by:
(a) Berzilius
(b) Kolbe
(c) Wohler
(d) Rutherford
Answer:
(a) Berzilius
Question 36.
Which catalyst is generally used in hydrogenation of oil?
(a) Pd
(b) Ni
(c) Fe
(d) V2Os
Answer:
(b) Ni

Question 37.
Which of the following is an example of zeolite?
(a) BaSO,
(b) BaCO3
(c) ZSM-5
(d) Al2O3
Answer:
(c) ZSM-5
Question 38.
The reaction, which is catalysed by any one of its product, is known as:
(a) negative catalysis
(b) auto-catalysis
(c) acidic catalysis
(d) induced catalysis
Answer:
(b) auto-catalysis
Question 39.
In hydrolysis of ethyl acetate, auto catalyst is:
(a) C2H2OH
(b) CH3COOC2H5
(c) H2O
(d) CH3COOH
Answer:
(d) CH3COOH
Question 40.
Finely divided Ni catalyst possessen high activity because:
(a) it has high surface area.
(b) it reacts faster.
(c) it forms an intermediate compound.
(d) the size of their particles is approximately equal to the size of reacting atoms.
Answer:
(a) it has high surface area.
Very Short Answer Questions:
Question 1.
Why is adsorption always exothermic?
Answer:
During adsorption, there is always a decrease in re- sidual or unbalanced forces of the surface. This re- sults in decrease in surface energy which appears as heat, therefore adsorption is an exothermic process.
Question 2.
Write one similarity between physisorption and chemisorption.
Answer:
Both physisorption and chemisorption depends on the surface area and increases with an increase in surface area of the adsorbent.
Question 3.
Physisorption is reversible while chemisorption is irreversible. Why?
Answer:
Physisorption is reversible due to the presence of weak van der Waals' forces between adsorbate and adsorbent. In chemisorption, the adsorbate and adsorbent are held together by strong chemical bonds thereby leading to the compound formation which is usually irreversible.

Question 4.
Define the following term by giving an example: Adsorption.
Answer:
The accumulation of molecular species at the sur- face rather than in the bulk of a solid or liquid is known as adsorption, e.g. accumulation of hydrogen on Pt or Pd surface.
Question 5.
What is the effect of temperature on chemisorption?
Answer:
Chemisorption is an exothermic process. Hence, according to LeChatelier's principle, rate of adsorption decreases with rise in temperature. However, rate of adsorption is very slow at low temperature, on account of high activation energy. Therefore, initially rate of chemisorption increases with increase in temperature but after attaining high activation energy rate of chemisorption decreases with increase in temperature.
Question 6.
What type of forces are responsible for the occurrence of physisorption?
Answer:
Physisorption occurs due to the presence of weak van der Waals forces present between adsorbate and adsor- bent.
Question 7.
Out of physisorption or chemisorption, which has a higher enthalpy of adsorption?
Answer:
Chemisorption has higher enthalpy of adsorption than physisorption as it involves chemical bond formation.
Question 8.
Out of NH3 and CO2, which gas will be adsorbed more readily on the surface of activated charcoal and why?
Answer:
Easily liquefiable gases, i.e. with higher critical temperatures are readily adsorbed as van der Waals forces are stronger near the critical temperature. Therefore, out of NH3 and CO2 gas, NH, gas will be adsorbed more readily on the surface due to its high critical tempera- ture than CO2 gas.
Question 9.
Adsorption of a gas on surface of solid is generally accompanied by a decrease in entropy, still it is a spon- taneous process. Explain.
Answer:
Adsorption is an exothermic process, i.e. energy factor control the process. For, AG = AH - TAS, in adsorption, though AS is negative but AH is also negative and AH > TAS in magnitude so that AG is negative. Hence, the process is spontaneous.
Question 10.
Write the two applications of adsorption.
Answer:
The two main applications of adsorption are:
- Chromatographic analysis based on the phenom- enon of adsorption finds a number of applications in analytical and industrial fields.
- Separation of inert gases due to the difference in degree of adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
Question 11.
Physisorption is multilayered, while chemisorption is monolayered.
Answer:
Physical adsorption occurs due to intermolecular attractive forces between the adsorbate and adsorbent. If the size of the adsorbent pores is close to the size of adsorbate molecules, multilayer adsorption takes place such that all the pores are filled with adsorbate molecules. Whereas in chemisorption, chemical bonds are formed between adsorbate and adsorbent molecules. Therefore, it is monolayered.

Question 12.
What is meant by chemisorption?
Answer:
When the gas molecules or atoms are held to the solid surface by chemical bonds, the adsorption is known as chemissorption.
Question 13.
Why is a finely divided substance more effective an adsorbent?
Answer:
A finely divided substance is more effective as an adsorbent because
- it has more surface area so, more adsorption occurs.
- the number of active sites (active centres) become more and the extent of adsorption increases.
Question 14.
Define the term of desorption.
Answer:
Desorption is the process of removing an adsorbed substance from a surface on which it is adsorbed.
Question 15.
Name two types of adsorption phenomena.
Answer:
Physisorption and chemisorption.
Question 16.
What is the sign of AH and AS when a gas is adsorbed by an adsorbent?
Answer:
Adsorption is an exothermic process, so AH is always negative and AS is also negative because after adsorption of gas, entropy decreases.
Question 17.
What is the basic difference between adsorption and absorption?
Answer:
In adsorption, the substance is concentrated only at the surface and does not penetrate through the surface to the bulk of the adsorbent while in absorption, the substance is uniformly distributed throughout the bulk of the solid, e.g. water vapours are adsorbed by silica gel but absorbed by anhydrous calcium chloride.
Question 18.
What are physisorption and chemisorption?
Answer:
Physisorption When the gas molecules or atoms on the surface of a solid are held to the surface by weak van der Waal's forces of attraction, the adsorption is called physisorption. When the gas molecules or atoms are held to the solid surface by chemical bonds, the adsorption is known as chemissorption.

Question 19.
Distinguish between physisorption and chemisorption.
Answer:
Differences between physisorption and chemisorption are: Physisorption It arises because of vander Waals' forces Chemisorption It is caused by chemical bond formation It is reversible in nature. It is irreversible.
Question 20.
CO(g) and H2(g) react to give different products in the presence of different catalysts. Which ability of the catalyst is shown by these reactions?
Answer:
CO(g) and H2(g) reacts to give different products in the presence of different catalysts.
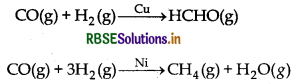
Selectivity of catalyst action is shown by these reactions.
Question 21.
What are biocatalysts? Give an example.
Answer:
Enzymes which help in catalysis of biochemical reactions that maintains the life process are called biocatalysts. e.g. Inversion of cane sugar with the help of enzyme 'invertase'. It converts cane sugar into glucose and fructose.
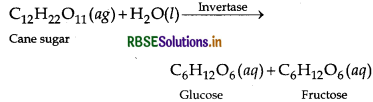
Question 22.
What are enzymes?
Answer:
Enzymes are complex nitrogeneous organic compounds which are produced by living plants and animals. They are actually protein molecules of high molecular mass. These are biochemical catalysts, highly efficient, highly specific in nature and highly active under optimum temperature and optimum pH.
Question 23.
What is meant by shape-selective catalysis?
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. Zeolites are good shape-selective catalysts.
Question 24.
Give an example of shape-selective catalysis.
Answer:
ZSM-5, a zeolite, shows shape-selective catalysis when it converts alcohol directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Question 25.
Define associated colloid with an example.
Answer:
Associated Colloid The colloids, produced by the aggregates of large number of ions because of attraction towards oppositely charged ions in a solution are called associated colloids or micelles. e.g. soap-solution.

Question 26.
Why are medicines more effective in colloidal state?
Answer:
Medicines are more effective in the colloidal form because they have large surface area per unit mass so they are easily assimilated in the body.
Question 27.
What is difference between an emulsion and a gel?
Answer:
An emulsion' is a colloidal dispersion in which both the dispersed phase and the dispersion medium are liquids (e.g. milk). Whereas, 'gel' is a colloidal dispersion in which liquid (dispersed phase) is dispersed in solid (dispersion medium) e.g. butter.
Question 28.
What type of colloid is formed when a liquid is dispersed in a solid? Give an example.
Answer:
The colloid which is formed when a liquid is dispersed in a solid is gel, e.g. butter.
Question 29.
What type of colloid is formed when a gas is dispersed in a liquid? Give an example.
Answer:
Foam is obtained when gas is dispersed in liquid e.g. whipped cream.
Question 30.
What is the reason for stability of colloidal sols?
Answer:
The presence of equal and similar charges on collotdal particles is largely responsible in providing stability to the colloidal solution. It is because the repulsive forces between charged particles having same charge prevent them from coalescing or aggregating or coagulating when they come closer to one another.

Question 31.
Out of BaCl, and KCl, which one is more effective in causing coagulation of a negatively charged colloidal soil? Give reason.
Answer:
According to Hardy-Schulze rule, the greater the valency of the flocculating ion added to the colloid, greater is its power to cause coagulation. Hence, BaCl2 (i.e. being divalent Ba2+ ion) is more effective in causing coagulation of negatively charged colloidal sol than monovalent K+ ion (i.e. KCl).
Question 32.
What are emulsions? Give an example.
Answer:
Emulsions are liquid-liquid colloidal systems, i.e. the dispersion of finely divided droplets in another liq- uid. In other words, we can say if a mixture of two immiscible or partially miscible liquid is shaken, a coarse dispersion of one liquid in the other is obtained which is called emulsion. e.g. oil in water and water in oil.
Question 33.
In reference to surface chemistry, define dialysis.
Answer:
Dialysis is the process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane. A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water flows continuously. The molecules or ions diffuse through membrane into the water and pure colloidal solution is left behind.
Question 34.
A delta is formed at the meeting point of sea water and river water. Why?
Answer:
River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in deposition with the formation of delta.
Question 35.
Write the dispersed phase and dispersion medium of butter.
Answer:
- Dispersed phase Liquid (water)
- Dispersion medium Solid (fat)
Question 36.
What are the dispersed phase and dispersion medium in milk?
Answer:
- Dispersed phase Liquid (fat)
- Dispersion medium Liquid (water)
Question 37.
Give one example each of 'oil in water' and 'water in oil' emulsion.
Answer:
Oil in water Milk, Water in oil Butter

Question 38.
Give one example of sol and gel.
Answer:
- Sol Paints, cell fluids (anyone)
- Gel Jellies, butter (anyone)
Question 39.
Give one example each of lyophobic sol and lyophiliccol.
Answer:
- Lyophobic sol Gold sol
- Lyophilic sol Starch sol
Question 40.
What are emulsions? Name an emulsion in which water is a dispersed phase.
Answer:
For emulsion: Easily liquefiable gases, i.e. with higher critical temperatures are readily adsorbed as van der Waals forces are stronger near the critical temperature. Therefore, out of NH3 and CO2 gas, NH, gas will be adsorbed more readily on the surface due to its high critical tempera- ture than CO2 gas. e.g. butter and cream.
Question 41.
Name the temperature about which the formation of micelles take place.
Answer:
Micelles can be formed only above a certain tempera- ture, called the Kraft temperature (TK).
Question 42.
Based on the type of dispersed phase, what type of colloid is micelles?
Answer:
Associated colloids form micelles There are some substances which at low concentration behave as normal strong electrolyte, but at higher concentration exhibit colloidal behaviour due to the formation of aggregates. The aggregated particles thus, formed are called micelles.
Question 43.
What is the difference between lyophobic sol and lyophilic sol?
Answer:
Lyophilic sol The colloidal sols directly formed by mixing substances like gum, gelatin, starch with a suitable liquid (the dispersion medium) are called lyophilic sols. If the dispersion medium is separated from dispersed phase, the sol can be reconstituted by simply mixing with the dispersion medium. That is why these sols are also called reversible sols. These sols are quite stable and cannot be easily coagulated.
Lyophobic sols The colloidal sols like gold do not formed directly by mixing with dispersion medium and can be prepared only by special methods. These are called lyophobic sols. These sols are readily coagulated on the addition of small amounts of electrolytes, or by heating or shaking and hence are not stable. Once coagulated, they do not give back the colloidal sol' by simple addition of the dispersion medium. Hence, these sols are called irreversible sols.
Question 44.
Which aerosol depletes ozone layer?
Answer:
Chlorofluorocarbons.

Question 45.
What is especially observed when a beam of light is passed through a colloidal solution?
Answer:
When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of incident light, the path of beam is illuminated by a bluish light. This phenomenon is called Tyndall effect. This is due to the fact that colloidal particles scattered light in all the directions in space.
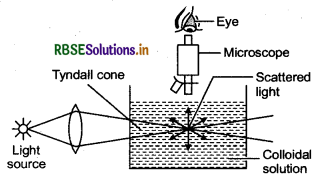
Question 46.
To which colloidal system does milk belong?
Answer:
Emulsion.
Question 47.
Define peptisation.
Answer:
The process of conversion of a fresh precipitate into colloidal sol by shaking it with dispersion medium in the presence of electrolyte is called peptisation.
Question 48.
How can a colloidal solution and true solution of the same colour be distinguished from each other?
Answer:
When a beam of light is passed through true and colloidal solutions each kept in a glass vessel, colloidal solution exhibits Tyndall effect whereas, true solution does not.
Question 49.
How is a sol different from an emulsion?
Answer:
Sol is a type of colloid in which the dispersed phase is solid and the dispersion medium is a liquid. e.g. mud, milk of magnesia. Emulsion is a type of colloid in which the dispersed phase is liquid and the dispersion medium is a liquid too, e.g. milk, hair cream.

Question 50.
Define electrophoresis.
Answer:
The movement of colloidal particles under an applied electric potential is called electrophoresis. Positively charged colloidal particles move towards the cathode, while negatively charged particles move towards the anode.
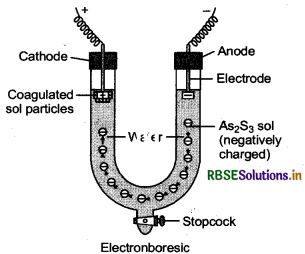
Short Answer Questions:
Question 1.
Explain how the phenomenon of adsorption finds application in the following processes?
1. Production of high vacuum
2. Heterogeneous catalysis
Answer:
- Production of high vacuum The traces of air can be adsorbed by charcoal from a vessel evacuated by a vacuum pump to give a very high vacuum.
- Heterogeneous catalysis Adsorption of reactants on the solid surface of the catalyst increases the rate of reaction. Use of finely divided nicked in the hydrogenation of oils is an excellent example of heterogeneous catalysis.
Question 2.
Write the differences between physisorption and chemisorption with respect to the following:
(i) Specificity
(ii) Temperature dependence
(iii) Reversibility
(iv) Enthalpy change
Answer:
|
Criteria |
Physisorption |
Chemisorption |
|
Specificity |
It is not specific in nature. |
It is highly specific in nature. |
|
Temperature dependence |
It decreases with increase in temperature. Thus, low temperature is favourable for physisorption. Reversible in nature. |
It occurs at moderate temperature. It first increases and then decreases within creae |
|
Reversibility Enthalpy change |
Low enthalpy of adsorption 20 - 40 kJ/mol-1. |
Irreversible in nature. High enthalpy of adsorption. (80-200KJ mol-1) |
Question 3.
Giving appropriate examples, explain how the two types of processes of adsorption (physisorption and chemisorption) are influenced by the prevailing temperature, the surface area of adsorbent and the activation energy of the process?
Answer:
Effect of temperature Physisorption decreases with increase of temperature and chemisorption first increases then decreases with increase of temperature. Effect of surface area Greater the surface area of adsorbent, greater is the physisorption and chemisorption. Effect of activation energy In physisorption, no appreciable activation energy is needed. In chemisorption, sometimes high activation energy is needed.

Question 4.
Name the two groups into which phenomenon of catalysis can be divided. Give an example of each group with the chemical equation involved.
Answer:
Catalysis can be broadly divided into two groups: Homogeneous catalysis When the reactants and the catalysts are in the same phase (i.e. liquid or gas), the catalysis is known as homogeneous catalysis.
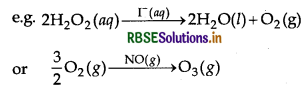
Heterogeneous catalysis When reactants and the catalysts are in different phases, the catalysis is known as heterogeneous catalysis. In most of these cases, the catalyst is solid, while reactants are either liquids or gases. Here, the catalyst is usually a metal or an oxide in finely divided form e.g.
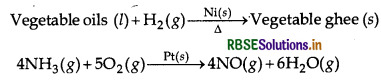
Question 5.
Explain the following:
1. Same substance can act both as colloids and crystalloids.
2. Artificial rain is caused by spraying salt over clouds.
Answer:
- The same substance can act as both colloid and crystalloid. It depends on the size of the particles. When the size of the particles lies between 1 to 1000 nm it behaves as a colloid. If particle size is less than 1 nm, it exists as a true solution and behaves like a crystalloid. e.g. NaCl in water behaves as a crystalloid while in benzene behaves as a colloid.
- Clouds are aerosols in which small droplets of water are suspended. When electrified sand or a sol carrying charge opposite to the one on clouds is sprayed from an aeroplane, the colloidal water particles of the clouds get neutralised and coagulate to form bigger water drops which causes artificial rain.
Question 6.
What is the difference between oil in water (O/W) type and water in oil (W/O) type emulsions? Give an example of each type.
Answer:
Oil in water (O/W) type emulsion: It has oil as dispersed phase and water as dispersion medium, e.g. milk, vanishing cream.
Water in oil (W/O) type emulsion: It has water as dispersed phase and oil as dispersion medium, e.g. cod liver oil.
Question 7.
Write the dispersed phase and dispersion medium of the following colloidal systems:
1. Smoke
2. Milk
Answer:
- Dispersed phase of smoke - Solid Dispersion medium of smoke = Gas
- Dispersed phase of milk = Liquid fat Dispersion medium of milk = Water (liquid)
Question 8.
How are the following colloidal solutions prepared?
(i) Sulphur in water
(ii) Gold in water
Answer:
(i) Sulphur sol is prepared by the oxidation of H2S with SO2.

(ii) Gold sol is prepared by Bredig's are process or by the reduction of AuCl3 with HCHO.
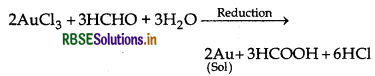
Question 9.
What is the difference between multimolecular and macromolecular colloids? Give one example of each.
Answer:
Differences between multimolecular and macromolecular colloids
Multimolecular colloids: When a large number of small molecules or atoms (diameter < 1 nm) of a substance combine together in a dispersion medium to form aggregates, which have size in the colloidal range, the colloidal solutions, thus formed, are known as multimolecular colloids. These colloids are not very much stable. e.g. gold sol, sulphur sol, etc.
Macromolecular Colloids: When substances which possess very high molecular masses are dispersed in suitable dispersion medium, the colloidal solutions, thus formed, are called macro molecular colloids. These colloids are quite stable.
e.g. cellulose, starch, etc.

Question 10.
Describe a conspicuous change observed when
1. a solution of NaCl is added to a sol of hydrated ferric oxide.
2. a beam of light is passed through a solution of NaCl and then through a sol.
Answer:
- Particles of hydrated ferric oxide sol are positively
- charged and contains Fe3+ ions on its surface. When NaCl is added to ferric oxide sol, it dissociates to give Na+ and Cl- ions. Thus, these Fe3+ get coagulated in the presence of negatively charged Cl- ions. When a beam of light is passed through a solution of NaCl no scattering of light, i.e. Tyndall effect is observed as NaCl solution is a true solution. But when light is passed through a sol, scattering of light is observed. This is known as the Tyndall effect. This scattering of light illuminates the path of the beam in the colloidal solution.
Question 11.
What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out.
Answer:
The process of aggregating together the colloidal particles or settling of colloidal particles is called coagulation of the sol. it is also known as precipitation. Following are the three methods by which coagulation of lyophobic sols can be carried out.
- Electrophoresis In this process, the colloidal particles move towards oppositely charged electrodes and get discharged resulting in coagulation.
- Mixing of two oppositely charged sols When equal proportions of oppositely charged sols are mixed, they neutralise each other resulting in coagulation.
- Prolonged dialysis By this method, electrolytes present in sol are removed completely and colloid becomes unstable resulting in coagulation.
Question 12.
Explain the following terms by giving one example for each.
Answer:
- Micelles Some substances at higher concentrations of aggregates. These aggregated particles are called micelles or associated colloids, e.g., soap at concentration 10-2 to 10-3 mol/L behaves as micelles.
- Aerosol It is a type of colloidal system in which dispersion medium is gas and dispersed phase is either solid or liquid, e.g. fog, smoke, mist, etc. Colloidal suspensions in the air are also called aerosols.
Question 13.
What is the difference between a colloidal solution and emulsion? What is the role of emulsifer in forming emulsion?
Answer:
Colloidal solution These are the solution in which the diameter of dispersed phase particles may range from 1 to 1000 nm. These are intermediate of true solutions and suspensions. The colloidal particles do not settle down under the force of gravity even on long standing. A colloid is a heterogeneous system, e.g. gold sol, sulphur sol, soap, etc. Emulsions are one of the many types of colloidal system, in which both the dispersed phase and dispersion medium are liquid, e.g. milk.
Role of emulsifier Emulsifying agents are added to emulsions to stabilise them. The emulsifying agent forms an interfacial film between suspended particles and the medium. For oil in water type emulsions, the principal emulsifying agents are gums, proteins, natural and synthetic soaps.
Question 14.
(i) What is the role of activated charcoal in gas mask?
(ii) A colloidal sol is prepared by the given method in figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
(iii) How does chemisorption vary with temperature?
Answer:
(i) Activated charcoal adsorbs various poisonous gases on its surface present in coal mines.
(ii) When ferric chloride solution is added to NaOH solution; a negatively charged sol is obtained due to adsorption of OH- ions.
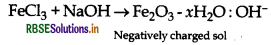
(iii) Chemisorption initially increases then decreases with rise in temperature. The initial increase is due to the fact that heat supplied acts as activation energy. The decrease afterward is due to the exothermic nature of adsorption equilibrium.
Question 15.
What happens when
(a) a freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution?
(b) persistent dialysis of a colloidal solution is carried out?
Answer:
(a) When a freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution, sol formation takes place. It is due to the preferential adsorption of Fe3 of FeCl over Fe(OH)3.
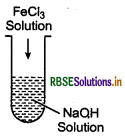
The resultant particles formed after adsorption are of colloidal size. Thus, forming sol with remaining dispersion medium. The complete reaction can be seen as
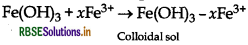
(b) Persistent and prolonged dialysis of colloidal sol remove even traces of electrolytes completely coagulate.
(c) When emulsion is centrifuged, demulsification occurs. Demulsification is defined as breaking of emulsion into its constituents.
Question 16.
Write one difference in each of the following:
(i) Solution and colloid.
(ii) Homogeneous catalysis and heterogeneous catalysis.
Answer:
(i) Solution It contains small solute particles dispersed throughout the solvent. The particle size is less than 1 nm. Colloid It contains particles of intermediate size. It is a heterogeneous solution. The particles of colloid have diameters between 1 to 1000 nm.
(ii) Homogeneous catalysis It is the phenomenon in which reactants and catalyst are present in the same phase.
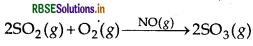
Heterogeneous catalysis It is the phenomenon in which reactants and catalyst are present in the different phase.
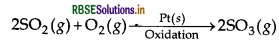
Question 17.
Write one difference in each of the following:
1. Multimolecular colloid and associated colloid.
2. Coagulation and peptisation.
3. Homogeneous catalysis and heterogeneous catalysis.
Answer:
- Multimolecular colloid These are aggregates of atoms or molecules with diameter less than 1 nm. Associated colloid These colloids are produced by the aggregates of a large number of ions because of the attraction towards oppositely charged ions in concentrated solution.
- Coagulation It is a process of aggregating together the colloidal particles so as to change them into large sized particles which ultimately settle as a precipitate. Peptisation It is the process of converting freshly prepared precipitate into colloidal sol by shaking it with the dispersion medium in the presence of electrolyte.
- heterogeneous reaction, the catalyst is in a different phase from the reactants. Example: 2SO2(g) + O2(g) → NO(gas)2 SO3(g) In a homogeneous reaction, the catalyst is in the same phase as the reactants. Example: 2SO(g) + O(g) → Pt(s)2 SO3(g)

Question 18.
Define the following terms
1. lyophilic colloid
2. zeta potential
3. associated colloid
Answer:
- Lyophilic colloids: are liquid loving colloids (Lyo means solvent and philic means loving). When these colloids are mixed with the suitable liquid, high force of attraction exists between colloidal particles and liquid.
- Zeta potential The type of potential difference produced between the fixed charge layer and diffused layer having opposite charges around the colloidal particles is called zeta potential.
- Associated Colloids (Micelles): Associated colloids are those colloids which behave as normal strong electrolytes at low concentrations but exhibit colloidal properties at higher concentrations due to the formation of aggregated particles. The aggregated particles thus formed are called micelles.
Question 19.
Give reasons for the following observations:
1. NH3 gas gets adsorbed more readily than N2 gas on the surface of charcoal.
2. Powdered substances are more effective adsorbents.
Answer:
- NH3 has higher critical temperature than N2, i.e. NH3 is more easily liquefiable than N2. Hence, NH, has greater intermolecular forces of attraction and thus will be adsorbed more readily on the surface of charcoal.
- Powdered substances have more surface area and the adsorption increases with increase in surface area of the adsorbent and hence, powdered substances are more effective adsorbents.
Question 20.
Give reasons for the following observations:
1. Lyophilic sol is more stable than lyophobic sol.
2. It is necessary to remove CO when ammonia is prepared by Haber's process.
Answer:
- Lyophilic sols are more stable than lyophobic sols. This is due to the fact that lyophilic colloids are exten- sively solvated, i.e. colloidal particles are covered by a sheath of the liquid in which they are dispersed.
- It is important to remove CO when ammonia is prepared by Haber's process because CO acts as a poison for the catalyst by lowering their activity.
Question 21.
1. In reference to Freundlich's adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
2. Write an important characteristic of lyophilic sols.
3. Based on the type of particles of dispersed phase, give one example each of associated colloid and mul- timolecular colloid.
Answer:
1. Freundlich's adsorption isotherm for adsorption of gases in the form of an euqation is x/m = kp1/n
where, m = mass of adsorbent,
x = mass of the gas adsorbed on mass m.
p = pressure and k,
n = constants
2. Lyophilic sols are reversible sols. These are quite stable and cannot be coagulated.
3. Associated colloid Soap solution, detergent. Multimolecular colloid Sulphur sol, gold sol.
Question 22.
Define the following terms:
(i) Sorption
(ii) Tyndall effect
(iii) Electrophoresis
Answer:
(i) Sorption It is the process in which adsorbate dissolves into adsorbent. Thus, adsorption changes into absorption. Moreover, both adsorption and absorption can take place simultaneously also.
(ii) Tyndall effect, also called Tyndall phenomenon, scattering of a beam of light by a medium containing small suspended particles e.g., smoke or dust in a room, which makes visible a light beam entering a window.
(iii) Electrophoresis is a laboratory technique used to separate DNA, RNA or protein molecules based on their size and electrical charge. An electric current is used to move the molecules through a gel or other matrix.

Question 23.
What are emulsions? What are their different types? Give one example of each type.
Answer:
Liquid - liquid colloidal system in which finely divided droplets of a liquid are dispersed into other liquid are called emulsion.
Types of emulsions:
- Oil in water, e.g. milk.
- Water in oil, e.g. butter.
Question 24.
Differentiate among a homogeneous solution, a suspension and a colloidal solution, give a suitable example of each.
Answer:
Homogeneous solution:
- Particle size < 1 nm.
- Constituent particles are ions or small molecules.
- e.g. sugar solution
Colloidal solution:
- Particle size between 1 to 1000 nm.
- Constituent particles are single macromolecule or ag- gregates of many atoms, ions or molecules.
- e.g. paints
Suspension:
- Particle size > 1000 nm.
- Constituent particles are molecules.
- e.g. sand in water
Question 25.
Classify colloids where the dispersion medium is water. State their characteristics and write an example of each of these classes.
Answer:
Following three colloids have water (liquid) as a dispersion medium.
- Sol When solid is dispersed in liquid, it is called sol, e.g. gold sol, starch sol.
- Emulsion When liquid is dispersed in another liquid, it is called emulsion, e.g. milk.
- Foam When as is dispersed in water, it is called foam or froth, e.g. soap lather, whipped cream.
Question 26.
Explain what is observed when
1. an electric current is passed through a sol?
2. a beam of light is passed through a sol?
3. an electrolyte (say NaCl or KCl) is added to ferric hydroxide sol (or hydrated ferric oxide sol)?
Answer:
- Colloidal particles move towards oppositely charged electrodes and get discharged and precipitated, i.e. coagulation takes place.
- The path of light becomes clearly visible due to scattering of light by colloidal particles.
- The ferric hydroxide sol gets precipitated because possitively charged ferric hydroxide sol is precipitated by the negatively charged Clions provided by NaCl or KCl.

Question 27.
How are the following colloids different from each other in respect of their dispersion medium and dispersed phase?
Give one example of each.
(i) Aerosol
(ii) Emulsion
(iii) Hydrosol
Answer:
|
Types of collids |
Dispersed phase |
Dispersion medium |
Examples |
|
Aerosol |
Solid or liquid |
Gas |
Smoke, dust fog and mist |
|
Emulsion |
Liquid |
Liquid |
Milk, hair cream |
|
Hydrosol |
Solid |
Water |
Gold sol, starch sol |
Long Answer Questions:
Question 1.
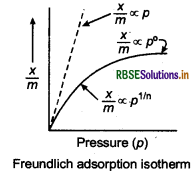
What is an adsorption isotherm? Describe Freundlich's adsorption isotherm.
Answer:
Adsorption isotherm The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve called adsorption isotherm.
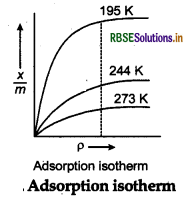
Freundlich's adsorption isotherm It is an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbed by unit mass of solid ad- sorbent and pressure at a particular temperature.
\(\frac{x}{m}=k p^{1 / n}(n>1)\)
when,
\(n=1, \frac{x}{m}=k p \text { or } \frac{x}{m} \propto p\)
where, x is the mass of gas adsorbed on mass m of the adsorbent at pressure p, k and n are constants which depends on the nature of the adsorbent and the gas at a particular temperature.
The relationship is generally represented in the form of a curve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure.
These curve indicate that at a fixed pressure, there is a decreases in physical adsorption with increase in tem- perature.
Taking log of eq. (i) we get
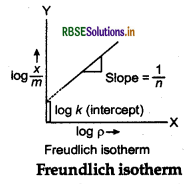
\(\log \frac{x}{m}=\log k+\frac{1}{n} \log p\)
The validity of Freundlich isotherm can be verified by plotting on Y-axis and logp on X-axis.
If it comes to be a straight line, the Freundlich isotherm is valid.
Question 2.
Discuss the effect of pressure and temperature on the adsorption of gases on solids. Describe the application of adsorption in controlling humidity.
Answer:
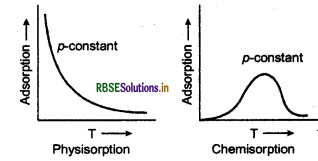
Effect of pressure on adsorption of gases on solids At constant temperature, the adsorption of gases increases with increase of pressure. This is further explained by Adsorption isotherm is the variation of the mass of the gas (adsorbate) adsorbed per gram of the adsorbent with pressure at constant temperature. The Freundlich adsorption isotherm is the mathematical representation for the variation of the extent of adsorption (x/m) with pressure (P) at a given temperature.
Effect of temperature Adsorption is an exothermic process. Therefore, in accordance with LeChatelier's principle, the magnitude of adsorption increases with decrease in temperature. Actually, physisorption increases with decrease in temperature but chemisorption first increases and then decreases with increase .in temperature.
Application of adsorption in controlling humidity Silica and aluminium gels are used as adsorbents for removing moisture and controlling humidity.
Question 3.
Explain the cleansing action of soap. Why do soaps not work in hard water?
Answer:
The cleansing action of soap is due to the fact that soap molecules form micelle around the oild droplet in such a way that hydrophobic part of the stearate ions [C17H3COO-] is in the oil droplet and hydrophilic part interact with water, the oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface. Thus, soap helps in emulsification and washing away of oils and fats. The negatively charged sheath around the globules prevents them from coming together and forming aggregates.
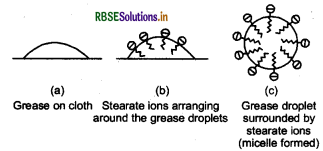
Grease on cloth Stearate ions arranging around the grease droplets Grease droplet surrounded by stearate ions (micelle formed) Grease on clothStearate ions arranging around the grease dropletsGrease droplet surrounded by stearate ions (micelle formed) Hard water contains calcium and magnesium ions. These ions form insoluble calcium and magnesium salt when sodium or potassium soaps are dissolved in hard water. These insoluble salts separate as scum in water, and are useless as cleansing agent.
Question 4.
Given reasons for the following:
(a) Leather gets hardened after tanning.
(b) FeCl3 is preferred over KCl in case of a cut leading to bleeding.
(c) Freundlich isotherm becomes independent of pressure at high pressure for a gas absorbed on a solid.
Answer:
(a) Leather gets hardened after tanning because of colloidal nature of animal-skin having positively charged particle, whereas tannin consists of negatively charged colloidal particles, which when combine, leather gets hardened.
(b) FeCl3 is preferred over KCI in case of cut leading to bleeding because FeCl3 has better coagulating efficiency than that of KCl due to more positive charge on Fe (i.e.+ 3) than on K (i.e.+1) in accordance with Hardly-Schulze rules.
(b) Freundlich isotherm can be expressed as:
\(\frac{x}{m}=k_p^{1 / n}\)
where, x/m = amount of the gas adsorbed per unit mass of the adsorbent.
At high pressure:

Question 5.
(i) Differentiate between adsorption and absorption.
(ii) Out of MgCl2 and AICI, which is more effective in causing coagulation of negatively charged solution and why?
(iii) Out of sulphur sol and proteins, which one forms multimolecular colloids?
Answer:
|
Absorption |
Adsorption |
|
1. It is bulk phenomenon, e.g. water vapours are absorbed by anhydrous calcium chloride. The concentration of solute is uniform throughout the bulk of the solid, e.g. when cotton is dipped in blue ink, it gets blue throughout. |
1. It is a surface phenomenon. e.g. water vapours are adsorbed by silica gel on its surface. The concentration of substance (adsorbate) is more on the surface and less in the bulk. The concentration increases only at the surface of adsorbent. e.g. when a chalk stick is dipped in ink, it is blue on the surface and white in bulk. |
(ii) According to Hardy-Schulze rule, "greater the valency of the flocculating ion added, the greater is its power to cause precipitation." As Al3+ ion due to greater valency has more flocculating power than Mg2+ ion. Therefore, AlCl, is more effective than MgCl2 in causing coagulation of negatively charged sol.
(iii) Sulphur sol forms multimolecular colloids. It consists of particles containing a thousand or more S sulphur molecules which associate together to form a multimolecular colloid.
COMPETITION CORNER:
Question 1.
The charge on As2S3 is because of the adsorption of:
(a) H+
(b) OH-
(c) O2-
(d) S2-
Answer:
(d) S2-
Question 2.
Which of the following property is similar in physical and chemical adsorption?
(a) attractive force
(b) enthalpy of adsorption
(c) temperature effect
(d) surface area effect
(e) number of adsorption layer
Answer:
(d) surface area effect
Question 3.
Equation IMM
(a) Gibb's adsorption isotherm
(b) Freundlich adsorption isotherm
(c) Langmuir adsorption isotherm
(d) BET equation
Answer:
(c) Langmuir adsorption isotherm
Question 4.
Which of the following statement is correct for the linear curve drawn between log (x/m) and log p in Freundlich adsorption isotherm equation?
(a) in the form of intercept
(b) in the form of slope
(c) Both of the above
(d) None of the above
Answer:
(b) in the form of slope

Question 5.
Which of the following is related to adsorption:
(a) ∆G is -ve but ∆H and ∆S is positive
(b) ∆G, ∆H and ∆S all are -ve
(c) ∆G and ∆H are negative while ∆S is positive
(d) ∆G and ∆S are negative and ∆H is positive
Answer:
(b) ∆G, ∆H and ∆S all are -ve
Question 6.
Which of the following statement is not correct with respect to physical adsorption?
(a) It is because of van der Waal's forces.
(b) Gases which are easily liquified are adsorbed easily.
(c) At high pressure multi layered molecular surface is formed.
(d) Enthalpy of adsorption is low and positive.
Answer:
(d) Enthalpy of adsorption is low and positive.
Question 7.
'Zeolite' catalysed reactions depend on the following factors:
(a) pores
(b) size of pore
(c) number of pores
(d) all of these
Answer:
(d) all of these
Question 8.
Milk is a colloid in which:
(a) a liquid is dispersed in liquid
(b) a solid is dispersed in liquid
(c) a gas is dispersed in liquid
(d) sugars are adsorbed in water
Answer:
(a) a liquid is dispersed in liquid
Question 9.
Sulphur sol has:
(a) discrete sulphur atoms
(b) discrete sulphur molecules
(c) conjugation of sulphur molecules
(d) solid sulphur is dispersed in water
Answer:
(c) conjugation of sulphur molecules
Question 10.
Fog is an example of colloidal solution of:
(a) liquid dispersed in gas
(b) gas dispersed in gas
(c) solid dispersed in gas
(d) gas dispersed in liquid
Answer:
(a) liquid dispersed in gas
Question 11.
Micelle is:
(a) gel
(b) associated colloid
(c) adsorbed catalyst
(d) true solution
Answer:
(b) associated colloid
Question 12.
Which one is false statement?
(a) Colloidal solutions are homogeneous
(b) Colloids have positive and negative charge
(c) Colloids show Tyndall effect
(d) The range of colloidal solution is 10 - 1000 Å
Answer:
(a) Colloidal solutions are homogeneous

Question 13.
The coagulation value of some electrolytes are given below which are used in the coagulation of As2S3:
L (NaCl)=52
II. (BaCl2)=0.69
III. (MgSO1) = 0.22
The order of coagulating power is
(a) III > I > II
(b) I > II > III
(c) II > I > III
(d) III > II > I
Answer:
(d) III > II > I
Question 14.
Suspension of slaked lime in water is known as:
(a) aqueous solution of slaked lime
(b) lime water
(c) unslaked lime
(d) milk of lime
Answer:
(d) milk of lime
Question 15.
In a flask, 3 g of activated charcoal is added to 50 mL of 0.06 N acetic acid. After one hour,it is filtered and the strength of filterate is found to be 0.042 N. The amount of adsorbed acetic acid per gram of activated charcoal is:
(a) 18 mg
(b) 36 mg
(c) 42 mg
(d) 54 mg
Answer:
(a) 18 mg

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम