RBSE Class 12 Chemistry Important Questions Chapter 4 Chemical Kinetics
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 4 Chemical Kinetics Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 4 Important Questions Chemical Kinetics
Multi Choice Questions (MCQ):
Question 1.
\(\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\) in the reaction N2+ 3H2 → 2NH3
(a) Rate of dissociation of ammonia.
(b) Rate of combination of N2.
(c) Rate of combination of H2.
(d) Rate of formation of ammonia.
Answer:
(d) Rate of formation of ammonia.
Question 2.
Rate of reaction
(a) increases with increase in temperature.
(b) decreases with increase in temperature.
(c) does not depend on temperature.
(d) does not depend on concentration.
Answer:
(a) increases with increase in temperature.

Question 3.
The rate constant of reaction depends on
(a) temperature
(b) mass
(c) weight
(d) catalyst
Answer:
(a) temperature
Question 4.
The rate of reaction of a substance depends on
(a) active mass
(b) molecular weight
(c) atomic weight
(d) equivalent weight
Answer:
(a) active mass
Question 5.
The rate constant and rate of reaction is similar when the concentration of reactants is
(a) unity
(b) zero
(c) both of these
(d) none of these
Answer:
(a) unity
Question 6.
The addition of a catalyst during a chemical reaction alters which of the following quantities?
(a) activation energy
(b) entropy
(c) internal energy
(d) enthalpy
Answer:
(a) activation energy
Question 7.
The rate of reaction on doubling the active mass of A by keeping active mass of B constant in the reaction
2A + B will be Product:
(a) twice as before
(b) four times as before
(c) half as before
(d) one fourth as before
Answer:
(b) four times as before
Question 8.
The rate of reaction becomes twice on increasing the concentration of A four times for the reaction. A → B. The order of reaction is.
(a) two
(b) one
(c) half
(d) zero
Answer:
(c) half
Question 9.
If volume of container becomes twice for the reaction 2SO2 + O2 → 2SO3, then the rate of reaction.
(a) will become one fourth of initial rate.
(b) will become eighth part of initial rate.
(c) will become four times of initial rate.
(d) will become eitht times of initial rate.
Answer:
(b) will become eighth part of initial rate.

Question 10.
The integrated rate law for the reaction H2 + I2 → 2HI will be
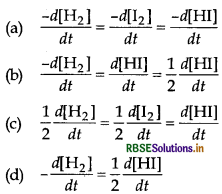
Answer:
\(\text { (d) }-\frac{d\left[\mathrm{H}_2\right]}{d t}=\frac{1}{2} \frac{d[\mathrm{HI}]}{d t}\)
Question 11.
Following equilibriums are given:
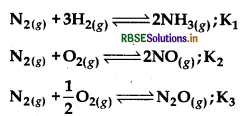
For following reaction,
\(2 \mathrm{NH}_{3(g)}+\frac{5}{2} \mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{NO}_{2(g)}+3 \mathrm{H}_3 \mathrm{O}_{(g)}\)
The equilibrium constant in terms of K1, K2, K3 is
(a) K1, K2, K3
(b) K1, K2/K3
(c) K1,K/K2
(d) K2, K/K1
Answer:
(c) K1,K/K2
Question 12.
Which of the following statement is correct in relation to equation k = AE/RT in chemical kinetics
(a) E, is activation energy.
(b) R is Rydberg constant.
(c) k is equilibrium constant.
(d) A is adsorption coefficient.
Answer:
(a) E, is activation energy.
Question 13.
The activation energy for forward reaction of a simple chemical reaction A → B is Ea. The activation energy for backward direction is.
(a) negative of E.
(b) always less than E.
(c) lesser or greater than E.
(d) always twice of E.
Answer:
(c) lesser or greater than E.
Question 14.
The threshold energy for a chemical reaction will be.
(a) activation energy + average energy of reactants.
(b) activation energy - average energy of reactants.
(c) average energy of reactants.
(d) activation energy.
Answer:
(a) activation energy + average energy of reactants.

Question 15.
The activation energy in the chemical reaction depends on.
(a) temperature.
(b) nature of reactants.
(c) collisions take place in unit time.
(d) concentration of reactants.
Answer:
(b) nature of reactants.
Very Short Answer Type Questions:
Question 1.
For the reaction A → B, the rate of reaction becomes three times when the concentration of A is increased by nine times. What is the order of reaction?
Answer:
T1 = k[A] ... (i)
T2 = k[9A]x ... (ii)
on dividing eqn. (ii) by eqn. (i), we get
\(\frac{r_2}{r_1}=\frac{k[9 A]^x}{k[A]^x}\)
⇒ 3 = (9)x (3)1 = (32)x
⇒ 2x = 1 ⇒ x = 1/2
Thus, the order of reaction is 1/2.
Question 2.
For a reaction A + B → P, the rate law is given by,r = k[A]1/2 [B]2. What is the order of this reaction?
Answer:
For a reaction, A + B → P
T = k[A]1/2[B]2
Given, rate of a reaction,
Order wrt
A = 1/2;
Order wrt B = 2
∴ Overall order of a reaction = 1/2 + 2 = 5/2
Question 3.
Express the rate of the following in terms of ammonia.
N2(g) + 3H2(g) → 2NH3(g)
Answer:
N2(g) + 3H2(g) →2NH3(g)
Rate = \(\frac{-d\left[\mathrm{~N}_2\right]}{d t}=-\frac{1}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}=+\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
Question 4.
Define order of a reaction.
Answer:
Order of a reaction The sum of the powers of the concentration i.e. stoichiometric coefficients of reactants of a chemical reaction in rate law expression is called the order of that chemical reaction. For example, for the reaction given below:
a A + bB → C2
Rate = k [A]x [B]y
Order of reaction = x + y
Where x and y are actual stoichiometric coefficients for the reaction.

Question 5.
Identify the reaction order from the following rate constant, k = 2.3 x 10-5 L mol-1 s-1.
Answer:
It is the second order reaction because unit of rate constant for second order reaction is L mol-1 S-1.
Question 6.
Why does the rate of a reaction not remain constant throughout the reaction process?
Answer:
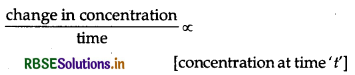
Hence, as time increase rate decreases.
Question 7.
Define rate of a reaction.
Answer:
Rate of a reaction Change in concentration of reactants or products in unit time is known as rate of a reaction. Its unit is (mol L-1)1-n S-1.
Question 8.
For a reaction RP, half-life (2) is observed to be independent of the initial concentration of reactants. What is the order of reaction?
Answer:
For a reaction R → P. half-life (1/2) is observed to be independent of the initial concentration of reactants. Thus, it follows first order reaction.
Question 9.
A first order reaction is found to have a rate constant, k = 5.5 x 10-14 S-1. Find the half-life of the reaction.
Answer:
Given, k = 5.5 x 10-14 S-1
For first order reaction,
\(t_{1 / 2}=\frac{0.693}{k}=\frac{0.693}{5.5 \times 10^{-14}}\)
= 0.126 x 1014 s = 1.26 x 1013 s
Question 10.
If half-life period of a first order reaction is x and 3/4th life period of the same reaction is y, how are y related to each other?
Answer:
(i) First, find out the value of k by using the formula,
\(k=\frac{0.693}{t_{1 / 2}}\)
where t1/2 = x.
(ii) Putting the value of k in the formula,
\(k=\frac{2.303}{t} \log \left(\frac{a}{a-x}\right)\)
find out the value of t which is given as y.
(iii) Then from the above points, relate x and y.
For first order reaction,
\(k=\frac{0.693}{t_{1 / 2}}=\frac{0.693}{x}\)
For 3/4 th life period, x = 3/4a
a - x = a - 3/4
a = 1/4 a
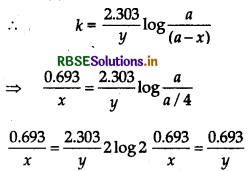
⇒ y = 2x
Question 11.
What is the effect of adding a catalyst on
(i) Activation energy (E) and
(ii) Gibbs energy (∆G) of a reaction?
Answer:
(i) Catalyst lowers the activation energy and changes the path of the reaction.
(ii) A catalyst does not alter Gibbs energy (∆G) of a reaction.

Question 12.
In some cases, it is found that a large number of colliding molecules have energy more than threshold energy, yet the reaction is slow. Why?
Answer:
It is due to improper orientation, or due to another factor P called steric factor, which refers to the orienta- tion of the colliding molecules. These are the two main factors which are responsible for a reaction to occur slowly.
Question 13.
Define activation energy.
Answer:
It is the extra energy contained by reactant molecules that results into effective collisions between them to form the products. It is denoted by Ea.
Short Answer Questions:
Question 1.
For a reaction,
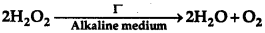
the proposed mechanism is as given below
(1) H2O2 + I → H2O + IO- (slow)
(2) H2O2 + IO → H2O + I + O2 (fast)
(i) Write rate law for the reaction.
(ii) Write the overall order of reaction.
(iii) Out of steps (1) and (2), which one is rate determining step?
Answer:
(i) Rate law for the given reaction is r = k[H2O2][I-]
(ii) Overall order of the reaction is 2. Since the reaction is first order w.r.t. H2O2 as well as w.r.t. I
(iii) The overall rate of the reaction is determined by the slowest step. Here, out of steps (1) and (2). H2O2 + I → H2O + IO- (slow) is the rate determined step.
Question 2.
For the reaction,
2N2O5(g) → 4NO2(g) + O2(g)
the rate of formation of NO2(g) is 2.8 × 10-3 Ms-1. Calculate the rate of disappearance of N2O5(g).
Answer:
For the reaction, 2N2O5(g) → 4NO2(g) + O2(g) overall rate of reaction is
\(-\frac{1}{2} \frac{d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}=+\frac{1}{4} \frac{d\left[\mathrm{NO}_2\right]}{d t}=+\frac{d\left[\mathrm{O}_2\right]}{d t}\)
Given,
\(\frac{d\left[\mathrm{NO}_2\right]}{d t}\) = 2.8 x 10-3 Ms-1 - \(\frac{d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}\) = ?
\(\frac{d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}\) = 1/4 x 2 x 2.8 x 10-3 Ms-1 = 1.4 x 10-3 Ms-1
Question 3.
For a reaction: \(\mathrm{H}_2+\mathrm{Cl}_2 \stackrel{h v}{\longrightarrow} 2 \mathrm{HCl}\) Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
Answer:
(i) For a reaction, \(\mathrm{H}_2+\mathrm{Cl}_2 \stackrel{h v}{\longrightarrow} 2 \mathrm{HCl}\)
Rate = k, suggests that the reaction is of zero order. Further, the molecularity of a given reaction is 2 as two molecules are participating in the reaction. Hence, order = zero and molecularity = two.
(ii) The unit of k for zero order reaction is equal to the rate of a reaction which is mol-1 s-1. Hence, the unit of k for the given reaction is mol L-1 S-1.
Question 4.
For a reaction, 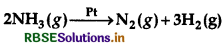
Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
Answer:
(i) Order of Reaction = Zero
Molecularity = Two
(ii) Unit = Mol L-1
Question 5.
Define rate of reaction. Write two factors that affect the rate of reaction.
Answer:
(i) Rate of a reaction Change in concentration of reactants or products in unit time is known as rate of a reaction. Its unit is (mol L-1)1-n S-1.
(ii) Two factors on which rate of reaction depends are:
(a) Concentration of reactants Generally rate increases with the increase in concentration.
(b) Temperature Generally rate of reaction increases about 2 - 3 times for every 10°C rise in temperature.
Question 6.
Write units of rate constants for zero order and for the second order reactions if the concentration is expressed in mol L-1 and time in seconds.
Answer:
Unit of rate constant (k)
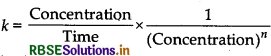
Unit of rate constant for zero order
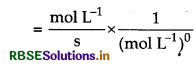
= mol L-1 S-1
Unit of rate constant for second order
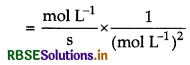
= mol L-1 S-1
Question 7.
Write two differences between 'order of reaction' and molecularity of reaction.
Or
List two main differences between order of a reaction and molecularity of a reaction.
Or
Distinguish between molecularity and order of a reaction.
Answer:
|
Molecularity of reaction |
Order of reaction |
|
1. The number of reacting species which must collide simultaneously in order to bring about a chemical reaction is called molecularity of a reaction. |
1. The sum of powers of the concentrations of the reactants in the rate law expression is called the order of that chemical reaction. |
|
2. Molecularity is always a whole number value. |
2. Order may have zero, whole number, negative or fractional, values. |
Question 8.
(i) For a reaction A + B → Product, The rate law is given by,
Rate = k[A]1 [B]2. What is the order of the reaction?
(ii) Write the unit of rate constant 'k' for the first order reaction.
Answer:
(i) The sum of the powers of the concentrations of reactants in the rate law expression is called order of that chemical reaction.
Rate = k[A]1 [B]2
Order of reaction = (1 + 2) = 3
(ii) Unit of rate constant for first order reaction is s1 or min-1 or time-1.

Question 9.
A reaction is of second order with respect to a reactant. How is its rate affected if the concentration of the reactant is
(i) doubled?
(ii) reduced to half?
Answer:
(i) Write the rate law for initial concentration of reactant and for the conditions, when concentration is doubled or reduced to half.
(ii) Now, compare the initial concentration condition with changed concentration to find the effect on rate. Let R be the initial concentration of reactant and r be the initial rate. So, the rate law for the second order reaction is,
Rate,
\(r \propto[R]^2\)
(i) When the concentration of the reactant is doubled, i.e. R1 = [2R]
Then new rate,
r' α [R']2 = [2R]2 = 4[R]2
From Eqs. (i) and (ii),
\(\frac{r}{r^{\prime}}=\frac{[R]^2}{4[R]^2}=\frac{1}{4}\)
Hence, the rate becomes four times of the initial rate on doubling the concentration of reactant.
(ii) When the concentration is reduced to half.
R = R/2 New rate, r" & [R"]2 \(\left[\frac{R}{2}\right]^2\) = 1/4[R]2 .........(iii)
From eqs. (i) and (iii),
\(\frac{r}{r^{\prime \prime}}=\frac{[R]^2}{\frac{1}{2}[R]^2}=4\)
or
\(r^{\prime \prime}=\frac{r}{4}\)
Hence, the rate of becomes one fourth of the initial rate when concentration of the reactant is reduced to half.
Question 10.
What do you understand by the rate law and rate constant of a reaction? Identify the order of a reaction, if the units of its rate constant are
(i) L-1 mol s-1
(ii) L mol-1 s-1
Or
Distinguish between rate expression and rate constant of a reaction.
Or
Express clearly, what do you understand by rate expression and rate constant of a reaction?
Answer:
Rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may be or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation. e.g. for a general reaction,
aA + bB → cC + dD Rate = k [A]d [B]b
Rate constant (k) is equal to the rate of reaction when molar concentration of reactants is unity. Its unit depends upon the order of reaction.
(i) Zero order - L-1 mol s-1
(ii) Second order - L mol-1 s-1
Question 11.
A reaction is of second order with respect to a reactant. How is the rate of reaction affected, if the concentration of the reactant is reduced to half? What is the unit of rate constant for such a reaction?
Answer:
A reaction is second order with respect to a reactant.
Rate = k[A]2
(i) If the concentration of the reactant is doubled, the rate of reaction becomes 4 times.
(ii) If the concentration of the reactant is reduced to half, the rate of reaction becomes one fourth.
The unit of rate constant is L mol-1 s-1 (second order reaction).
Question 12.
Define
(i) Order of reaction
(ii) Elementary step in a reaction.
Answer:
(i) Order of a reaction The sum of the powers of the concentration i.e. stoichiometric coefficients of reactants of a chemical reaction in rate law expression is called the order of that chemical reaction. For example, for the reaction given below:
a A + bB → C2
Rate = k [A]x [B]y
Order of reaction = x + y
Where x and y are actual stoichiometric coefficients for the reaction.
(ii) Each step of a complex reaction is called the elementary step of the reaction.
Question 13.
Identify giving reasons, the reaction order from each of the following rate constants.
(i) k = 2.3 x 10-5 L mol-1 s-1
(ii) k = 3.0 × 10-4 s-1
Answer:
(i) Unit of k indicates that the reaction is of second order reaction.
(ii) Unit of k indicates that it is a first order reaction.
Question 14.
Explain the following terms.
(i) Rate determining step of a reaction.
(ii) Molecularity of a reaction.
Answer:
(i) The slowest step in the reaction mechanism is the rate determining step of a reaction.
(ii) The molecularity of a reaction is defined as the number of reacting molecules which collide simultaneously to bring about a chemical reaction. In other words, the molecularity of an elementary reaction is defined as the number of reactant molecules taking part in the reaction.

Question 15.
A reaction is of first order in reactant A and of second order in reactant B. How is the rate of this reaction affected when
(i) the concentration of B alone is increased to three times?
(ii) the concentrations of A as well as B are doubled?
Answer:
(i) Write the rate law for initial concentration of reactants and for the conditions when concentration of reactants are changed.
(ii) Now compare them.
Since, the reaction is of first order wrt A and second order wrt B, then the rate law can be given as, (Rate) = k[A] [B]2.
(i) When the concentration of B is increased to three times (3B), the rate would be
(Rate)2 = k[A] [3B]2
(Rate)2 = 9k [A] [B]2 = 9 x (Rate)1
∴ Rate is increased by 9 times.
(ii) (Rate)3 = k [24] [2B]2
(Rate)3 = 2 x 2 x 2 x k [A] [B]2 = 8x (Rate)
∴ Rate is increased by 8 times.
Question 16.
Discuss any four factors which affect the rate of a chemical reaction.
Answer:
Factors influencing the rate of a chemical reaction are
(i) Nature of reactants Different reactants require different amount of energies for breaking the old bonds and for the formation of new bonds.
Hence, the reactivity of a substance is related to the ease with which the specific bonds are broken or formed.
e.g. 2NO + O2 → 2NO2 (fast)
2CO + O2 → 2CO2(slow)
(ii) Concentration of reactants Rate of reaction is directly proportional to the concentration of the reactants.
(iii) Temperature Rate of reaction increases with increase in temperature.
(iv) Catalyst It alters the rate of reactions without being consumed in the reaction. It provides an alternative path to the reaction with a low energy barrier.
Question 17.
Explain the difference between the average rate and instantaneous rate of a chemical reaction.
Answer:
Average rate of reaction It is defined as the change in the concentration of any one of the reactants or prod- ucts over a long time interval. Average rate of reaction.
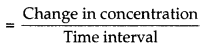
For a reaction, R →P
\(r_{a v}=-\frac{\Delta[R]}{\Delta t}=\frac{+\Delta[P]}{\Delta t}\)
Instantaneous rate of reaction It is defined as the rate of change in concentration of any one of the reactants or products at that particular instant of time.
For a reaction R→ P
\(r_{\text {inst }}=-\frac{d[R]}{d t}=+\frac{d[P]}{d t}\)
(dt = very small interval of time)
Question 18.
A reaction is first order in A and second order in B.
(i) Write the differential rate equation.
(ii) How is the rate affected on increasing the concentration of B three times?
(iii) How is the rate affected when the concentration of both A and B are doubled?
Answer:
A reaction is first order in A and second order in B
(i) Differential rate equation = (Rate),
= \(\frac{-d[R]}{d t}=k[A][B]^2\)
(ii) When concentration of B is tripled, rate of reaction increases by 9 times.
(iii) First, we have to write the given rate law. Then, we will triple the concentration of A and double the concentration of B. Hence, the reaction rate would be increased by a factor of 12.
Question 19.
A reaction is second order w.r.t. A and first order w.r.t. B.
(i) Write the differential rate equation.
(ii) How is the rate affected on increasing the concentration of A three times?
(iii) How is the rate affected when the concentrations of both A and B are doubled?
Answer:
A reaction is second order w.r.t. A and first order w.r.t. B.
(i) Differential rate equation = (Rate)1 = \(\frac{-d[R]}{d t}\)
= k[A]2[B]
(ii) When the concentration of A is increased three times, i.e. 3A then
(Rate)2 = k [34]2 [B] = 9k [A]2 [B] = 9 (Rate)1
This shows that rate will be increased 9 times to the initial rate.
(iii) When concentrations of both A and B are doubled then,
(Rate) = k [2, A]2 [2B] = k8[A]2 [B] = 8k [A] [B] = 8 (Rate)1
This shows that rate will be increased 8 times to the initial rate.

Question 20.
For the reaction,
2NO(g) + Cl2(g) → 2NOCl(g)
The following data were collected. All the measurements were taken at 263 K.
|
Intial [NO] (M) |
Intial [Cl2] (M) |
Intial rate of disappearance of Cl2 (M/min) |
|
1. 0.15 |
0.15 |
0.60 |
|
2. 0.15 |
0.30 |
1.20 |
|
3. 0.30 |
0.15 |
2.40 |
|
4. 0.25 |
0.25 |
? |
(i) Write the expression for rate law.
(ii) Calculate the value of rate constant and specify its unit.
(iii) What is the initial rate of disappearance of Cl2 in experiment 4?
Answer:
First, write the rate law, then find out the order w.r.t. each reactant, then find out the value of rate constant, put the values of concentration of reactants in any experiment and find its value.
Rate law may be written as
Rate = k [NO]" [Cl2]"
The initial rate becomes
(Rate) = k [NO] [Cl2]
Comparing experiments 1 and 2, (Rate),k (0.15) (0.15) = 0.60 .... (i)
(Rate)2 = k(0.15) (0.30) = 1.20 ...... (ii)
Dividing Eq. (ii) by Eq. (i),
\(\frac{(\text { Rate })_2}{(\text { Rate })_1}=\frac{k(0.15)^p(0.30)^q}{k(0.15)^p(0.15)^q}=\frac{1.20}{0.60}\)
or 24 = 21=> q=1
∴ Order with respect to Cl2 = 1
Comparing experiments 1 and 3.
(Rate) = k (0.15) (0.15)= 0.60
(Rate) = k(0.30) (0.15) = 2.40
Dividing Eq. (iv) by Eq. (iii)
\(\frac{(\text { Rate })_3}{(\text { Rate })_1}=\frac{k(0.30)^p(0.15)^q}{k(0.15)^p(0.15)^q}=\frac{2.40}{0.60}\)
or 2P = 4 => 2P = 22
⇒ p = 2
Thus, order with respect to NO is 2.
(i) The rate law for the given reaction
Rate = k [NO]2 [Cl2]
(ii) Raté constant can be calculated by substituting the value of rate, [NO] and [Cl2] for any of the experiments.
\(k=\frac{\text { Rate }}{[\mathrm{NO}]^2\left[\mathrm{Cl}_2\right]}=\frac{0.60}{(0.15)^2(0.15)}=\frac{0.60}{0.00338}\)
= 177.51 mol-2 L2 min-1
(iii) Let initial rate of disappearance of Cl2 in experiment 4 is r4.
r4 = k [NO]2 [Cl2]
= 177.51 x (0.25)2 (0.25)
= 2.77 M/min
Question 21.
Consider the reaction,
2A + B → C + D
Following results were obtained from experiments designed to study the rate of reactions.
|
Intial [A] |
Concentration(mol L-1) |
Intial rate of formation [D](M/min) |
|
1. 0.10 |
0.10 |
1.5 x 10-3 |
|
2. 0.20 |
0.20 |
3.0 x 10-3 |
|
3. 0.20 |
0.40 |
6.0 x 10-3 |
(i) Write the rate law for the reaction.
(ii) Calculate the value of rate constant for the reaction.
(iii) Which of the following possible reaction mechanisms is consistent with the rate law?
I. A + B → C + E (slow)
A + E → D (fast)
II.B → C + E (slow)
A + E → F (fast)
A + F → D (fast)
Answer:
Rate law may be written as Rate = k [A]p [B]q
Comparing experiments (2) and (3), we get
(Rate)2 = k (0.2) (0.2) = 3 x 10-3 ...(i)
(Rate)3 = k(0.2) (0.4) = 6× 10-3 ... (ii)
Dividing Eq. (ii) by Eq. (i), we get
\(\frac{(\text { Rate })_3}{(\text { Rate })_2}=\frac{k(0.2)^p(0.4)^p}{k(0.2)^q}=\frac{6 \times 10^{-3}}{3=10^{-3}}\)
or 2q = 2, q = 1
Comparing experiments (1) and (2)
(Rate)1 = k (0.1) (0.1) = 1.5 × 10-3 ... (iii)
(Rate)2 = k(0.2) (0.2) = 3.0 × 10-3 ... (iv)
Dividing Eq. (iv) by Eq. (iii), we get
\(\frac{3 \times 10^{-3}}{1.5 \times 10^{-3}}=\frac{k(0.2)^p(0.2)^q}{k(0.1)^p(0.1)^q}\)
where, q = 1,1 = 2p, 2o, 2p, p = 0
(i) Thus, the rate law is rate = k [A] [B] = k[B]
(ii) Rate = k[B]
\(k=\frac{\text { Rate }}{[\mathrm{B}]}=\frac{3 \times 10^{-3}}{0.2}\) min-1
(iii) B → C + E (slow) is the possible reaction which is consistent with the rate law.
rate = k[B].
Question 22.
The following results have been obtained during ki- netic studies of the reaction:
2A + B → C + D
|
Intial [A] |
Concentration(mol L-1) |
Intial rate of formation [D](M/min) |
|
1. 0.1 M |
0.1 M |
6.0 x 10-3 M min-1 |
|
2. 0.3 M |
0.2 M |
7.2 x 10-3 M min-1 |
|
3. 0.3 M |
0.4 M |
2.88 x 10-3 M min-1 |
|
4. 0.4 M |
0.1 M |
2.40 x 10-3 M min-1 |
Determine rate law and the rate constant for the reaction.
Answer:
Let the rate law in terms of rate of formation of D be
\(\frac{d[D]}{d t}=k[A]^a[B]^b\)
1.6.0 × 103 = k (0.1)a (0.1)b ...... (i)
2.7.2 × 10-3 = k (0.3)a (0.2)b ....... (ii)
3.2.88 x 10-2 = k(0.3)a (0.4)b ........ (iii)
4.2.40 x 10-2 = k(0.4)a (0.1)b ......... (iv)
Divide Eq. (iv) by Eq. (i), we get
4 = (4)a
∴ a = 1
Divide Eq. (iii) by Eq. (ii), we get 4 = (2)
4 = (2)b
22 = (1)b
∴ b = 2
Order with respect to A = 1
Order with respect to B = 2
Rate law = \(\frac{d[D]}{d t}\) = k[A] [B]2
On putting the value of 'A' and 'B' in any equation, say (i)
6.0 x 10-3 M min-1 = k (0.1 M) (0.1 M)2
∴k = 6 M-2 min-1

Question 23.
Define the following terms.
(i) Half-life of a reaction (t1/2)
(ii) Rate constant (k)
Answer:
(i) Half-life (1/2) of a reaction is the time in which the concentration of a reactant is reduced to one half of its initial concentration.
(ii) Rate.constant (k) is equal to the rate of reaction when molar concentration of reactants is unity. Its unit depends upon the order of reaction.
Question 24.
For a chemical reaction, R → P, the variation in the concentration of R versus time (t) plot is given as
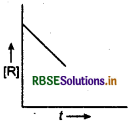
(i) Predict the order of the reaction.
(ii) What is the slope of the curve?
Answer:
(i) The reaction is of zero order.
(ii) For zero order reaction, [R] = -kt + [R], Compare with equation of a straight line (y = mx + c). If we plot [R] against t, we get a straight line with slope = -k and intercept equal to [R]o
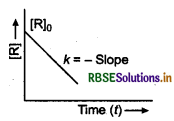
Variation in the concentration vs time plot for a zero order reaction.
Question 25.
Hydrogen peroxide, H2O2 (aq) decomposes to H2O(l) and O2(g) in a reaction that is first order in H2O2 and has a rate constant k = 1.06 x 10-3 min1.
(i) How long will it take for 15% of a sample of H2O to decompose?
(ii) How long will it take for 85% of the sample to decompose?
Answer:
(i) When 15% of a sample of H2O2 is decomposed. For a first order reaction,
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
Given, k = 1.06 × 10-3 min-1, [R] = 100 M, [R] (after time, t) = 100 - 15 = 85 M
(ii) When 85% of a sample of H2O is decomposed, [R] = 100 - 85 = 15 M
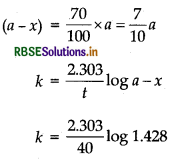
= 1790.05 min
Question 26.
Define half-life of a reaction. Write the expression of half-life for
(i) zero order reaction
(ii) first order reaction
Answer:
Half-life (t1/2) of a reaction is the time in which the concentration of reactant is reduced to one half of its initial concentration.
(i) For a zero order reaction, \(t_{1 / 2}=\frac{[R]_0}{2 k}\)
(ii) For first order reaction, \(t_{1 / 2}=\frac{0.693}{k}\)
Question 27.
A first order reaction takes 40 min for 30% decompo- sition. Calculate t1/2 for this reaction. (Given, log 1.428 = 0.1548)
Answer:
(i) To find t1/2, first calculate k by using the formula.
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
(ii) Calculate t1/2 by using the formula, t1/2 = 0.693/K
For a reaction,
\(t_{1 / 2}=\frac{0.693}{k}\)
For 30% decomposition, it takes 40 min which means after 40 min, reactant left is 70% of its initial concen- tration.
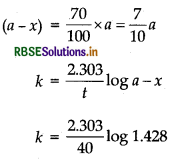
k = 0.00891 min-1
∴ Half-life of the reactant (1/2)
= \(\frac{0.693}{k}=\frac{0.693}{0.00891}\)
= 77.76 min
Question 28.
A reactant has a half-life of 10 min.
(i) Calculate the rate constant for the first order reaction.
(ii) What fraction of the reactant will be left after an hour of the reaction has occurred?
Answer:
\(k=\frac{0.693}{t_{1 / 2}}=\frac{0.693}{10}\)
[where, No initial amount of reactant and N = amount of reactant left after time, t]
Question 29.
What are pseudo first order reactions? Give one example of such reactions.
Answer:
Pseudo first order reaction The reaction which is bimolecular but has order one, is called pseudo first order reaction, e.g. acidic hydrolysis of ester.
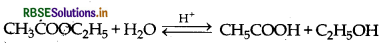
Question 30.
In a first order reaction, the concentration of the reactant is reduced from 0.6 mol L-1 to 0.2 mol L-1 in 5 min. Calculate the rate constant of the reaction.
Answer:
Rate constant,
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
Given, [R] = 0.6 mol L-1
[R] = 0.2 mol L-1, t = 5 min
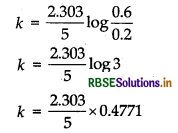
= 0.2197 min-1
Question 31.
The rate constant for a zero order reaction in A is 0.0030 mol L-1 s1. How long will it take for the initial concentration of A to fall from 0.10 M to 0.075 M?
Answer:
For zero order reaction.
rate constant, k = \(\frac{[\mathrm{R}]_0-[\mathrm{R}]}{t}\)
Given, [R]0 = 0.10 M,
[R] = 0.075 M and k = 0.0030 mol L-1 s-1

= 8.33s
Question 32.
The decomposition of NH3 on platinum surface is zero order reaction. If rate constant (k) is 4 × 10-3 MS-1, how long will it take to reduce the initial concentration of NH3 from 0.1 M to 0.064 M.
Answer:
2NH3 (g) → N2(g) + 3H2(g)
Given
k = 4 × 10-3 Ms-1
[A] = 0.1 M; [A] = 0.064 M
∵ \(t=\frac{\left[\mathrm{A}_0\right]-[\mathrm{A}]}{k}\)
[For zero order reaction]
∵ \(t=\frac{0.1-0.064}{4 \times 10^{-3}}=\frac{0.036 \times 1000}{4}\) = 9s

Question 33.
A first order reaction takes 20 minutes for 25% decomposition. Calculate the time when 75% of the reaction will be completed.
Given: log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021
Answer:
For a first order reaction, k = \(\frac{2.303}{t} \log \frac{a}{a-x}\)
where, k = Rate constant
a = initial concentration
(a - x) = concentration after time't'.
When a first order reaction is 25% completed in 20 min.
a = 100, a - x = 100 - 25 = 75, t = 20 min.
∴ \(k=\frac{2.303}{t} \log \frac{a}{a-x}=\frac{2.303}{20} \log \frac{100}{75}\)
= \(\frac{2.303}{20}[\log 4-\log 3]\)
= 0.0143 min-1
For 75% completion of reaction.
∴ \(t=\frac{2.303}{k} \log \frac{a}{a-x}=\frac{2.303}{0.0143} \log ; \frac{100}{25}\)
= \(\frac{2.303}{0.0143} \log 4\)
= 96.968 min.
Question 34.
Following data are obtained for the reaction
N2O5 → 2NO2 + 1/2O2
|
t/s |
0 |
300 |
600 |
|
[N2O5]/mol L-1 |
1.6 X 10-2 |
0.8 X 10-2 |
0.4 X 10-2 |
(i) Show that it follows first order reaction.
(ii) Calculate the half-life.
(Given: log 2 = 0.3010, log4 = 0.6021)
Answer:
Using data, to find out the rate constant.
|
t/s |
0 |
300 |
600 |
|
[N2O5]/mol L-1 |
1.6 X 10-2 |
0.8 X 10-2 |
0.4 X 10-2 |
Using hit and trial method,
For first order reaction, \(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{\mathrm{IR}]}\)
Here, t = 300s, [R] = 1.6 x 10-2 mol L1 and [R] = 0.8 x 10-2 mol L-1
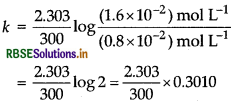
= 2.31 x 10-3 s-1
Similarly,
\(k=\frac{2.303}{600} \log \frac{\left(1.6 \times 10^{-2}\right) \mathrm{mol} \mathrm{L}^{-1}}{\left(0.4 \times 10^{-2}\right) \mathrm{mol} \mathrm{L}^{-1}}\)
k = 2.31 x 10-3.
Thus, it is proved that reaction proceed through first order kinetics as the rate constant remains same.
(b) Half-life(t1/2) = \(\frac{0.693}{k} t_{1 / 2}=\frac{0.693}{2.31 \times 10^{-3}} \mathrm{~s}\)
Question 35.
For the first order thermal decomposition reaction, the following data obtained:
C2H5Cl(g) → C2H4(g) + HCl(g)
Calculate the rate constant.
(Given: log2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021)
Answer:
C2HCl (g) → C2H4(g) + HCl (g)
Initial pressure at t = 0,0.30 atm
After 300s, Ptotal = (0.30 - p + p + p) atm = (0.30 + p) atm
Ptotal (given) = 0.50 - 0.30 + p ⇒ p = 0.50 - 0.30 atm = 0.20 atm
Pressure of C2H5Cl (g) at 300 sec
(PC2H5Cl) = 0.30 - 0.20 atm = 0.10 atm
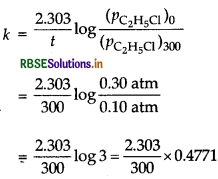
= 3.66 × 10-3 S-1
Question 36.
The rate constant for a first order reaction is 60s-1. How much time will it take to reduce the initital concentration of the reactant to its 1/10th value?
Answer:
For a reaction,
Here, a - x = a/10
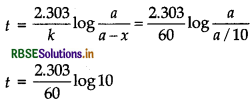
t = 0.0384 s
Question 37.
The followings data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume:
SO2Cl2(g) → SO2(g) + Cl2(g)
|
Experiment |
Time |
Total pressure/atm |
|
|
0 |
0.4 0.7 |
Calculate the rate constant.
(Given, log 4 = 0.6021, log 2 = 0.3010)
Answer:
SO2Cl2(g) → SO2(g) + Cl2(g)
Total pressure after time t,
ie. Pt = Pi - p + p + p = pi + p
=> P = P - Pi
Thus, a = pi and a - x = pi - p = pi - (Pi - Pi)
= Pi - P + Pi = 2pi - Pt
Substituting the values of a and (a - x) in equation,
\(k=\frac{2.303}{t} \log \frac{a}{a-x} k=\frac{2.303}{t} \log \frac{p_i}{\left(2 p_i-p_t\right)}\)
Calculating rate constant (k), when t = 100s Given pi = 0.4 atm and pt = 0.7 atm
Then,
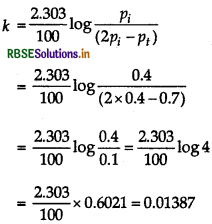
= 1.387 x 10-2 s-1.

Question 38.
A first order reaction takes 100 min for completion of 60% of the reaction. Find the time when 90% of the reaction will be completed.
Answer:
(i) First, find out the value of a - x from the given percentage, then the value of k by using the formula,
\(k=\frac{2.303}{t} \log \frac{a}{a-x}\) and keeping the value of k in this formula.
For the first order reaction, when t = 100 min
rate constant, k
\(k=\frac{2.303}{100} \log \frac{a}{a-x}\)
For 60% completion of the reaction, if a = 100% a - x = 100 - 60 = 40%
Then,
\(k=\frac{2.303}{100} \log \frac{100}{40}\) ..... (i)
For 90% completion of the reaction,
and
Then,
a = 100%
a - x = 100 - 90 = 10%
Then
\(k=\frac{2.303}{t} \log \frac{100}{10}\) ..... (ii)
Substituting the value of k in Eq. (ii), we have,
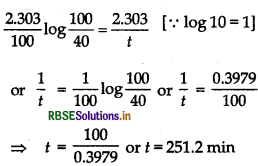
Question 39.
Nitrogen pentoxide decomposes according to the equation 2N2O5(g) → 4NO2(g) + O2(g)
This first order reaction was allowed to proceed at 40°C and the data below were collected.
|
[NO5]M |
Time (min) |
|
1. 0.400 |
0.00 |
|
2. 0.289 |
20.0 |
|
3. 0.209 |
40.0 |
|
4. 0.151 |
60.0 |
|
5. 0.109 |
80.0 |
(i) Calculate the rate constant. Include units with your answer.
(ii) What will be the concentration of N2O5 after 100 min?
(iii) Calculate the initial rate of reaction.
Answer:
(i) Find rate constant of each experiment using the formula, k imm then find the average value of k.
(ii) Find the concentration of N2O5 after the given time by putting the value of k in the above formula.
(iii) Then find the initial rate of reaction.
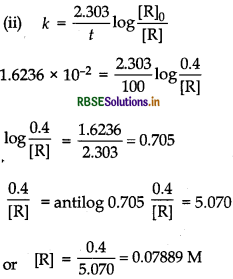
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
[R]o = 0.400 M
When [R] = 0.289 M, t = 20 min

When
t = 40 min, [R] = 0.209 M
\(k=\frac{2.303}{40} \log \frac{0.400}{0.200}=\frac{2.303}{40} \log 1.914\)
\(=\frac{2.303}{40} \times 0.2819\)
= 0.01623 min-1
Similarly, when t = 60 min, [R] = 0.151
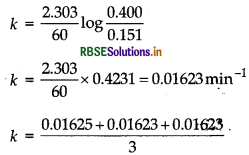
= 0.016236 min-1
(iii) Initial rate = k[N2O5] = 0.016236 × 0.4
= 0.00649 mol L-1 min-1
Question 40.
The thermal decomposition of HCOOH is a first order reaction with a rate constant of 2.4 × 10-3 s-1 at a certain temperature. How long will it take for three fourth of initial quantity of HCOOH to decompose? (log 0.25 = -0.6021)
Answer:
Three fourth of initial quantity of HCOOH is decomposed means that (where, initial concentration = [R]o) concentration after t time.
\([R]=\frac{[R]_0}{4}\)
k = 2.4 x 10-3 s-1
Thus, for the first order reaction, rate constant,
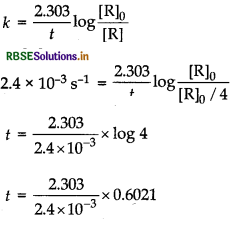
= 5.77 x 102 s
Question 41.
Nitrogen pentoxide decomposes according to equation, 2N2O3(g) → 4NO2(g) + O2(g) This first order reaction was allowed to proceed at 40°C and the data below were collected
|
[NO5]M |
Time (min) |
|
1. 0.400 |
0.00 |
|
2. 0.289 |
20.0 |
|
3. 0.209 |
40.0 |
|
4. 0.151 |
60.0 |
|
5. 0.109 |
80.0 |
(i) Calculate the rate constant. Include units with your answer.
(ii) Calculate the initial rate of reaction.
(iii) After how many minutes will [N2O5] be equal to 0.350 M?
Answer:
(i)
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
[R]o = 0.400 M
When [R] = 0.289 M, t = 20 min

When
t = 40 min, [R] = 0.209 M
\(k=\frac{2.303}{40} \log \frac{0.400}{0.200}=\frac{2.303}{40} \log 1.914\)
\(=\frac{2.303}{40} \times 0.2819\)
= 0.01623 min-1
Similarly, when t = 60 min, [R] = 0.151

= 0.016236 min-1
(ii) Initial rate = k[N2O5] = 0.016236 × 0.4
= 0.00649 mol L-1 min-1


Question 42.
A first order reaction has a rate constant value of 0.00510 min-1. If we begin with 0.10 M concentration of the reactant, how much of the reactant will remain after 3.0 h?
Answer:
Given, k = 0.00510 min-1
[R] = 0.10 M and
t = 3h = 3 x 60 min
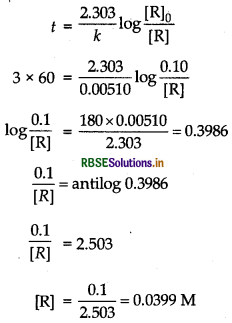
Question 43.
The decomposition of phosphine, PH3 proceeds according to the following equation:
4PH3(g) → P4(g) + 6H2(g)
It is found that the reaction follows the following rate equation
Rate = k[PH3]
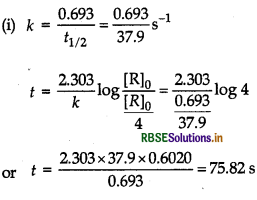
The half-life of PH3 is 37.9 s at 120°C.
(i) How much time is required for 3/4th of PH3 to decompose?
(ii) What fraction of the original sample of PH3 remains behind after 1 min?
Answer:
(i) Find the value of k by using formula,
k = 0.693/t1/2, then the value of t by using formula,
\(t=\frac{2.303}{k} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
(ii) From the value of k and t = 60s, find the % concentration of PH3 by using the above formula. Given, t1/237.9s, initial concentration = [R]o
concentration after time, t = \(\frac{[R]_0}{4}\)
(becauseth of PH 3/4 is decomposed).
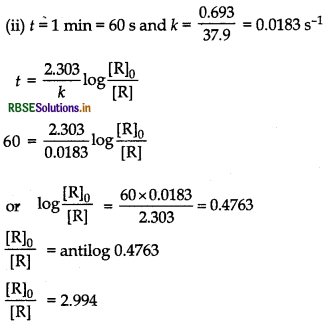
Let the initial amount is 100,
∴ \(\frac{100}{2.994}=[R]\)
⇒ [R] = 33.40%
Question 44.
The decomposition of a compound is found to follow a first order rate law. If it takes 15 min for 20% of original material to react, calculate
(i) the rate constant.
(ii) the time at which 10% of the original material remains unreacted.
Answer:
(i) First, find rate constant by using formula,
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
Where [R] = a - x and [R]0 = a
(ii) From the value of k and using the above formula, find the value of time at which 10% of the original material remains unreacted.
(i) 20% decomposition means [R] = 100,
and [R] 100 - 20 = 80
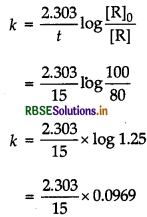
= 0.0148 min-1
(ii) Time at which 10% of the original material remains unreacted,
i.e.
[R] = 100, [R] = 10

= 155.6 min
Question 45.
In a pseudo first order hydrolysis of ester in water, the following results are obtained.
|
t/s |
0 |
30 |
60 |
90 |
|
Ester M |
0.55 |
0.31 |
0.17 |
0.085 |
(i) Calculate the average rate of reaction between the time interval 30 to 60 s.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
Answer:
(1) Average rate of reaction between the time interval
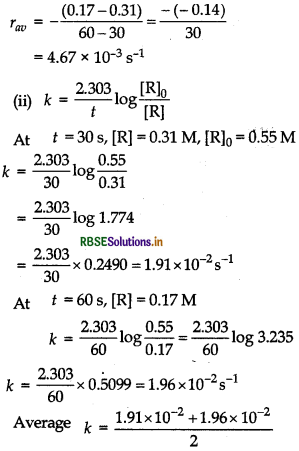
= 1.93 x 10-2 s-1
Question 46.
Define the following terms.
(i) Rate constant (k)
(ii) Activation energy (E)
Answer:
(i) Rate constant is the coefficient of proportionality related to the rate of reaction at a given temperature to the concentrations of reactants.
(ii) It is the extra energy contained by reactant molecules that results into effective collisions between them to form the products. It is denoted by Ea.
Question 47.
Define each of the following.
(i) Specific rate of a reaction.
(ii) Energy of activation of reaction.
Answer:
(i) Rate of chemical reaction when concentration of each reactant is unity is called rate constant. It is also known as specific rate of reaction.
(ii) It is the extra energy contained by reactant molecules that results into effective collisions between them to form the products. It is denoted by Ea.
Question 48.
How does a change in temperature affect the rate of a reaction? How can this effect on the rate constant of a reaction be represented quantitatively?
Or
What is the effect of temperature on the rate constant of a reaction? How can this temperature effect on rate constant be expressed quantitatively?
Answer:
Rate of reaction increase with temperature. Temperature coefficient is the ratio of rate constant at temperature (T + 10) K to the rate constant at temperature (1)K.
Temperature coefficient =

It is observed that for a chemical reaction with rise in temperature by 10°, the rate constant is nearly doubled.

Question 49.
With the help of diagram, explain the role of acti- vated complex in a reaction.
Answer:
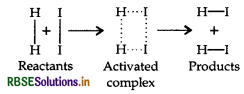
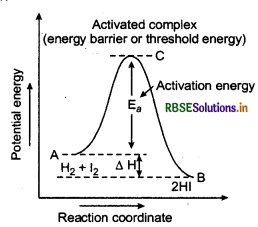
When the colliding molecules possess the kinetic energy less than the threshold Energy (E,), they require the energy to achieve E,. This amount of energy (energy required) is known as activation energy (E). After achieving the activation energy (i.e. E), they reach to an activated stage. This stage is different from the reactant as well as from the product.
e.g. in the reaction between H2(g) and I2(g), activated complex has configuration in which H - H and I - I bonds are breaking and H - I bonds are forming as shown below:
Potential energy diagram of this reaction is shown below.
Question 50.
The rate of most reactions become double when their temperature is raised from 298 K to 308 K. Calculate their activation energy.
(Given, R = 8.314J mol-1 K-1)
Answer:
Given T1 = 298 K, T2 = 308 K and k2/k1 = 2 and R = 8.314J mol-1 K-1
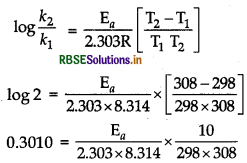
EA = 52903.05 J mol-1
Question 51.
The rate of a reaction becomes four times when the temperature changes from 300 K to 320 K. Calculate the energy of activation of the reaction, assuming that it does not change with temperature.
(R = 8.314 JK-1 mol-1)
Answer:
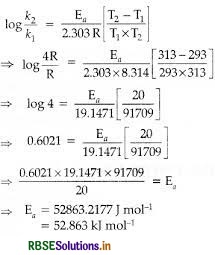
Question 52.
A first order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given: log 2 = 0.3010, log4 = 0.6021, R = 8.314 JK-1 mol-1)
Answer:
Given, t1/2 = 40 min at T = 300 K
∴ For first order,
\(k_1=\frac{0.693}{\left(t_{1 / 2}\right)_1}=\frac{0.693}{40 \mathrm{~min}}\)
k1 = 0.0173 min-1
Similarly, at T = 320 K
\(k_2=\frac{0.693}{\left(t_{1 / 2}\right)_1}=\frac{0.693}{20 \mathrm{~min}}\)
= 0.0346 min-1
Now, for calculation of activation energy
we know,
\(\log \frac{k_2}{k_1}=\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{~T}_1 \mathrm{~T}_2}\right)\)
Putting values in above equation
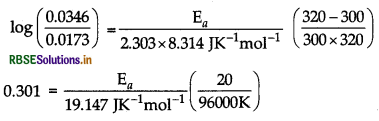
Ea = 27663.58 J or 27.663 kJ mol-1
Question 53.
The rate constant for the first order decomposition of H2O2 is given by the following equation:
\(\log k=14.2-\frac{1.0 \times 10^4}{T} K\)
Calculate Ea for this reaction and rate constant k if its hlf-life period be 200 min. (Given, R = 8.314 JK-1 mol-1)
Answer:
Calculation of activation energy, Ea
According to Arrhening equation, k = Ae-E/RT
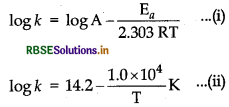
On comparing Eqs. (i) and (ii)
\(\frac{\mathrm{E}_a}{2.303 \mathrm{RT}}=\frac{1.0 \times 10^4}{\mathrm{~T}} \mathrm{~K}\)
Ea = 2.303 R x 1.0 × 104 K
= 2.303 × 1.0 × 104K x 8.314 JK-1 mol-1
= 19.14 x 104 J mol-1
= 191.4 k J mol-1
Calculation of rate constant, k
Given, t1/2 = 200 min = 200 × 60s
\(k=\frac{0.693}{t_{1 / 2}}=\frac{0.693}{200 \times 60}\)
⇒ k = 5.775 x 10-5 s-1.
Question 54.
The rate constants of a reaction at 500 K and 700 K are 0.02 s-1 and 0.07 s-1 respectively. Calculate the value of activation energy,
Ea (R = 8.314 JK-1 mol-1)
Answer:
Use formula, \(\log \frac{k_2}{k_1}=\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{~T}_1 \times \mathrm{T}_2}\right)\) to calculate Ea
Given
k1 = 0.02 s-1,
k2 = 0.07 s-1
T1 = 500 K,
T2 = 700 K
R = 8.314 JK-1 mol-1

Ea = 18230.35J mol-1
Question 55.
For a decomposition reaction, the values of k at two different temperatures are given below.
k1 = 2.15 x 10-8 L/(mol.s) at 650 K
k2 = 2.39 x 10-7L/(mol.s) at 700 K
Calculate the value of E, for the reaction.
(Given, log 11.11 = 1.046 R = 8.314 JK-1 mol-1)
Answer:
Given k1 = 2.15 x 10-8 L/(mol s) at 650 K
k2 = 2.39 x 10-7 L/(mols) at 700 K
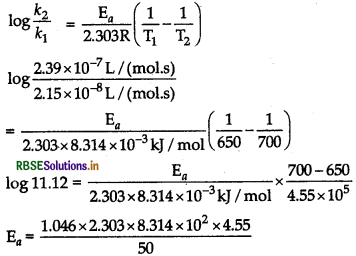
Ea = 182.25 kJ/mol
Question 56.
The decomposition of A into products has a value of k as 4.5 x 103 s-1 at 10°C and energy of activation 60 kJ mol-1. At what temperature would k be 1.5 x 104 s-1?
Answer:
Using formula, \(\log \frac{k_2}{k_1}=\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{~T}_1 \mathrm{~T}_2}\right)\) first find out the value of T2 in Kelvin, then in Celsius.
Given,
T1 = 10°C + 273 = 283 K
KT1 = 4.5 x 103 s-1
Ea = 60 kJ mol-1
= 60 × 103 J mol-1
T2 =?
KT2 =1.5 x 104 s-1
We know that,
\(\log \frac{k_{\mathrm{T}_2}}{k_{\mathrm{T}_1}}=\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{~T}_1 \mathrm{~T}_2}\right)\)
On putting values,
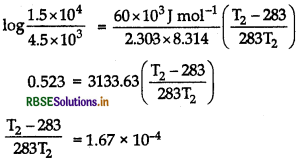
T2 - 283 = 0.0472T2
0.953T2 = 283
T2 = 297 K or T2 = 24°C
Question 57.
Rate constant 'K' of a reaction varies with temperature T according to the equation log k = log A \(\frac{\mathrm{E}_a}{2.303 \mathrm{R}}\left(\frac{1}{\mathrm{~T}}\right)\) where Ea is the activation energy.
When a graph is plotted for logk us 1/T, a straight line with a slope of -4250 K is obtained. Calculate 'E,' for the reaction. (R = 8.314 K-1 mol-1)
Answer:
Given, Slope = -4250 K,
R = 8.314 JK-1 mol-1
From equation,
\(\log k=\log \mathrm{A}-\frac{\mathrm{E}_a}{2.303 \mathrm{RT}}\)
Comparing with straight line equation, y = mx + c
\(-\frac{E_a}{2.303 R}\) = -4250
⇒ Ea = 2.303 × 8.314 × 4250 = 81.37 kJ mol-1
Question 58.
The rate of a reaction becomes four times when the temperature changes from 293 K to 313 K. Calculate the energy of the activation (E) of the reaction assuming that it does not change with temperature. (R = 8.314 JK-1 mol-1, log4 = 0.6021)
Answer:
Given T1 = 293 K
T2 = 313 K
k2/k1 = 4
R = 8.314 JK-1 mol-1
log 4 = 0.6021
= 52862.94 J mol-1.

Question 59.
The activation energy for the reaction 2HI (g) → H2 (g) + I2 (g) is 209.5 kJ mol-1 at 581 K. Calculate the fraction of molecules having energy equal to or greater than activation energy.
(R = 8.31 JK-1 mol-1)
Answer:
Given, Ea = 209.5 kJ mol-1 = 209500 J mol-1
R = 8.314 JK-1 mol-1
T = 581 K
Fraction of molecules = x
\(\log x=\frac{-\mathrm{E}_a}{2.303 \mathrm{RT}}\)
\(\log x=\frac{-209500}{2.303 \times 8.314 \times 581}\)
log x = -18.8323
x = antilog (19.1677)
= 1.471 × 10-19
Alternative method
Fraction of molecules = e-Ea/RT
= \(e^{\frac{-209.5 \times 1000}{8.314 \times 581}}\)
= e-43.37
= 1.461 × 10-19
Long Answer Type Questions:
Question 1.
For the hydrolysis of methyl acetate in aqueous solu- tion, the following results were obtained.
(i) Show that it follows pseudo first order reaction, as the concentration of water remains constant.
(ii) Calculate the average rate of reaction between the time interval 10 to 20 s.
(Given: log2 = 0.3010, log4 = 0.6021)
Answer:
(i) For the hydrolysis of methyl acetate to be a pseudo first order reaction, the reaction should be first order with respect to ester when [H2O] is constant. The rate constant (k) for a first order reaction is given by
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
where, [R]0 = initial concentration of the reactant
[R] = final concentration of the reactant
At t1 = 10s
\(k_1=\frac{2.303}{10} \log \frac{0.10}{0.05}\)
= 6.93 x 10-2 s-1
t2 = 20s
\(k_2=\frac{2.303}{20} \log \frac{0.10}{0.025}\)
= 6.93 x 10-2 s-1
It can be seen that the rate constant (k) for the reaction has a constant value under any given time interval. Hence, the given reaction follows the pseudo first order kinetics.
(ii) Average rate of reaction between the time interval of 10-20s is given by
Average rate = \(\frac{-\Delta[\text { Ester }]}{\Delta t}\)
= \(-\left(\frac{0.025-0.05}{20-10}\right)\)
= 0.0025 mol L-1 s-1
Question 2.
(i) For a reaction, A + B → P, the rate is given by Rate = k[A][B]2
(a) How is the rate of reaction affected if the concentration of B is doubled?
(b) What is the overall order of reaction if A is present in large excess?
(ii) A first order reaction takes 30 min for 50% completion. Calculate the time required for 90% completion of this reaction.
(log 2 = 0.3010)
Answer:
(i) A + B → P
Raté = k[A][B]2 (given)
(a) If concentration of B is doubled, then rate of reaction= k[A][2B]2 = 4k[A][B]2
∴ Rate becomes 4 times the original rate.
(b) If A is present in large excess, then the reaction will be independent of the concentration of A and will be dependent only on the concentration of B. As [B]2 will be the only determining factor in the order of reaction, the overall order of the reaction will be two.
(ii) For the given first order reaction, the rate constant for 50% completion is given by
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
Here, t = time taken for 50% completion = 30 min
[R]0 = initial concentration of reactant
[R] = final concentration of reactant
Let [R]o be 100 and due to 50% completion of reaction, [R] will be
100 - 50, i.e. 50
Putting values in (i), we get
\(k=\frac{2.303}{30} \log \frac{100}{50}\)
= \(\frac{2.303}{30}\) log 2
= 0.023 min1
For same reaction, the time required for 90% comple- tion of reaction can be computed using the expression,
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
Here, [R] = final concentration of reactant = 100 - 90 =
\(0.023=\frac{2.303}{t} \log \frac{100}{10}\)
= \(t=\frac{2.303}{0.023}\) log 10
= 100.13 min
Therefore, the time required for 90% completion of the given first order reaction is 100.13 min.

Question 3.
For the hydrolysis of methyl acetate in aqueous solu- tion, the following results were obtained.
|
t/s |
0 |
30 |
60 |
|
[CH3COOCH3]/mol L-1 |
0.30 |
0.15 |
0.60 |
(i) Show that it follows pseudo first order reaction, as the concentration of water remains constant.
(ii) Calculate the average rate of reaction between the time interval 30 to 60 s.
(Given: log 2 = 0.3010, log4 = 0.6021)
Answer:
(i) \(\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{A}]_0}{[\mathrm{~A}]}\)
Where [A]n = Intial concentration of rectant
[A] = Final concentration of rectant
At t1 = 30 sec
\(\mathrm{k}=\frac{2.303}{30} \log \frac{0.60}{0.30}\)
K = 0.07677 log 2
K = 0.0231 s-1
for t = 60 sec
\(\mathrm{k}=\frac{2.303}{30} \log \frac{0.30}{0.15}\)
K = 0.07677 log 2
k = 0.0231 s-1
∴ K same for both the case hence it is pseudo first order reaction.
(ii) Average rate of reaction between the time interval of 30 - 60 seconds is given by
\(\begin{aligned} &\mathrm{k}=\frac{-\Delta\left[\mathrm{CH}_3 \mathrm{COOCH}_3\right]}{\Delta \mathrm{t}} \\ &\mathrm{k}=-\left(\frac{0.15-0.30}{60-30}\right) \\ &=\frac{0.15}{30}=0.005 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1} \end{aligned}\)
Question 4.
(i) For a chemical reaction R → P, the variation in the concentration, In [R] us time(s) plot is given as
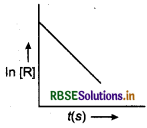
(a) Predict the order of the reaction.
(b) What is the slope of curve?
(c) Write the unit of rate constant for this reaction.
(ii) Show that the time required for 99% completion is double of the time required for the completion of 90% reaction.
Answer:
(i) The first order rate constant is given by,
\(k=\frac{1}{t} \cdot \operatorname{In} \frac{[\mathrm{R}]_0}{[\mathrm{R}]}\)
where, [R]o is the initial
concentration of reactant and [R] is the concentration of reactant at time t.
∴ In[R] = In[R]o - kt
y = c + mx
(a) The order of the reaction is first order.
(b) Slope of the curve = -k.
(c) Unit rate constant for first order reaction = time"1 or s-1 or min-1.
(ii) Let [R] = 100
For the reaction which is 99% completed, [R] = 1% of
[R]o = 1and for the reaction which is 90% completed,
[R] = 10% of [R] = 10.
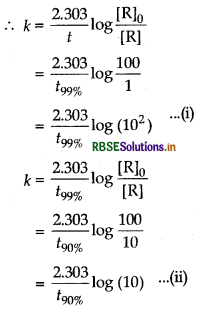
Equating Eqs. (i) and (ii)

Hence, time required for 99% completion of a reaction of first order is double the time required for 90% completion of the reaction.
Question 5.
(i) Define the following terms.
(a) Activation energy
(b) Rate constant
(ii) A first order reaction takes 10 min for 25% decomposition. Calculate t1⁄2 for the reaction.
(Given, log2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021)
Answer:
(i) It is the extra energy contained by reactant molecules that results into effective collisions between them to form the products. It is denoted by Ea.
(b)Rate of reaction increase with temperature. Temperature coefficient is the ratio of rate constant at temperature (T + 10) K to the rate constant at temperature (1)K.
Temperature coefficient =

It is observed that for a chemical reaction with rise in temperature by 10°, the rate constant is nearly doubled.
(ii) Given, t = 10 min,
amount of reactant decomposed in time (t = 10 min) = 25% if [R] = 100, then amount of reactant left = 75% of [R]o = 75
\(k=\frac{2.303}{t} \log \frac{[\mathrm{R}]_0}{\mathrm{R}}=\frac{2.303}{10} \log \frac{100}{75}\) = 0.02303 x 0.124
k = 0.028 min-1
\(t_{1 / 2}=\frac{0.693}{k}=\frac{0.693}{0.028}\)
= 24.75 min
Question 6.
(a) Consider the reaction R → P for which the change in concentration of R with time is shown by the following graph:
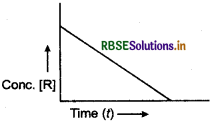
(i) Predict the order of reaction.
(ii) What does the slope of the curve indicate?
(b) The rate of reaction quadruples when temperature changes from 293 K to 313 K. Calculate E, assuming that it does not change with time.
[R = 8.314 JK-1 mol-1]
Answer:
The reaction is of zero order. Since, the integrated rate equation for zero order reaction is \(k=\frac{\left[\mathrm{R}_0\right]-[\mathrm{R}]}{t}\)
(i) If we plot [R] against t, we get a straight line with slope equal to -k and intercept equals to [Ro] as shown below.
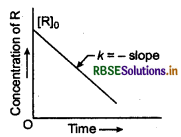
Variation in the concentration vs time plot for a zero order reaction
(ii) Slope of curve indicate that concentration of reactant decrease with the time (t) and is numerically equal to the value of k.
i.e. slope of the graph = \(k=\frac{d[\mathrm{R}]}{d t}\)
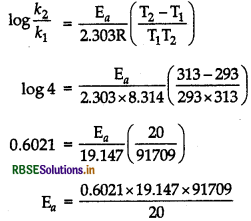
(b) Given, T1 = 293 K, T2 = 313 K, k2/k1 = 4. R = 8.314 JK-1 mol-1, log4 = 0.6021
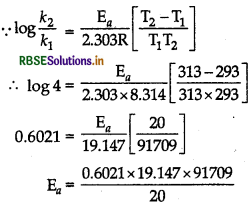
= 52862.94 J mol-1
Hence
Ea = 52.86294 kJ mol-1
Question 7.
(a) Draw the plot of Ink vs 1/T for a chemical reaction. What does the intercept represent? What is the relation between slope and Ea?
(b) A first order reaction takes 30 minutes for 20% decomposition. Calculate t1/2.
[log 2 = 0.3010]
Answer:
(a) The plot of Ink vs 1/T gives a straight line. Slope of this plot gives the value of imm and the intercept gives the value of In A.
Ea and A can also be determined from the rate constants at two different temperatures.
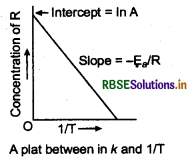
A plot between ink and 1/T
(b) Given, reactant decomposes 20% in 30 minutes (x)
Let initial quantity (a) = 100.
Then after 30 minutes (a - x) = 100 - 20 = 80.
Time (t30) = 30 minutes
For first order reaction,

On substituting the value of k in above equation, we get
\(t_{1 / 2}=\frac{0.693}{7.439 \times 10^{-3}}\)
= 9315 minutes.
COMPETITION CORNER:
Question 1.
According to Arrhenius equation
(a) high activation energy generally shows rapid reaction.
(b) the rate constant increases with increase in temperature. It is due to increase in number of those collisions whose energy is more than activation energy.
(c) greater the activation energy, dependence of rate constant on temperature will be high.
(d) It is a measurement of rate of collisions of powers factor without considering their energy.
Answer:
(c) greater the activation energy, dependence of rate constant on temperature will be high.
Question 2.
The decomposition of H2O2 is of first order reaction. In this type of decomposition, the concentration of H2O2 decreases from 0.5 M to 0.125 M in 50 mins. When the concentration of H2O reached to 0.5 M, then the rate of formation of O2 will be
(a) 6.93 x 10-4 mol min-1
(b) 2.66 L min-1 at STP
(c) 1.34 x 10-2 mol min-1
(d) 6.93 x 10-2 mol min-1
Answer:
(a) 6.93 x 10-4 mol min-1
Question 3.
Which of the following quantity changes by addition of catalyst in a chemical reaction?
(a) Entropy
(b) Internal energy
(c) Enthalpy
(d) Activation energy
Answer:
(b) Internal energy

Question 4.
The decomposition of phosphine (PH3) on tungsten at low pressure is of zero order reaction, because.
(a) the rate of decomposition is very slow.
(b) the rate is directly proportional to surface area.
(c) the rate is inversely proportional to surface area.
(d) the rate is free from surface area.
Answer:
(d) the rate is free from surface area.
Question 5.
The rate of reaction is 0.04 mol L-1 s-1 after 10 s and 0.03 mol L-1 s-1 after 20 second after initiating first order reaction. The half-life period of this reaction is
(a) 24.1 s
(b) 34.1 s
(c) 44.1 s
(d) 54.1 s
Answer:
(a) 24.1 s
Question 6.
High order reaction (> 3) is rare, why?
(a) The probability of collisions of all species in a reaction is low.
(b) Entropy and activation energy increase due to large number of molecules.
(c) Equilibrium is transferred in the direction of reactants due to ineffective collisions.
(d) There is decay of active species through collision.
Answer:
(a) The probability of collisions of all species in a reaction is low.
Question 7.
In which of the following graph, activation energy of reaction can be determined from slope?
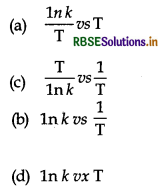
Answer:
\(\text { (b) } \ln k \text { vs } \frac{1}{\mathrm{~T}}\)
Question 8.
The rate constant 'k' for the reaction
N2O5(g) → 2NO2(g) + 1/2O2(g)
Is 23. x 10-2 s1. Which of the following equation shows the change of [N2O5] with time, [N2O5]10 corresponds to initial concentration and [N2O5]t after time 't'
(a) [N2O5] = [N2O5]10 + kt
(b) [N2O5]10 = [N2O5]t ekt
(c) log10 [N2O5]t = log10 [N2O5]10 - kt
\(\text { (d) } \ln \frac{\left[\mathrm{N}_2 \mathrm{O}_5\right]_0}{\left[\mathrm{~N}_2 \mathrm{O}_5\right]_t}=k t\)
Answer:
\(\text { (d) } \ln \frac{\left[\mathrm{N}_2 \mathrm{O}_5\right]_0}{\left[\mathrm{~N}_2 \mathrm{O}_5\right]_t}=k t\)
Question 9.
The rate constant for the reaction A → B is 0.6 x 10-3 mol s-1. If the concentration of A is 5 M, then the concentration of B after 20 min is
(a) 1.08 M
(c) 0.36 M
(b) 3.60 M
(d) 0.72 M
Answer:
(d) 0.72 M

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम