RBSE Class 12 Chemistry Important Questions Chapter 13 Amines
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 13 Amines Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 13 Important Questions Amines
Objective Questions:
Question 1.
The product 'D' in following reaction is:
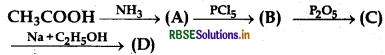
(a) Ester
(b) Amine
(c) Acid
(d) Alcohol
Answer:
(b) Amine
Question 2.
The correct order of basicity is:
(a) CH3NH2 > (CH3)2NH > (CH3)3N > NH3
(b) (CH3)3N > (CH3)2NH > CH3NH2 > NH3
(c) (CH3)2NH > CH3NH2 > (CH3)3N > NH3
(d) NH3 > (CH3)3N > CH3NH2 > (CH3)2NH
Answer:
(c) (CH3)2NH > CH3NH2 > (CH3)3N > NH3
Question 3.
In the following reaction (C) is:
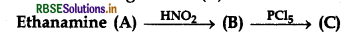
(a) Ethylcyanide
(b) Ethylamine
(c) Methanamine
(d) Acetamide
Answer:
(d) Acetamide

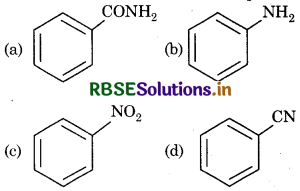
Question 4.
Which of the following reaction does not form amine?
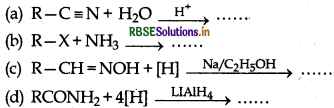
Answer:
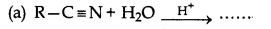
Question 5.
The correct order of boiling point is:
(a) RCH2NH2 > RCOOH > RCH2OH
(b) RCH2NH2 > RCH2OH > RCOOH
(c) RCH2NH2 < RCOOH < RCH2OH
(d) RCH2NH2 < RCH2OH < RCOOH
Answer:
(d) RCH2NH2 < RCH2OH < RCOOH
Question 6.
CH3NH2 + CHCl3 3KOH → X + Y + 3H2O here compounds 'X' and 'Y' are:
(a) CH3CN + 3KCl
(b) CH3NC + 3KCl
(c) CH3CONH2 + 3KCl
(d) CH3NC + K2CO3
Answer:
(b) CH3NC + 3KCl
Question 7.
'A' in following reaction is:
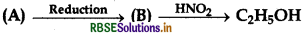
(a) CH3CN
(b) CH3NC
(c) C2H5CN
(d) CH3NO2
Answer:
(a) CH3CN
Question 8.
How many isomers will be possible for compound having molecular formula C7H9N?
(a) 4
(b) 5
(c) 6
(d) 7
Answer:
(b) 5
Question 9.
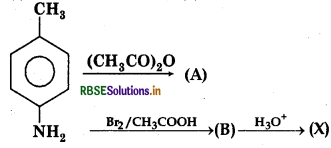 Here 'X' is:
Here 'X' is:
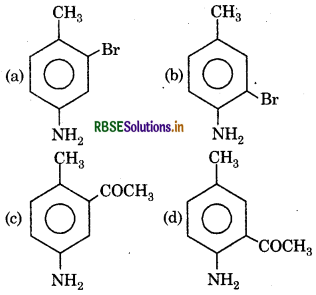
Answer:
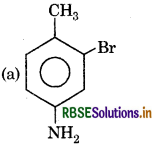
Question 10.
In Hinsberg's test, the product of which amine does not dissolve in NaOH?
(a) CH3CH2NH2
(b) (CH3)2CHNH2
(c) CH3NHCH3
(d) CH3N(CH3)2
Answer:
(c) CH3NHCH3

Question 11.
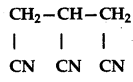 has IUPAC name:
has IUPAC name:
(a) 1,2,3-Tricyano propane
(b) Propane-1,2,3-tricarbonitrile
(c) 3-Cyanopentane-1, 5-dinitrile
(d) 1,2,3-Propane trinitrile
Answer:
(c) 3-Cyanopentane-1, 5-dinitrile
Question 12.
The product obtained on acidic hydrolysis of methyl isocyanide is:
(a) CH3NH2 + HCOOH
(b) C2H5NH2 + HCOOH
(c) CH3NH2 + HCOOH
(d) CH3NH2 + CH3CH2COOH
Answer:
(a) CH3NH2 + HCOOH
Question 13.
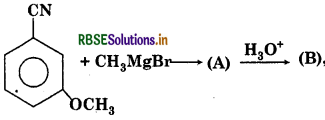
The product (B) is:
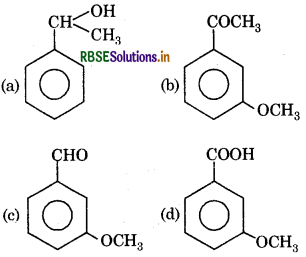
Answer:
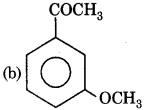
Question 14.
Which of the following is strong base?
(a) FCH2NH2
(c) C6H5NH2
(b) FCH2CH2NH2
(d) C6H5CH2NH2
Answer:
(d) C6H5CH2NH2
Question 15.
Which of the following does not show by molecular formula C3H9N?
(a) 1° amine
(c) 3o amine
(b) 2o amine
(d) quaternary ammonium salt
Answer:
(d) quaternary ammonium salt
Very Short Answer Questions:
Question 1.
Why is an alkylamine more basic than ammonia.
Answer:
Due to electron releasing inductive effect (+1) of alkyl group, the electron density on the nitrogen atom increases and thus, it can donate the lone pair of electrons more easily than ammonia.
Question 2.
Arrange the following compounds in an increasing order of basic strengths in their aqueous solutions:
NH3, CH3NH2, (CH3)2NH, (CH3)3N
Answer:
Basicity order (due to stability of ammonium cation
(CH3)2 NH > CH3NH2 > (CH3)3 N > NH3

Question 3.
Give the IUPAC name of H2N - CH2 - CH2 - CH = CH2.
Answer:
IUPAC name: But-3-ene-1-amine
Question 4.
Arrange the following compounds in an increasing order of their solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2
Answer:
CH3NH2 < (C2H3)2NH < C2H2NH2
Question 5.
Give a chemical test to distinguish between ethylamine and aniline.
Answer:
Ethylamine and aniline:
By Azo dye test: It involves the reaction of any aromatic primary amine with HNO2 (NaNO2 + dil. HCI) at 273 - 278 K followed by treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange or red coloured dye is obtained.
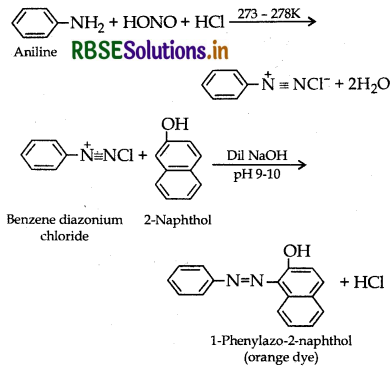
Aliphatic 1° amines under these conditions give a brisk evolution of N2, gas with the formation of 1° alcohol i.e., solution remains clear.
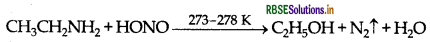
Question 6.
Arrange the following in the decreasing order of their basic strength in aqueous solutions:
CH3NH2, (CH3)2, NH, (CH3)3N and NH3
Answer:
(CH3)2NH > CH3NH2 > (CH3)3 N > NH3

Question 7.
Arrange the following in increasing order of their basic strength in aqueous solution:
CH3NH2,(CH3)3N, (CH3)2NH
Answer:
(CH3)3N < CH3NH2 < (CH3)2NH
3° amine 1° amine 2° amine
Question 8.
Write the structure of 2-aminotoluene.
Answer:
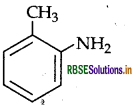
Question 9.
Write the structure of n-methylethanamine.
Answer:
Structure of n-methylethanamine:
H3C - H2C - NH - CH3
Question 10.
Write the structure of prop-2-en-1-amine.
Answer:
H2C = CH - H2C - NH2
Question 11.
How may methyl amine be preferentially converted to methyl isocyanide?
Answer:
By carbylamine reaction:
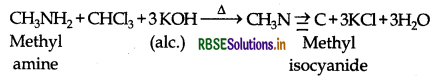
Question 12.
Arrange the following compounds in increasing order of solubility in water:
C6H5NH2, (C2H3)2NH, C2H5NH2
Answer:
C6H5NH2 < (C2H3)2NH < C2H5NH2
Question 13.
Arrange the following in increasing order of basic strength:
C6H5NH2, C6H5NHCH3, C6H5CH2NH2
Answer:
C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2
Question 14.
Arrange the following in increasing order of basic strength:
CH5NH2 CH5NHCH5 CH5N(CH3)2
Answer:
C6H5N(CH3)2 > C2H2NHCH3 > C2H2NH2
Question 15.
The conversion of primary aromatic amines into diazonium salts is known as .............
Answer:
Diazotization.

Question 16.
Out of CH3-NH2 and (CH3)3N, which one has higher boiling point?
Answer:
CH3-NH2 has higher boiling point than (CH3)3N.
Question 17.
Complete the following reaction equation:
C6H5N2Cl + H3PO2 + H2O
Answer:
C6H5N2Cl + H3PO2 + H2O → C6H6 + N2 + H3PO3 + HCl
Benzene diazonium chloride Hypophosphorous acid Benzene Phosphorous acid.
Question 18.
Arrange the following in increasing order of basic strength
Aniline, p-Nitroaniline and p-Toluidine
Answer:
p-Nitroaniline < Aniline < p-Toluidine
(Show - I effect) (Show + I effect)
Question 19.
Write the IUPAC name of the given compound:
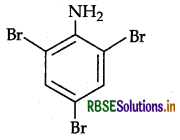
Answer:
2, 4, 6-Tribromoaniline
Question 20.
Write IUPAC name of the following compound:
(CH3CH2)2NCH3
Answer:
N-Ethyl-N-methylethanamine
Question 21.
Write the IUPAC name of the following compound:
CH3NHCH(CH3)2
Answer:
IUPAC name: N-Methylpropan-2-amine
Question 22.
Write the IUPAC name of the following compound:
(CH3)2N - CH2CH3
Answer:
IUPAC name: N,N-Dimethylethanamine
Question 23.
Write IUPAC name of the following compound:

Answer:
IUPAC name: N,N-Dimethylbutanamine,
Short Answer Type Questions:
Question 1.
Give the chemical tests to distinguish between the following pairs of compounds:
(i) Ethyl amine and Aniline
(ii) Aniline and Benzylamine
Answer:
(i) Ethylamine and aniline:
By Azo dye test: It involves the reaction of any aromatic primary amine with HNO2 (NaNO2 + dil. HCI) at 273 - 278 K followed by treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange or red coloured dye is obtained.

Aliphatic 1° amines under these conditions give a brisk evolution of N2, gas with the formation of 1° alcohol i.e., solution remains clear.
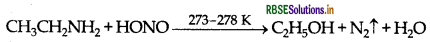
(ii) Distinction between Aniline and Benzylamine: By Nitrous acid test: Benzylamine reacts with HNO2 to form a diazonium salt which being unstable even at low temperature, decom poses with evolution of N2 gas.
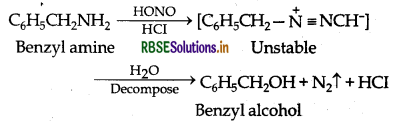
Aniline on the other hand, reacts with HNO2 to form benzene diazonium chloride which is stable at 273 - 278 K and hence does not decompose to evolve N2 gas.
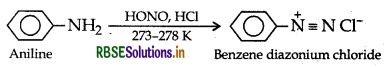

Question 2.
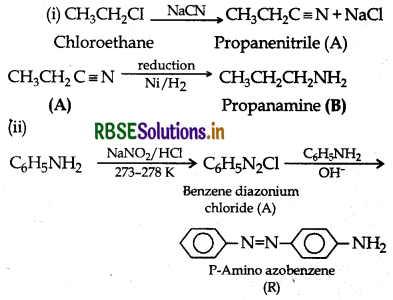
Identify A and B in each of the following processes:
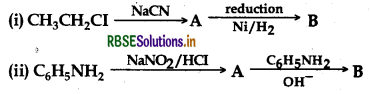
Answer:
Question 3.
Give the chemical tests to distinguish between the following pairs of compounds:
(i) Methylamine and Dimethylamine
(ii) Aniline and N-methylaniline
Answer:
(i) Methylamine and Dimethylamine:
By Carbylamine test: Methylamine being a primary amine gives this test but Dimethylamine being a secondary amine does not.
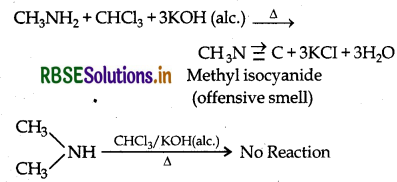
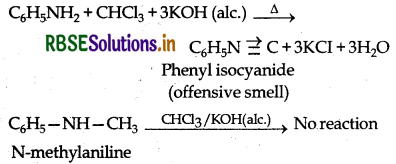
(ii) Aniline and N-methylaniline
By Carbylamine test: Aniline is a 1° aromatic amine while N-methylaniline is a secondary aromatic amine. Therefore only 1° aromatic amine gives this test.
Question 4.
Describe the following giving the relevant chemical equation in each case:
(i) Carbylamine reaction
(ii) Hofmann's bromamide reaction
Answer:
(i) Carbylamine reaction: Aliphatic and aromatic primary amines on heating with chloroform and ethanolic KOH form isocyanides or carbylamines which are foul smelling substances. This reaction is known as carbylamines reaction.
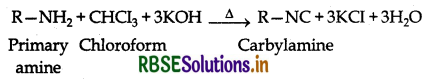
(ii) Hofmann's bromamide reaction: Primary amines can be prepared by treating an amide with Br2 in an aqueous or alcoholic soln of NaOH.

Question 5.
Complete the following reaction equations:
(i) C6H5N2Cl + H3PO2 + H2O
(ii) C6H5NH2 + Br2 (aq) →
Answer:
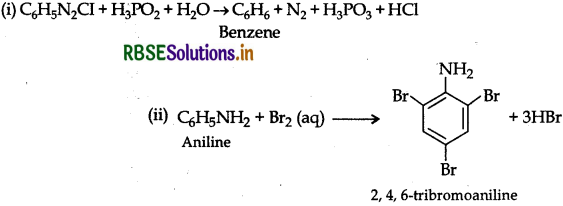
Question 6.
Give IUPAC names of the following compounds :
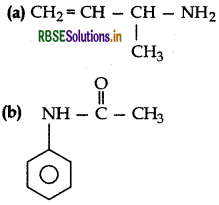
Answer:
(a) IUPAC name: Methyl prop-2-en-1-amine
(b) IUPAC name: Phenyl acetamide
(b) Ethanoyl chloride to Ethanenitrile.

Question 7.
How are the following conversions carried out:
(a) Aniline to p-hydroxyazobenzene
Answer:
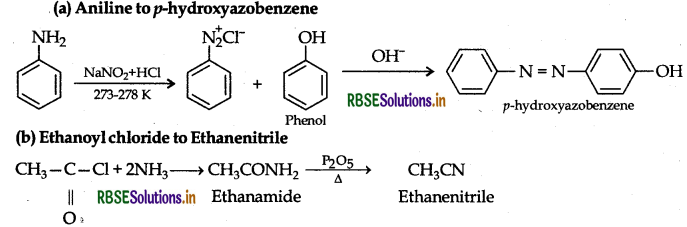
Question 8.
How are the following conversions carried out?
(i) CH3-CH2Cl to CH3CH2CH2NH2
(ii) Benzene to aniline
Answer:
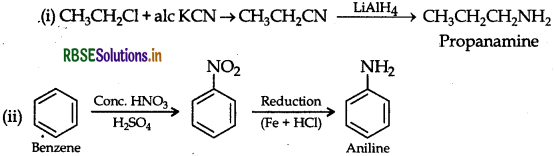
Question 9.
How would you account for the following:
(a) Aniline is a weaker base than cyclohexyl amine.
(b) Methylamine in aqueous medium gives reddish brown precipitate with FeCl3.
Answer:
(a) In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen decreases.
But in cyclohexylamine, the lone pair of electrons on N-atom is readily available due to absence of π-electrons. Hence aniline is weaker base than cyclohexylamine.
(b) Methylamine being more basic than H2O, it accepts a proton from water liberating OH- ions.
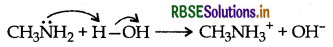
These OH- ions combine with Fe+3 ions present in H2O to form reddish brown precipitate of hydrated ferric oxide.
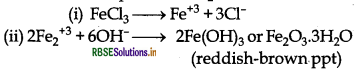
Question 10.
How would you account for the following:
(a) Electrophilic susbstitution in case of aromatic amines takes place more readily than benzene.
(b) Ethanamide is a weaker base than ethanamine.
Answer:
(a) Aniline exists as a resonance hybrid of the following five structures:
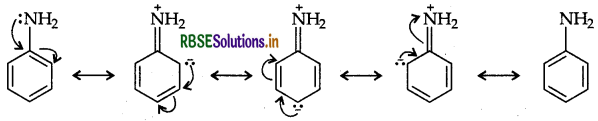
The electron density is maximum at ortho and para positions to the -NH2 group. But in benzene there is no delocalisation of electron at any position and hence electrophilic substitution in case of aromatic amines takes place more readily than benzene.
(b) In ethanamide the lone pair of electron of N-atom is not available due to resonance structure
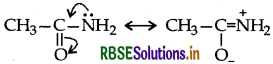
Question 11.
Illustrate the following reactions:
(a) Sandmeyer's reaction
(b) Coupling reaction
Answer:
(a) Sandareyer's reaction: Aniline reacts with NaNO2 in HCl at 273-278 K giving diazonium salt which further reacts with cuprous chloride/bromide to give chloro or bromo benzene.
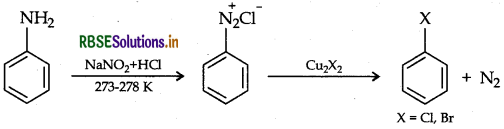
This reaction is Sandmeyer's reaction.
(b) Coupling reaction: Arene diazonium salts react with highly reactive aromatic compounds such as phenols and amines to form brightly coloured azo compounds.
Ar - N - N - Ar. This reaction is known as coupling reaction.
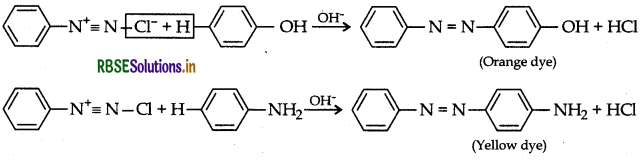

Question 12.
Give chemical tests to distinguish between the following pairs of compounds:
(a) Aniline and Ethylamine
(b) Ethylamine and Dimethylamine
Answer:
(a) Ethylamine and aniline:
By Azo dye test: It involves the reaction of any aromatic primary amine with HNO2 (NaNO2 + dil. HCI) at 273 - 278 K followed by treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange or red coloured dye is obtained.

Aliphatic 1° amines under these conditions give a brisk evolution of N2, gas with the formation of 1° alcohol i.e., solution remains clear.
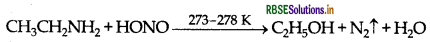
(b) Ethylamine and dimethylamine can be distinguished by the carbylamine test. Carbylamine test: Aliphatic and aromatic amines on heating with chloroform and ethanolic potassium hydroxide form foul smelling isocyanides or carbylamines. Ethylamine (being an aliphatic primary amine) gives a positive carbylamine test, but dimethylamine does not.
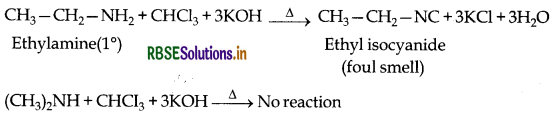
Question 13.
Explain the following reactions:
(a) Gabriel Phthalimide reaction
(b) Coupling reaction
Answer:
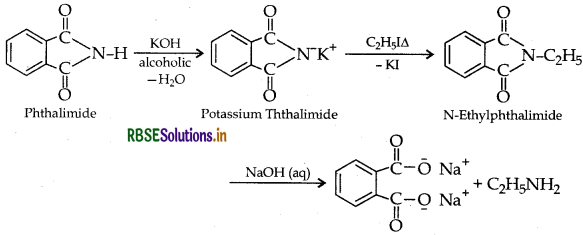
(a) Gabriel phthalimide synthesis: It is used to prepare 1° amine (Primary amine). The starting compound is a phthalimide. But aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Example:
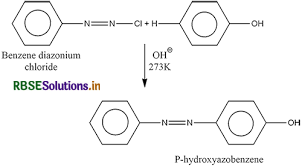
(b) Coupling reaction is a class of organic reaction in which two chemical species are joined together with the help of a metal catalyst. When benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position coupled with the diazonium salt to form p- hydroxyazobenzene.
Question 14.
Give reasons:
(a) Aniline is a weaker base than cyclohexyl amine.
(b) It is difficult to prepare pure amines by ammonolysis of alkyl halides.
Answer:
(a) In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen decreases.
But in cyclohexylamine, the lone pair of electrons on N-atom is readily available due to absence of л-electrons. Hence aniline is weaker base than cyclohexylamine.
(b) Because the primary amine formed by ammonolysis itself acts as a nucleophile and produces further 2° and 3° alkyl amine.

Question 15.
Give reasons:
(i) Electrophilic substitution in aromatic amines takes place more readily than benzene.
(ii) CH3CONH2 is weaker base than CH3CH2NH2.
Answer:
(i) Due to the strong activating effect of the NH2 group, aromatic amines undergo electrophilic substitution reactions readily than benzene.
(ii) In case of acetamide due to resonance, the lone pair of electrons on the nitrogen atom is delocalized over keto group which decreases electron density hence less basic while in ethylamine due to + I effect of ethyl group electron density increases on N-atom and hence basic character increases.
Question 16.
(i) Arrange the following compounds in an increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2 (C2H5)2NH and CH3NH2
(ii) Arrange the following compounds in a decreasing order of pK values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Answer:
(i) Increasing order of basic strength:
C6H5NH2 < C6H5N(CH3)2 < CH3NH2 < (C2H5)2NH
More + I effect
(ii) Decreasing order of pK values :
C6H5NH2 < C6H5NHCH3 < C2H5NH2 < (C2H5)2NH
Question 17.
Give a chemical test to distinguish between each of the following pairs of compounds:
(i) Ethylamine and Aniline
(ii) Anline and Benzylamine
Answer:
Ethylamine and aniline:
By Azo dye test: It involves the reaction of any aromatic primary amine with HNO2 (NaNO2 + dil. HCI) at 273 - 278 K followed by treatment with an alkaline solution of 2-naphthol when a brilliant yellow, orange or red coloured dye is obtained.

Aliphatic 1° amines under these conditions give a brisk evolution of N2, gas with the formation of 1° alcohol i.e., solution remains clear.
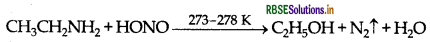
(ii) Distinction between Aniline and Benzylamine: By Nitrous acid test: Benzylamine reacts with HNO2 to form a diazonium salt which being unstable even at low temperature, decom poses with evolution of N2 gas.

Aniline on the other hand, reacts with HNO2 to form benzene diazonium chloride which is stable at 273 - 278 K and hence does not decompose to evolve N2 gas.

Question 18.
Write the chemical equations involved in the following reactions:
(i) Hoffmann-bromamide degradation reaction
(ii) Carbylamine reaction
Answer:
(i) Hoffmann's bromamide reaction: In this reaction, migration of an alkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom. Therefore the amine so formed has one carbon atom less than that of amide.
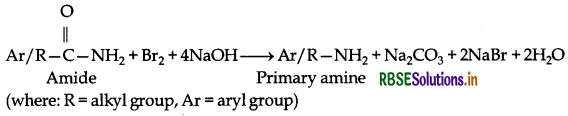
Example
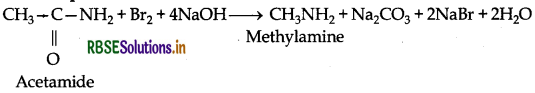
(ii) Carbylamine reaction: This reaction is used to distinguish primary amines from 2o and 3o amines as it is only given by 1° amines with the production of a very bad smelling organic compound.
For example:
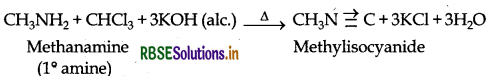

Question 19.
Giving an example for each describe the following reactions:
(i) Hoffmann's bromamide reaction
(ii) Gatterman reaction
(iii) A coupling reaction
Answer:
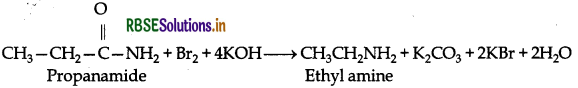
(i) Hoffmann's bromamide reaction: When amide is treated with bromide in alkaline solution, an amide yields an amine containing one carbon less than the starting amide.
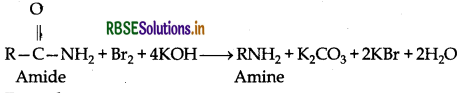
Example:
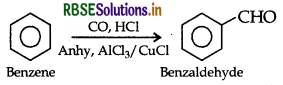
(ii) Gattermann reaction: When benzene or its derivative is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
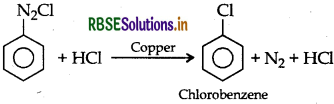
Galtermann Koch reaction: Diazonium salt reacts with hydrogen halide in presence of copper powder giving haloarene.
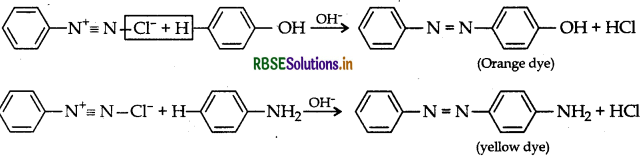
(iii) A coupling reaction: Arene diazonium salts react with highly reactive aromatic compounds such as phenols and amines to form brightly coloured azo compounds.
Ar - NN - Ar. This reaction is known as coupling reaction.
Question 20.
Complete the following reaction equations :
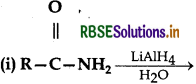
(ii) C6H5N2Cl + H3PO2 + H2O →
(iii) C6H5NH2 + Br2(aq) →
Answer:
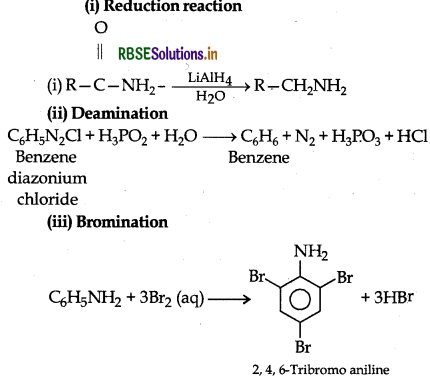
Question 21.
Complete the following reaction equations:
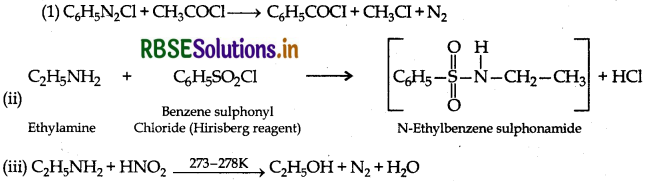
(i) C6H5N2Cl + CH3COCl →
(ii) C2H5NH2 + C6H5SO2C →
(iii) C2H5NH2 + HNO2 →
Answer:
Question 22.
In the following cases rearrange the compounds as directed:
(i) In an increasing order of basic strength: C6H5NH2, C6H5, N(CH3)2, (C2H5)2NH and CH2NH2
(ii) In a decreasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
(iii) in an increasing order of pKb values: C2H5NH2, C6H5, NHCH3, (C2H5)2NH
Answer:
(i) Order of basic strength: C6H5NH2 < C6H5N(CH3)2 < CH3NH2 < (C2H5)2NH
(ii) The decreasing order of basic strength:

(iii) Increasing order of pK values: (C2H5)2NH < C2H5NH2 < CHÁNHCH3 < C6H5NH22 Since a stronger base has a lower pKb value therefore basic strength order. (C2H5)2NH > C2H5NH2 > C6H5NHCH3 > C6H5NH2
Question 23.
Complete the following chemical equations:
(i) C6H5N2Cl + C6H5NH2OH
(iii) RNH2 + CHCI3 + KOH-

Answer:
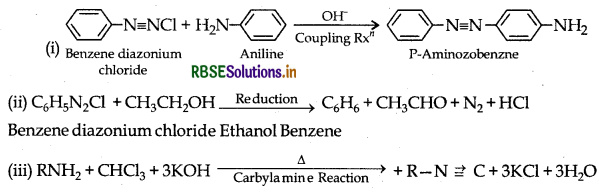
1° Amine Chloroform Alkyl isocyanide (offensive smell)

Question 24.
(a) Explain why an alkylamine is more basic than ammonia?
(b) How would you convert
(i) Aniline to nitrobenzene?
(ii) Aniline to iodobenzene?
Answer:
(a) This is because of electron releasing tendency of alkyl group. The alkyl group shows +I effect which increases the electron density at N atom in alkyl amine which makes it more basic than ammonia.
(b) (i) Aniline to nitrobenzene
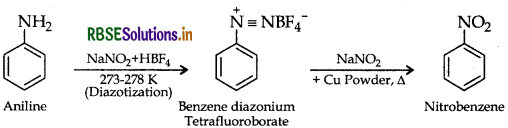
(ii) Aniline to iodobenzene
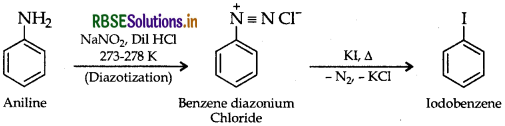
Question 25.
Complete the following chemical equations:
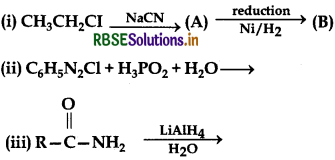
Answer:
(i)
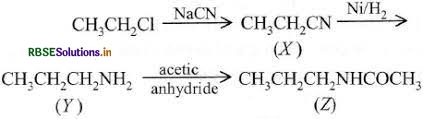
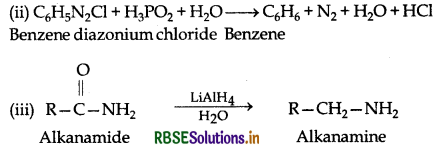
Question 26.
State reasons for the following:
(i) pK value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.
Answer:
(i) Higher the pK, value, lower will be the basicity therefore aniline is less basic than methylamine because the lone pair of electrons on nitrogen atom gets delocalized over the benzene ring are unavailable for protonation due to resonance in aniline which is absent in case of alkylamine.
(ii) Ethylamine is soluble in water due to its capability to form H-bonds with water while aniline is insoluble in water due to larger hydrocarbon part which tends to retard the formation of H-bonds.
(iii) Due to presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H-bonding while tertiary amines due to the absence of a H-atom on the N-atom, do not undergo H bonding. As a result, primary amines have higher boiling points than 3° amines.
Question 27.
Write chemical equations for the following conversions:
(i) Nitrobenzene to benzoic acid.
(ii) Benzyl chloride to 2-phenylethanamine
(iii) Aniline to benzyl alcohol.
Answer:
(i) Nirtobenzene to benzoic acid: NO2
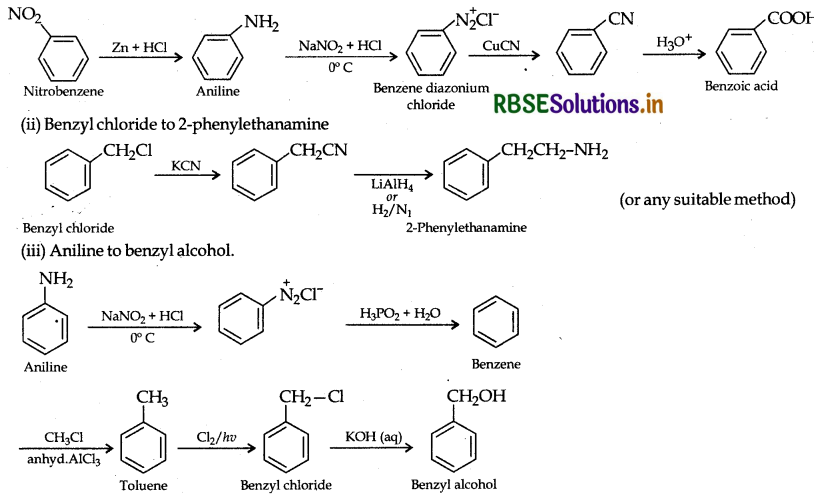
Question 28.
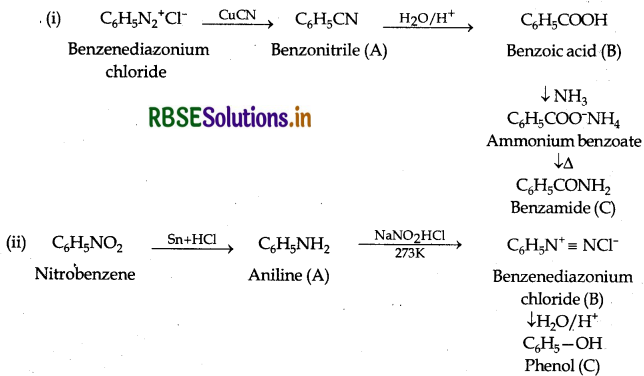
Give the structure of A, B and C in the following reactions:
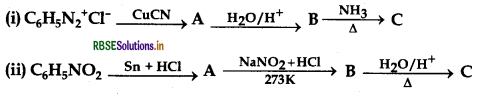
Answer:
Question 29.
Give the structures of products A, B and C in the following reactions:
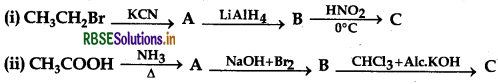
Answer:
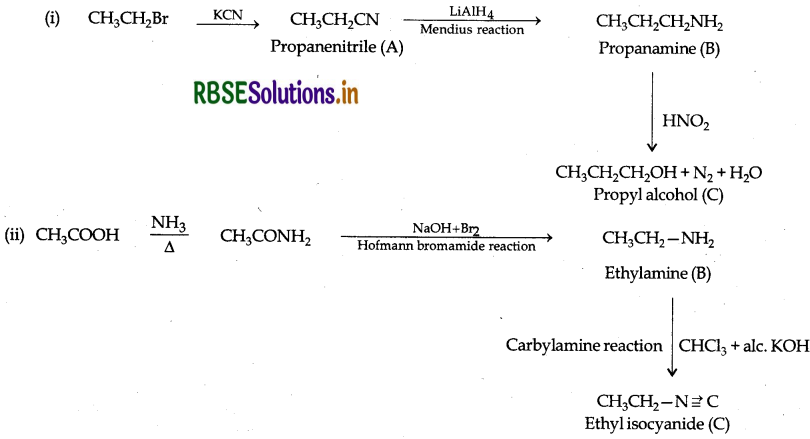

Question 30.
Complete the following reactions:
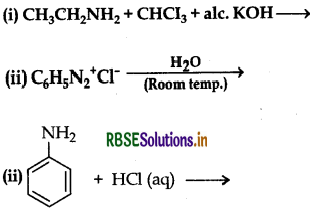
Answer:
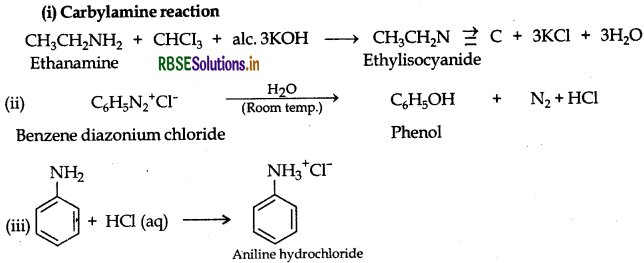
Question 31.
Write the main products of the following reactions:
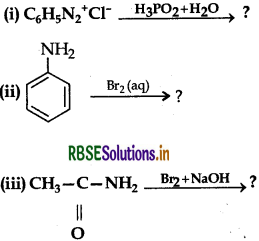
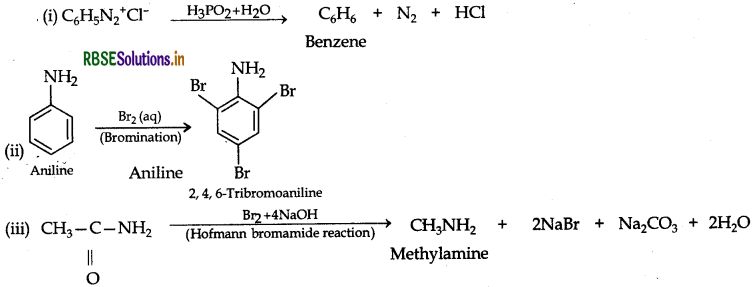
Answer:
Question 32.
Give the structures of A, B and C in the following reactions:
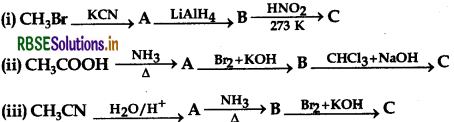
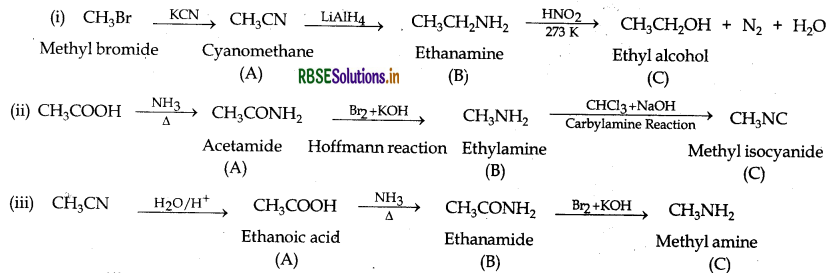
Answer:
Question 33.
How will you convert the following:
(i) Nitrobenzene into aniline
(ii) Ethanoic acid into methanamine
(iii) Aniline into N-phenylethanamide (Write the chemical equations fnvolved).
Answer:
(i) Nitrobenzene into aniline
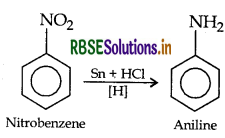
(ii) Ethanoic acid into methanamine
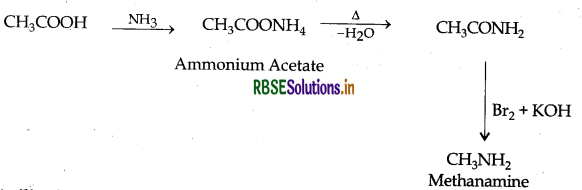
(iii) Aniline into N-phenylethanamide
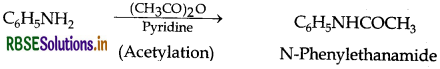
Question 34.
Account for the following:
(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).
(ii) Aniline does not undergo Friedel - Crafts reaction.
(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Answer:
(i) Due to presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H- bonding while tertiary amines due to the absence of a H-atom on the N-atom, do not undergo H bonding. As a result, primary amines have higher boiling points than 3° amines.
(ii) Aniline being a Lewis base reacts with Lewis acid AlCl3 to form a salt.
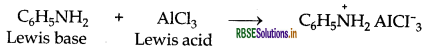
As a result, N of aniline acquires positive charge and hence it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Freidel Craft reaction.
(iii) Due to more steric hindrance in (CH3)3N it is less basic than (CH2)2NH.

Question 35.
Account for the following:
(i) Aniline does not give Friedel-Crafts reaction.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) pK of methylamine is less than that of aniline.
Answer:
(i) Aniline being a Lewis base reacts with Lewis acid AICl3 to form a salt.
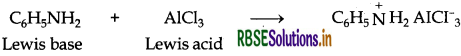
As a result, N of aniline acquires positive charge and hence it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Freidel Craft reaction.
(ii) Ethylamine is soluble in water due to its capability to form H-bonds with water while aniline is insoluble in water due to larger hydrocarbon part which tends to retard the formation of H-bonds.
(iii) In aniline due to resonance lone pair of electron of nitrogen atom is delocalised due to which it is weaker base than methyl amine. Hence Aniline has high pKb molecule i.e., methylamine has less pK molecule.
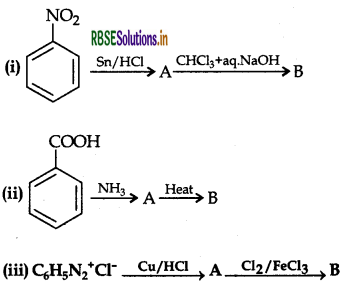
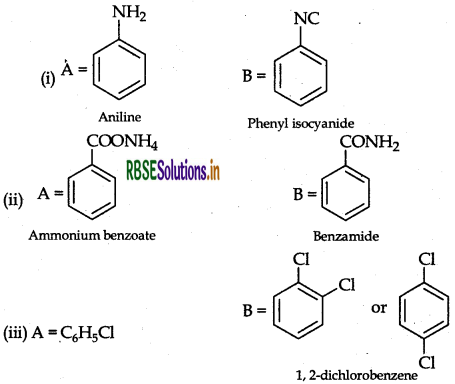
Question 36.
An aromatic compound 'A' on treatment with aqueous ammonia and heating forms compound 'B' which on heating with Br2 and KOH forms a compound 'C' of molecular formula CHN. Write the structures and IUPAC names of compounds A, B and C.
Answer:
The data shows that C6H7N may be C6H5NH2 i.e. Aniline. Since it is obtained by heating with Br2 and KOH (Hoffmann bromamide reaction), then the compound 'B' is Benzamide C6H5CONH2 which is in turn obtained by reaction with aqueous ammonia then the compound 'A' can be Benzoic acid i.e. C6H5COOH
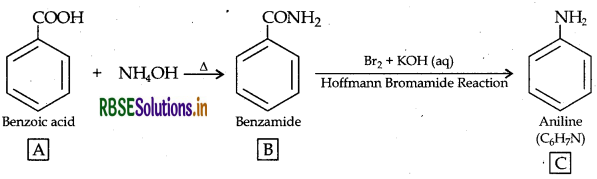
A → Benzoic acid, B → Benzamide, C → Aniline
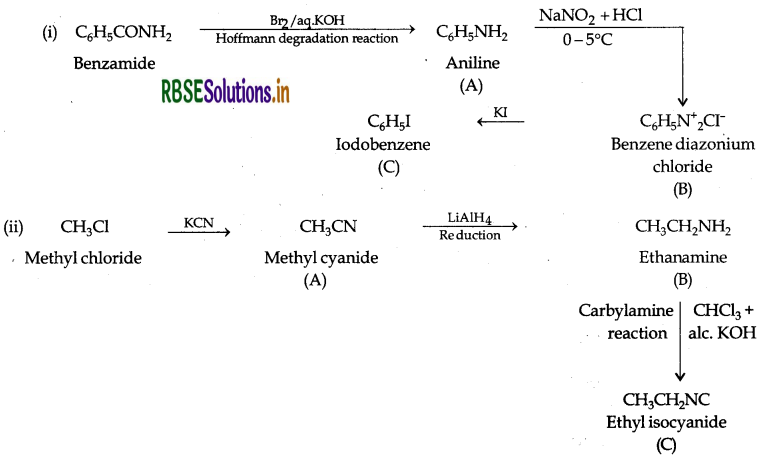
Question 37.
Write the structures of A, B and C in the following:
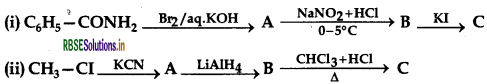
Answer:
Question 38.
Give reasons for the following:
(i) Aniline does not undergo Friedal-Crafts reaction.
(ii) (CH3)2NH is more basic than (CH3)3 N in an aqueous solution.
(iii) Primary amines have higher boiling point than tertiary amines.
Answer:
(i) Aniline being a Lewis base reacts with Lewis acid AlCl3 to form a salt.
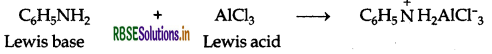
As a result, N of aniline acquires positive charge and hence it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Freidel Crafts reaction.
(ii) In (CH3)N there is maximum steric hindrance and least solvation but in (CH3)2NH the solvation is more and the steric hindrance is less than in (CH3)3NH; although + I effect is less, since there are two methyl groups; di-methyl amine is still a stronger base than tri-methyl.
(iii) Tertiary amines have no hydrogen atoms bonded to the nitrogen atom and therefore are not hydrogen bond donors. Thus, tertiary amines cannot form intermolecular hydrogen bonds. As a result, they have lower boiling points than primary and secondary amines of comparable molecular weight.
Question 39.
Write major product(s) in the following reactions:
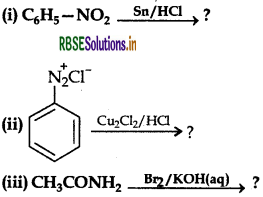
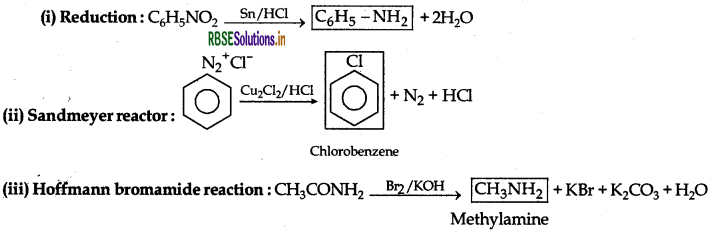
Answer:
Question 40.
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reaction involved.
Answer:
Benzenesulphonyl chloride (C6H5SO2Cl), which is also known as Hinsberg's reagent, reacts with primary and secondary amines to form sulphonamides and tertiary amine does not react.
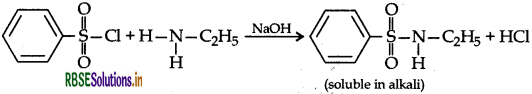
The product will precipitate after addition of HCI.
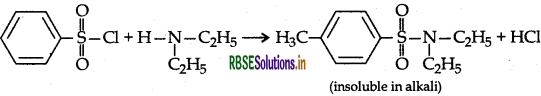
If does not react with tertiary amines.
Question 41.
Write the products A and B in the following:
Answer:
Question 41.
Give reasons:
(i) Acetylation of aniline reduces its activation effect.
(ii) CH3NH2 is more basic than C6H5NH2.
(iii) Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline.
Answer:
(i) Acetylation of aniline reduces its activation effect because acetyl group being electron withdrawing group attracts the lone pair of electrons of the N-atom towards carboxyl group and the lone pair of electrons on N is less available for donation to benzene ring by resonance.
(ii) CH3NH2 is more basic than aniline due to availability of lone pair of electrons for donation while in aniline lone pair of electrons on the nitrogen atom is delocalised over benzene ring and thus unavailable for donation.
(iii) Because of nitration in an acidic medium, aniline gets protonated to give anilinium ion which is m directing.

Question 42.
Give reasons for the following:
(a) Acetylation of aniline reduces its activation effect.
(b) CH3NH2 is more basic than C6H5NH2.
(c) Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline.
Answer:
(a) Acetylation of NH2 group of aniline reduces its activating effect because the lone pair on the nitrogen atom is in conjugation with the carbonyl group in acetylated aniline. As a result, the activating effect of NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance.
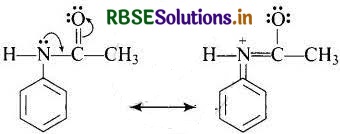
(b) In aniline lone pair of electron on the nitrogen atom is delocalised over the benzene ring due to resonance and thus making it less available for donation. That is why CH3-NH2 is more basic than C6H5 - NH2.
(c) Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ion (which is meta-directing).
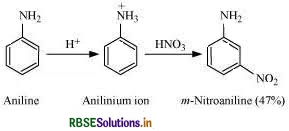
Question 43.
Write the structures of compounds A, B and C in each of the following reactions:
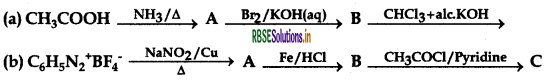
Answer:
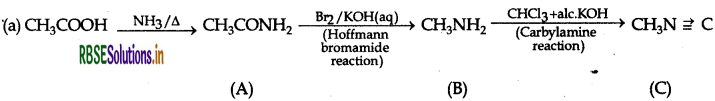
A = Ethanamide
B = Methanamine
C = Methyl isocyanide
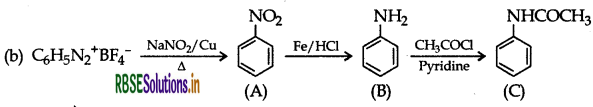
A = Nitrobenze
B = Aniline
C = N- phenylanimine or Acetanide
Question 44.
Illustrate Sandmeyer's reaction with the help of a suitable example.
Answer:
Sandmeyer's reaction: The substitution of diazo group of benzene diazonium chloride by Chloro, Bromo and Cyano group with the help of solution of CuCl dissolved in HCI, CuBr/HBr and CuCN/KCN respectively is known as Sandmeyer's reaction.
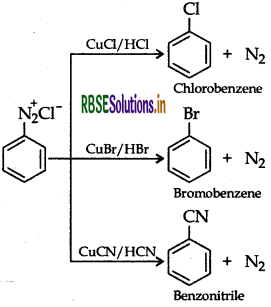
Question 45.
Identify A, B and C in the following reactions:
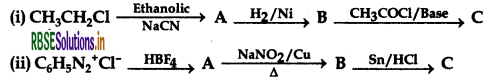
Answer:
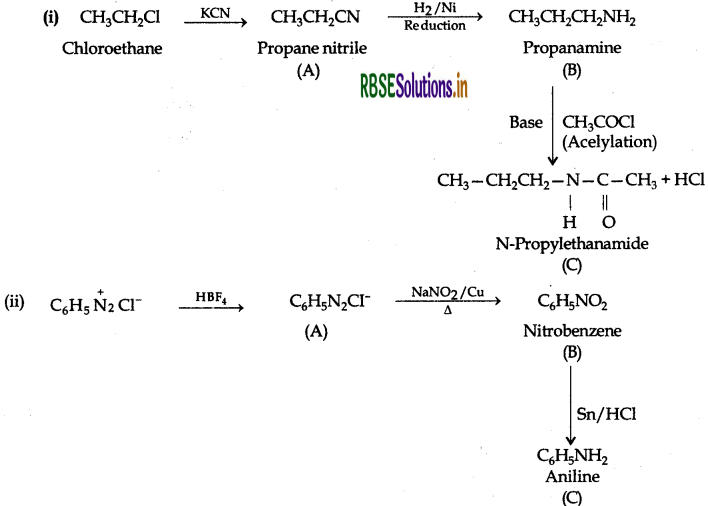
Question 46.
Identify A, B and C in the following reactions:
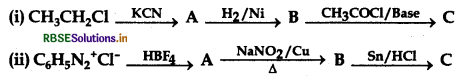
Answer:

Long Answer Type Questions:
Question 1.
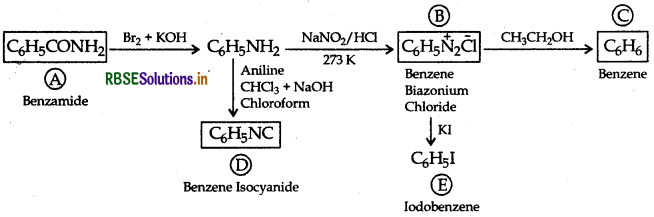
An aromatic compound 'A' of molecular formula C-H-ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions:
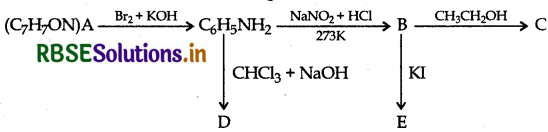
Answer:

Question 2.
(a) Write the structures of main products when aniline reacts with the following reagents:
(i) Br2 water
(ii) HCl
(iii) (CH3CO)2 O/pyridine
(b) Arrange the following in the increasing order of their boiling point:
C2H5NH2 C2H5OH, (CH3)3N
(c) Give a simple chemical test to distinguish between the following pair of compounds:
(CH3)2NH and (CH3)2N
Answer:
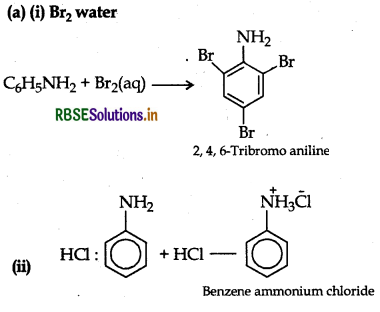
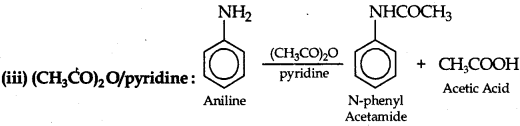
(b) Increasing order of boiling point:
(CH3)3N < C2H5NH2 < С2H5OH
(c) By Hinsberg test, secondary amines or (CH3)2NH shows precipitate formation which is insoluble in KOH. Tertiary amines or (CH3)3N do not react with Hinsberg's reagent (benzene sulphonyl chloride).
Question 3.
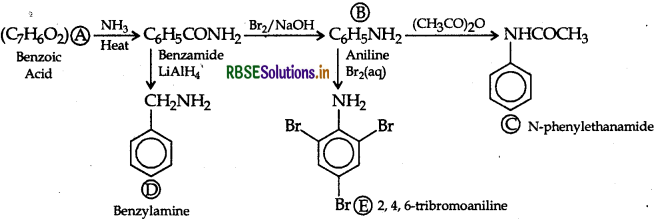
An aromatic compound 'A' of molecular formula C7H6O2 undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions:
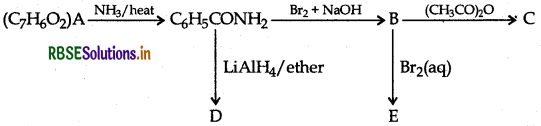
Answer:
Question 4.
(a) Write the structures of main products when benzene diazonium chloride reacts with the following reagents:
(i) H3PO2 + H2O
(ii) CuCN/KCN
(iii) H2O
(b) Arrange the following in the increasing order of their basic character in an aqueous solution:
C2H5NH2 (C2H5)2NH, (C3H5)3N
(c) Give a simple chemical test to distinguish between the following pair of compounds:
C6H5-NH2 and C6H5-NH-CH3
Answer:
(a) The structure of main products when aniline (benzene diazonium chloride) reacts with the following reagents:
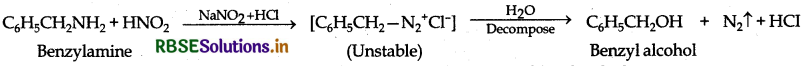
(b) C2H5NH2 < (C3H5)3N < (C2H5)2NH
(c) Aniline and Benzylamine can be distinguished by the Nitrous acid test. Benzylamine reacts with HNO2 to form a diazonium salt which being unstable even at low temperature, decomposes with evolution of N2 gas.
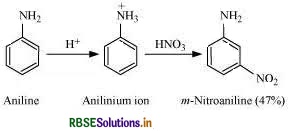
Aniline on the other hand, reacts with HNO2 to form benzene diazonium chloride which is stable at 273 - 278 K and hence does not decompose to evolve N2 gas.
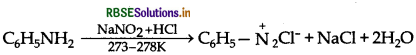
COMPETITION CORNER:
Question 1.
The order of basicity of amine in gaseous phase is:
(a) 3° > 2° > 1° > NH3
(b) 1°> 2° >3° > NH3
(c) 2° > 3° > 1° > NH3
(d) 2° >1° > 3° > NH3
Answer:
(a) 3° > 2° > 1° > NH3

Question 2.
Which of the following can be prepared by Gabriel phthalimide reaction?
(a) Aniline
(b) Benzylamine
(c) o-toluidine
(d) N-methylethanamine
(e) 4-bromoaniline
Answer:
(c) o-toluidine
Question 3.
Amine 'A' when reacts with nitrous acid forms a yellow oily substance. Amine A is:
(a) Triethylamine
(b) Aniline
(c) Dimethylamine
(d) Methylphenylamine
Answer:
(d) Methylphenylamine
Question 4.
Choose the compound which reacts with nitrous acid and release nitrogen:
(a) Nitroethane
(b) Triethylamine
(c) Diethylamine
(d) Ethylamine
Answer:
(d) Ethylamine
Question 5.
The product formed by reaction of aldehyde and primary amine is:
(a) Schiff base
(b) ketone
(c) carboxylic acid
(d) Aromatic acid
Answer:
(a) Schiff base
Question 6.
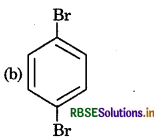
The product of following reaction sequence is are:
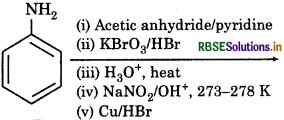
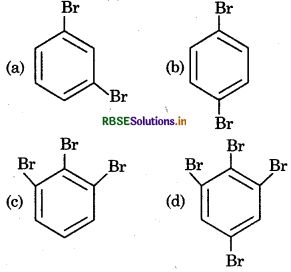
Answer:
Question 7.
An organic compound ' A' on reduction gives compound 'B' which reacts with trichloromethane and caustic potash and forms 'C'. Compound 'C' on catalytic reduction gives N-methylbenzene. Compound 'A ' is :
(a) Nitrobenzene
(b) Methanamine
(c) Nitromethane
(d) Benzenamine
Answer:
(a) Nitrobenzene

Question 8.
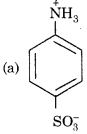
Which of the following is zwitter ion?
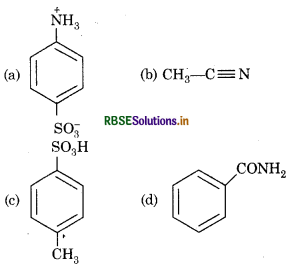
Answer:
Question 9.
The reaction 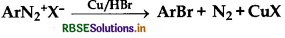
is termed as:
(a) Gattermann Koch reaction
(b) Gattermann reaction
(c) Sandmeyer's reaction
(d) Savazier-Santes reaction
Answer:
(b) Gattermann reaction
Question 10.
The correct statement for basicity of arylamines is:
(a) Generally arylamines are less basic than alkylamines because lone pair of electrons of nitrogen becomes delocalized with π -electrons of aromatic ring.
(b) Generally arylamines are more basic than alkylamines because lone pair of electrons of nitrogen do not delocalized with π -electrons of aromatic ring.
(c) Due to aryl group, arylamines are generally more basic than alkylamine.
(d) Generally arylamine is more basic than alkylamine as nitrogen atom is sp-hybridisied in arylamines.
Answer:
(a) Generally arylamines are less basic than alkylamines because lone pair of electrons of nitrogen becomes delocalized with π -electrons of aromatic ring.
Question 11.
A given compound 'A' having nitrogen, gives unstable compound 'B' on reaction with HCl and then HNO2. 'B' reacts with phenol and forms a beautiful coloured compound 'C' whose molecular formula is C12H10N2O. The structure of compound 'A' is:
Answer:
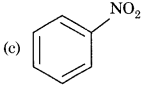

Question 12.
Which of the following nitro compound does not react with nitrous acid?
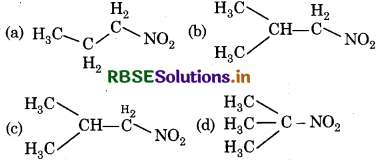
Answer:
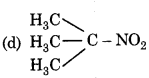
Question 13.
Primary, secondary and tertiary amines can be differentiated by:
(a) Schiff test
(b) Fehling's test
(c) Tollen's test
(d) Hinsberg's test
Answer:
(d) Hinsberg's test
Question 14.
Possible number of structural isomers of molecular formula C3H9N is:
(a) 4
(b) 5
(c) 2
(d) 3
Answer:
(a) 4
Question 15.
In the given sequence of reaction, 2-bromopropane
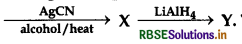
The IUPAC name of product y is:
(a) Butane-2-amine:
(b) N-methyl propanamine
(c) N-methyl propane-2-amine
(d) N-isopropyl methanamine
Answer:
(c) N-methyl propane-2-amine
Question 16.
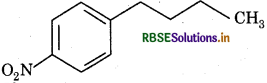
Choose the correct method from the following options for synthesis of compound shown above:
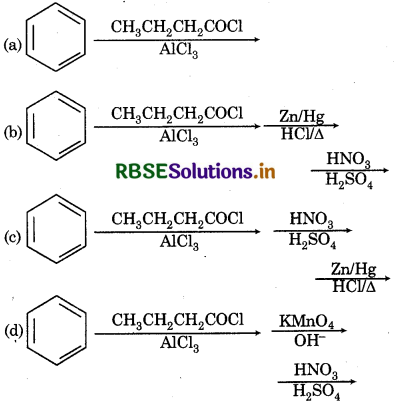
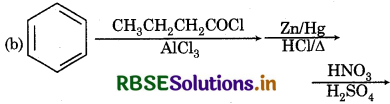
Answer:

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम