RBSE Class 12 Chemistry Important Questions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 12 Aldehydes, Ketones and Carboxylic Acids Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 12 Important Questions Aldehydes, Ketones and Carboxylic Acids
Question 1.
Which compound produces acetaldehyde on heating with aqueous potassium hydroxide:
(a) CH3COCl
(b) CH3CH2Cl
(c) CH2ClCH2Cl
(d) CH3CHCl2
Answer:
(d) CH3CHCl2
Question 2.
General formula of aliphatic aldehyde is:
(a) CnH2n + 2O
(b) CnH2nO
(c) CnH2n-2O
(d) CH2n-1O
Answer:
(b) CnH2nO
Question 3.
Whose aqueous solution is formalin?
(a) Formic acid
(b) Formaldehyde
(c) Fluorine
(d) Furfural
Answer:
(b) Formaldehyde

Question 4.
Which of the following can differentiate between aldehyde and ketone:
(a) Fehling solution
(b) Hydrazine
(c) Pyrogallol
(d) Grignard Reagent
Answer:
(a) Fehling solution
Question 5.
Formaldehyde on heating with KOH gives:
(a) Acetylene
(b) Methane
(c) Methanol
(d) Ethyl Formate
Answer:
(c) Methanol
Question 6.
Aldehyde on oxidation:
(a) Acid
(b) Ether
(c) Seleniumdioxide
(d) Alcohol
Answer:
(a) Acid
Question 7.
Dry calcium acetate on heating gives:
(a) acetic acid
(b) acetaldehyde
(c) acetone
(d) ethane
Answer:
(c) acetone
Question 8.
Formaldehyde on reacting with ammonia gives:
(a) Paraformaldehyde
(b) Urotropin
(c) Formose
(d) Bakelite
Answer:
(b) Urotropin
Question 9.
The compound which gives iodoform test:
(a) Pentane-1-one
(b) Pentane-2-one
(c) Pentane-3-one
(d) Pentanal
Answer:
(b) Pentane-2-one
Question 10.
Which of the following does not give Cannizzaro reaction:
(a) trimethyl acetaldehyde
(b) benzaldehyde
(c) acetaldehyde
(d) formaldehyde
Answer:
(c) acetaldehyde
Question 11.
Which of the following is chloral:
(a) CCl3CH3
(b) Cl2CHCOOH
(c) CCl3CHO
(d) Cl3CCOOH
Answer:
(c) CCl3CHO
Question 12.
Which of the following does not have -OH group:
(a) alcohol
(b) phenol
(c) aldehyde
(d) carboxylic acid
Answer:
(c) aldehyde
Question 13.
Benedict's solution has:
(a) Ag+
(b) La+
(c) Cu2+
(d) Ba2+
Answer:
(c) Cu2+
Question 14.
From which formaldehyde reacts and form bakelite:
(a) Phenol
(b) Chlorobenzene
(c) Nitrobenzene
(d) Aniline
Answer:
(a) Phenol
Question 15.
At room temperature formaldehyde is:
(a) gas
(b) liquid
(c) solid
(d) none of these
Answer:
(a) gas
Very Short Answer Type Questions:
Question 1.
Write the structure of 3-oxopentanal.
Answer:
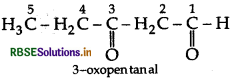
Question 2.
Write the structural formula of 1-phenylpentan-1-one.
Answer:1
-Phenylpentan-1-one
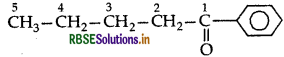
Question 3.
Draw the structural formula of 1-phenyl propan- 1-one molecule.
Answer:1
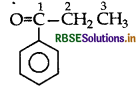
Question4.
What is Tollen's reagent? Write one usefulness of this reagent.
Answer:
Ammonial silver nitrate solution is called Tollen's reagent.
Uses: It is used to test aldehydes. Both aliphatic and aromatic aldehydes reduce Tollen's reagent to shining silver mirror. It is also used to distinguish aldehydes from ketones.
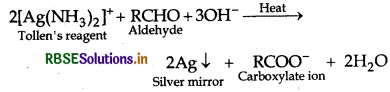
Question 5.
Draw the structure of 3-methylbutanal.
Answer:
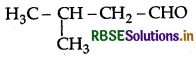
Question 6.
Draw the structure of 4-chloropentan-2-one.
Answer:
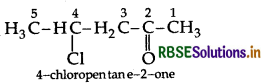

Question 7.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions ethanal, propanal, propanone, butanone.
Answer:
Butanone < Propanone < Propanal< Ethanal
Question 8.
Write the IUPAC name of the following:

Answer:
IUPAC name: Pent-2-enal
Question 9.
Write the IUPAC name of Ph-CH=CH-CHO.
Answer:
IUPAC name: 3-phenylprop-2-enal.
Question 10.
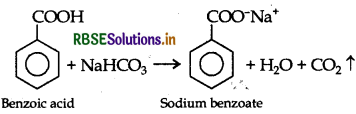
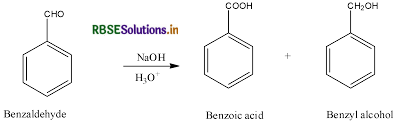
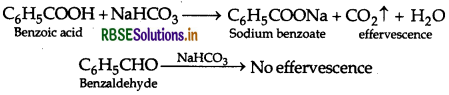
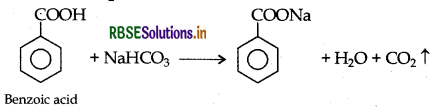
Give a chemical test to distinguish between Benzoic acid and Phenol.
Answer:
Benzoic acid forms a brisk effervescence with NaHCO3 solution but phenol does not respond to this test.
Question 11.
Give a chemical test to distinguish between Ethanal and Propanal.
Answer:
Ethanal on heating with 12 in NaOH gives a yellow ppt of iodoform but propanal does not respond to this test.
Question 12.
Give a chemical test to distinguish between Propanal and Propanone.
Answer:
Propanone on reacting with 12 and NaOH gives a yellow ppt of iodoform but propanal does not respond to this test.
Question 13.
Formaldehyde does not take part in Aldol condensation. Why?
Answer:
Formaldehyde does not contain a-hydrogen atom. Therefore it does not take part in aldol condensation.
Question 14.
Write the IUPAC name of the following:
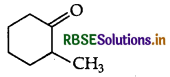
Answer:
IUPAC name 2-methylcyclohexanone
Question 15.
Aldehydes and Ketones have lower boiling points than corresponding alcohols. Why?
Answer:
It is due to weak molecular association in aldehydes and ketones arising out of the dipole dipole interactions.

Question 16.
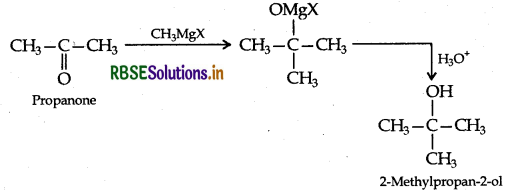
Give the structure and IUPAC name of the product formed when propanone is reacted with methylmagnesium bromide followed by hydrolysis.
Answer:
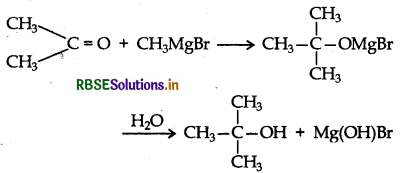
IUPAC name: 2-methylpropan-2-ol.
Question 17.
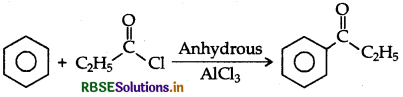
Write the structure of the product formed in the following reaction:
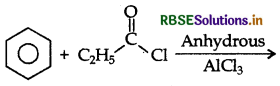
Answer:
Question 18.
Write the structure of 3-methylbutanal.
Answer:
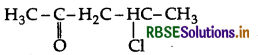
Question 19.
Write the structure of 4-chloropentan-2-one.
Answer:
Question 20.
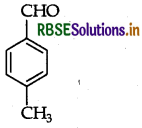
Write the structure of p-Methylben-zaldehyde molecule.
Answer:
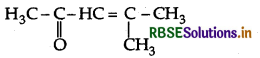
Question 21.
Rearrange the following compounds in the increasing order of their boiling points:
CH3 –CHO, CH3 –CH2– OH, CH3 – CH2 - CH3
Answer:
CH3CH2CH3 < CH3CHO < CH3CH2OH
Question 22.
Ethanal is soluble in water. Why?
Answer:
Ethanal is soluble in water due to H-bonding between the polar carbonyl group and water molecules.
Question 23.
Draw the structure of the compound named 4-methylpent-3-en-2-one.
Answer:
4-Methylpent-3-en-2-one
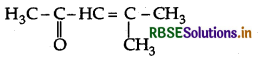
Question 24.
Carboxylic acids do not give characteristic reactions of carbonyl group. Explain why?
Answer:
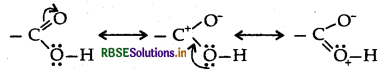
The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure.
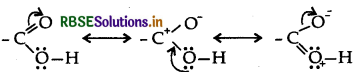
Question 25.
Give a chemical test to distinguish between benzoic acid and phenol.
Answer:
On addition of NaHCO3 to both solutions carbon dioxide gas is evolved with benzoic acid while phenol does not form CO2
Question 26.
Write IUPAC name of the following.
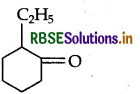
Answer:
IUPAC name: 2-Ethyl cyclohexanone.

Question 27.
Write the IUPAC name of the compound.
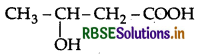
Answer:
IUPAC name: 3-Hydroxybutanoic acid
Question 28.
Write the IUPAC name of the compound.
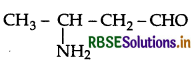
Answer:
IUPAC name: 3-Aminobutanal
Question 29.
Write the IUPAC name of the compound
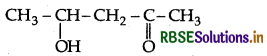
Answer:
IUPAC name: 4-Hydroxypontan-2-one
Question 30.
Write the structure of p-methylben-zaldehyde.
Answer:
p-methylbenzaldehyde
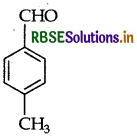
Question 31.
Write the structure of 4-chloropentan-2-one.
Answer:
4-Chloropentan-2-one:
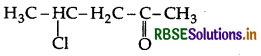
Question 32.
Write the structure of 2-hydroxybenzoic acid.
Answer:
2-hydroxybenzoic acid:
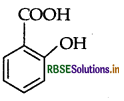
Question 33.
Write the IUPAC name of the following compound:
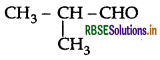
Answer:
IUPAC name: 2-methylpropanal
Question 34.
Give a test to distinguish between propan-2- one and pentan-3-one.
Answer:
Propan-2-one and pentan-3-one can be distinguished by lodoform test.
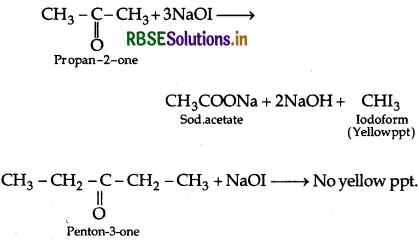
Question 35.
Draw the structure of 3-methylpentanal.
Answer:
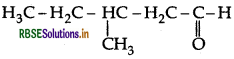
Question 36.
Write the IUPAC name of the following.
CH3 – CH2 – CHO
Answer:
IUPAC name: Propan-1-al
Question 37.
Draw the structure of 2-methylbutanal.
Answer:
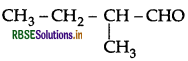
Question 38.
What type of aldehydes undergo Cannizzaro reaction?
Answer:
Aldehydes which do not contain a-hydrogen atom undergo Cannizzaro reaction e.g Formaldehyde (HCHO) and Benzaldehyde (C6H5CHO).

Question 39.
What type of aldehydes undergo Aldol condensation?
Answer:
Aldehydes with a hydrogen atom undergo Aldol condensation.
Question 40.
Arrange the following compounds in the increasing order of their property indicated:
Propanol. Propane, Propanal (boiling point)
Answer:
Propanol > Propanal > Propane (Boiling point)
Short Answer Type Question:
Question 1.
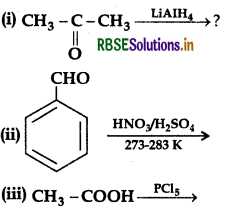
Name the reagents used in the following reactions:
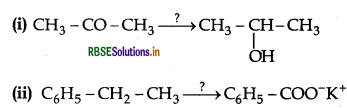
Answer:
(i) LIAIH4 (Lithium Aluminium hydride)
(ii) KMnO4 (Alkaline Potassium permanganate)
Question 2.
Write the reagents required in the following reactions:
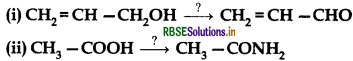
Answer:
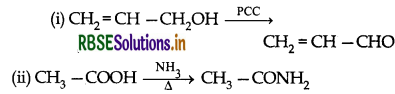
Question 3.
Arrange the following compounds in increasing order of their property as indicated:
(i) CH3COCH, C6H5COCH3, CH3CHO
(reactivity towards nucleophilic addition)
(ii) Cl - CH2 - COOH, F - CH2 - COOH, CH3 - COOH (acidic character)
Answer:
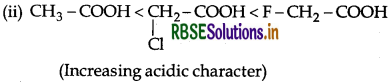
Question 4.
Write the equations involved in the following reactions:
(i) Wolff-Kishner reduction
(ii) Etard reaction
Answer:
(i) Wolff-Kishner reduction
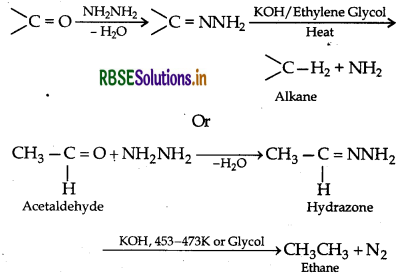
(ii) Etard reaction
Question 5.
Write the reactions involved in the following:
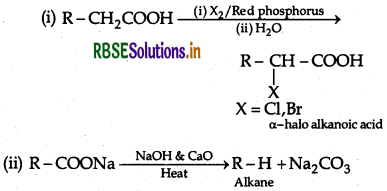
(i) Hell-Volhard Zelinsky reaction
(ii) Decarboxylation reaction
Answer:
Question 6.
Write the reactions involved in the following reactions:
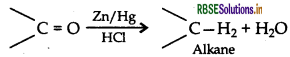
(i) Clemmensen reduction
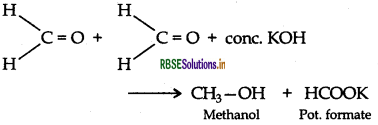
(ii) Cannizzaro reaction
Answer;
(i) Clemmensen reduction: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid.
(ii) Cannizzaro reaction: Aldehydes, which do not have an a hydrogen atom undergo self oxidation and reduction on treatment with conc. alkali and produce alcohol and carboxylic acid salt.
Question 7.
Predict the products of the following reactions:
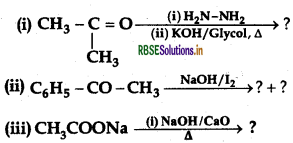
Answer:
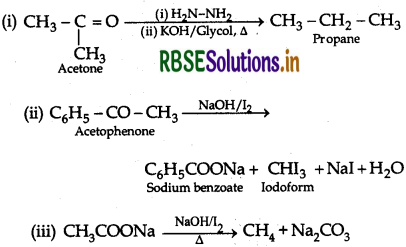
Question 8.
Predict the products of the following reactions:
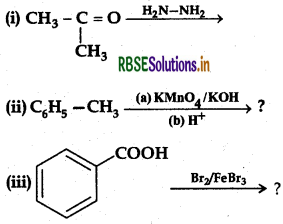
Answer:
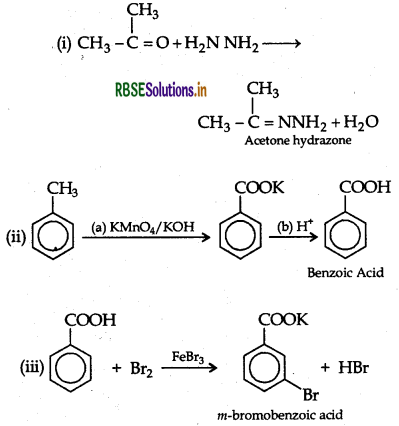

Question 9.
Write structures of compounds A, B and C in cach of the following reactions:

Answer:
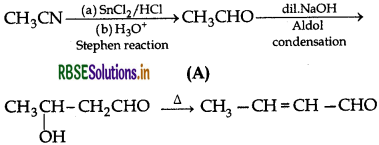
Question 10.
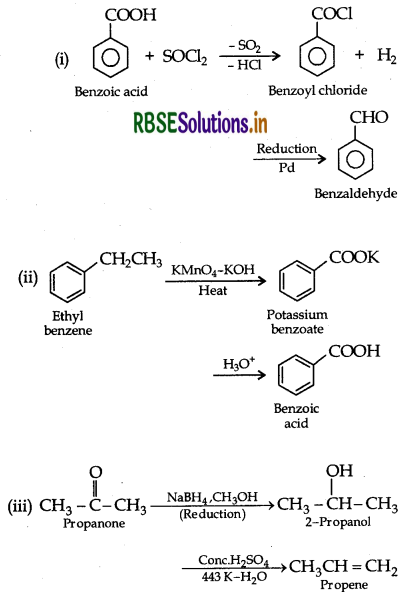
Do the following conversions in not more than two steps:
(i) Benzoic acid to benzaldehyde
(ii) Ethyl benzene to Benzoic acid
(iii) Propanone to Propene
Answer:
Long Answer Type Questions:
Question 1.
(a) How are the following obtained?
(i) Benzoic acid from ethyl benzene.
(ii) Benzaldehyde from toluene.
(b) Complete each synthesis by giving the missing material, reagent or products:
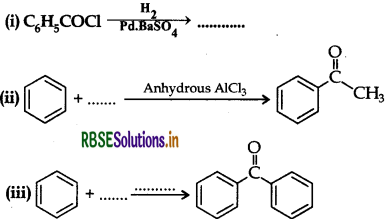
Answer:
(a) (i) Benzoic acid from ethyl benzene:
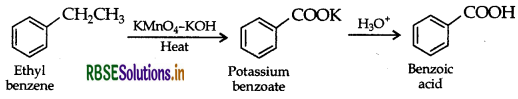
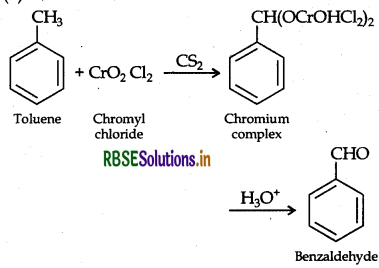
(ii) Benzaldehyde from toluene: It can be obtained by Etard reaction.
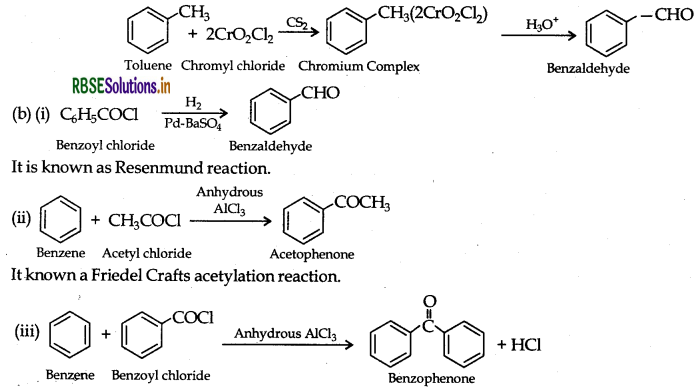
It is also known as Friedel Crafts acetylation reaction.
Question 2.
(a) Write chemical equations to illustrate the following name reactions:
(i) Cannizzaro reaction
(ii) Hell-Volhard-Zelinsky reaction
(b) Give chemical tests to distinguish between the following pairs of compounds:
(i) Propanal and Propanone
(iii) Phenol and Benzoic acid
Answer:
(a) (i) Cannizzaro reaction is a chemical reaction named after Stanislao Cannizzaro that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield a carboxylic acid and a primary alcohol.
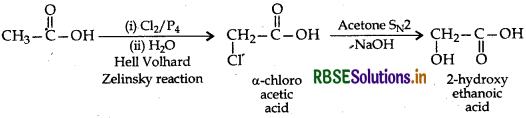
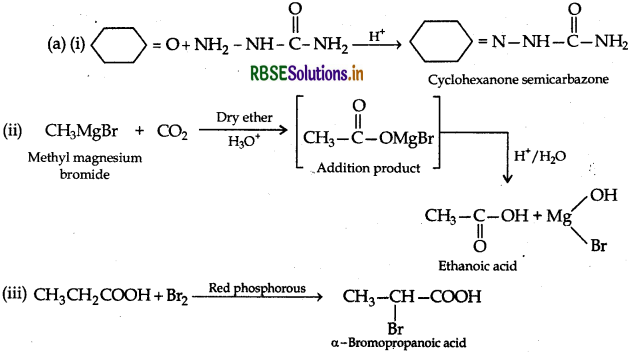
(ii) Hell-Volhard-Zelinsky reaction: Carboxylic acid reacts with chlorine or bromine in presence of small quantities of red phosphorus to give exclusively a-chloro or a-bromo acids.
Example:
(b) (i) Propanal and Propanone: Propanal gives positive test with Fehling solution in which a red ppt. of cuprous oxide is obtained while propanone does not respond to test.
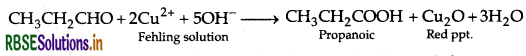
(ii) Acetophenone and Benzophenone: They can be distinguished by iodoform test which is given by only acetophenone with the formation of yellow ppt. while benzophenone does not respond to iodoform test.
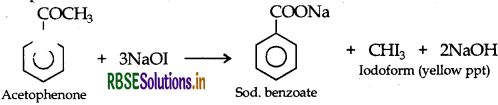
(iii) Phenol and Benzoic acid : On addition of NaHCO3 to both solutions carbon dioxide gas is evolved with benzoic acid while phenol does not form CO2.
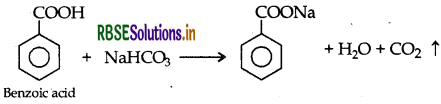
Question 3.
(a) How will you bring about the following conversions:
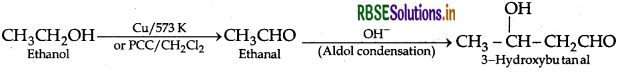
(i) Ethanol to 3-hydroxybutanal
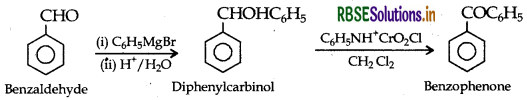
(ii) Benzaldehyde to Benzophenone
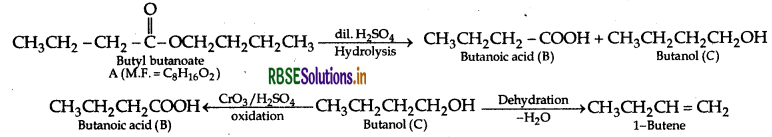
(b) An organic compound A has the molecular formula C6H6O2. It gets hydrolysed with dilute sulphuric acid and gives a carboxylic acid B and an alcohol C. Oxidation of C with chromic acid also produces, B. C on dehydration reaction gives but-1-ene. Write equations for the reactions involved.
Answer:
(a) (i) Ethanol to 3-hydroxybutanal:
(ii) Benzaldehyde to Benzophenone
(b) The compound A with molecular formula is Butyl butanoate.
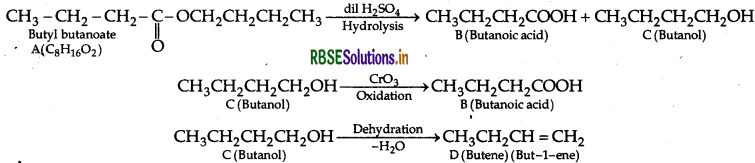
Question 4.
(a) Give chemical tests to distinguish between compounds in the following pairs of substances:
(i) Ethanol and Propanal
(ii) Benzoic acid and Ethyl benzoate
(b) An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen's reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the structure of the compound.
Answer:
(a) (i) Ethanol and Propanal:
Ethanol: Ethanol does not form silver mirror with Tollen's reagent.
Propanal: Propanal being an aldehyde forms silver mirror with Tollen's reagent (Silver mirror Test).
(ii) Benzoic acid and Ethyl benzoate :
By lodoform test: Ethyl benzoate on boiling with excess of NaOH solution gives ethyl alcohol which on heating with iodine gives yellow ppt of iodoform.
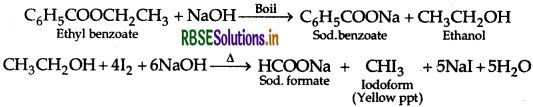
Benzoic acid does not show this test.
(b) % oxygen = 100 - (%C + %H)
= 100 - (69.77 + 116) = 18.6%
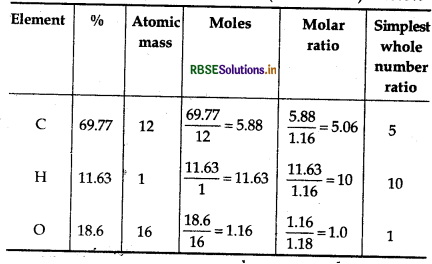
Thus, empricial formula of the compound = C5H10O
∴ Empirical formula mass = 5 x 12 + 10 x 1 + 1 x 16 = 86
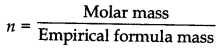
= \(\frac{86}{86}\) = 1
∴ Molecular formula = n x Empirical formula = C5H10O
Step II : To determine the structure of the compound
(i) Since the given compound forms addition compound with NaHSO3 therefore it must be either an aldehyde or methyl ketone.
(ii) As the compound does not reduce Tollen's reagent therefore it can not be an aldehyde and hence it must be a methyl ketone.
(iii) Since the given compound gives positive iodoform test, therefore the given compound is a methyl ketone.
(iv) Since the given compound on vigorous oxidation gives a mixture of ethanoic acid and propanoic acid therefore the methyl ketone is pentan-2-one.
Step III : To explain the reactions involved in the given problem:
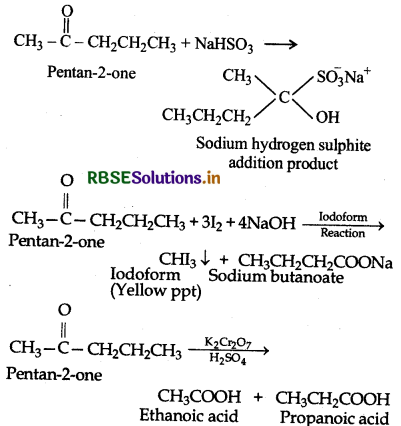

Question 5.
(a) Arrange the following compounds in an increasing order of their indicated property:
(i) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
(ii) CH2CH2CH(Br)COOH, CH2CH(Br) CH2COOH (CH3)2CHOOH,CH3CH2CH2COOH (acid strength)
(b) How would you bring about the following conversions:
(i) Propanone to Propene
(iii) Bromobenzene to 1-phenylethanol.
(ii) Benzoic acid to Benzaldehyde
Answer:
(a) (i) 4-Methoxy benzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3, 4-Dinitrobenzoic acid. The order is due to increasing -I effect.
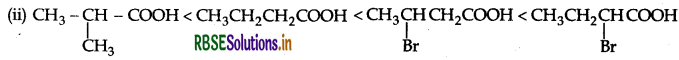
The above order is due to increased-I effect with decrease of distance and decrease of +I effect.
(b) (i) Propanone to Propene
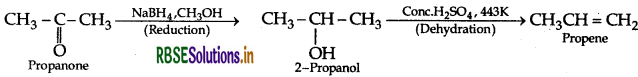
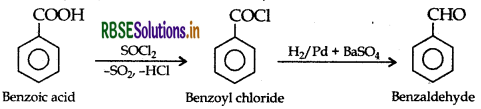
(ii) Benzoic acid to Benzaldehyde (Rosenmund reduction)
(iii) Bromobenzene to 1-phenylethanol
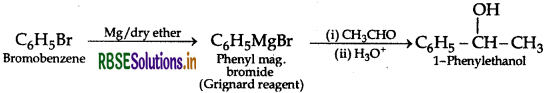
Question 6.
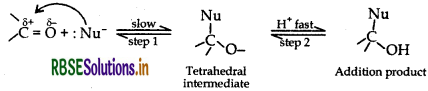
(a) Explain the mechanism at a nucleophilic attack on the carbonyl group of an aldehyde or a ketone.
(b) An organic compound (A) (molecular formula C.H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C)
Answer:
(a) Mechenishm of a nucleophilic attack on the carbonyl group C = O is a polar group in which carbon acquires positive charge and O acquires negative charge due to more electronegativity of oxygen. The Nu attacks on carbon and forms a tetrahedral intermediate and then electrophile attacks on axygen and forms a compound.
(b) Since the organic compound (A) with molecular formula (M.F.) CHO2 upon hydrolysis with dil. H2SO4 gives carboxylic acid (B) and the alcohol (C) therefore it must be an ester. Further since oxidation of (C) with chronic acid produces the acid (B), therefore both the carboxylic acid (B) and the alcohol (C) must contain the same number of carbon atoms.
Question 7.
(a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Phenol and Benzoic acid
(b) How will you bring about the following conversions?
(i) Benzoic acid to benzaldehyde
(ii) Ethanal to but-2-enal
(iii) Propanone to propene
Give complete reaction in each case.
Answer:
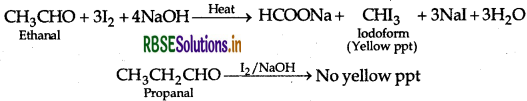
(a) (i) Ethanal and Propanal: Ethanal and propanal can be distinguished by iodoform test. Warm each compound with iodine and sodium hydroxide solution in water. Ethanal gives yellow crystal of iodoform while propanal does not respond to iodoform test.
(ii) Phenol and Benzoic acid : On addition of NaHCO3 to both solutions, carbon dioxide gas is evolved with benzoic acid while phenol does not form CO2.
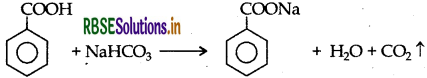
(b) (i) Benzoic acid to benzaldehyde :
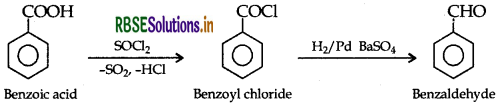
(ii) Ethanal to but-2-enal
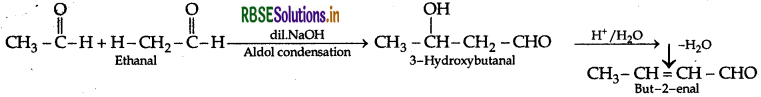
(iii) Propanone to propene
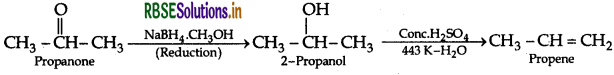
Question 8.
(a) Illustrate the following name reactions giving a chemical equation in each case:
(i) Clemmensen reaction
(ii) Cannizzaro reaction
(b) Describe how the following conversions can be brought about:
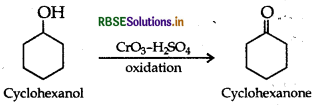
(i) Cyclohexanol to cyclohexan-1-one
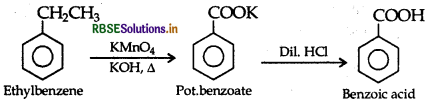
(ii) Ethylbenzene to benzoic acid
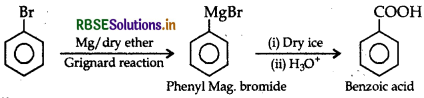
(iii) Bromobenzene to benzoic acid
Answer:
(a) (i) Clemmenson's reduction is most commonly used to convert acyl benzenes to alkyl benzenes. - Benzaldehyde undergoes Clemmenson's reduction in the presence of zinc amalgam and concentrated hydrochloric acid to form toluene.
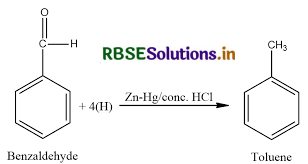
(ii) Cannizzaro reaction is a chemical reaction named after Stanislao Cannizzaro that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield a carboxylic acid and a primary alcohol.

(b) (i) Cyclohexanol to cyclohexan-1-one
(ii) Ethylbenzene to benzoic acid
(iii) Bromobenzene to benzoic acid
Question 9.
(a) Illustrate the following name reactions
(i) Hell-Volhard-Zelinsky reaction
(ii) Wolff-Kishner reduction
(b) How are the following conversions carried out :
(i) Ethylcyanide to ethanoic acid
(ii) Butan-1-ol to butanoic acid
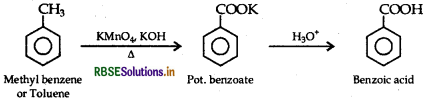
(iii) Methylbenzene to benzoic acid
Write chemical equations for the involved reactions.
Answer:
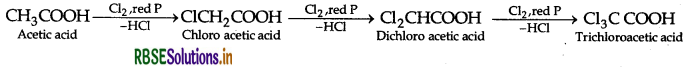
(a) (i) Hell-Volhard-Zelinsky reaction: Carboxylic acid reacts with chlorine or bromine in presence of small quantities of red phosphorus to give exclusively a-chloro or a-bromo acids.
Example:

(ii) Wolff-Kishner reduction: The reduction of aldehydes and ketones to the corresponding hydrocarbons by heating them with hydrazine and KOH or potassium tert-butoxide in a high boiling solvent like ethylene glycol is called Wolff- Kishner reduction.
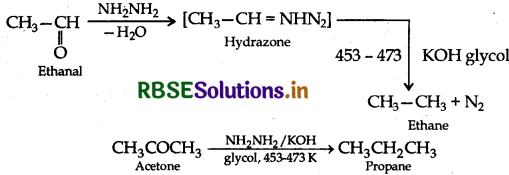
(b) (i) Ethylcyanide to ethanoic acid
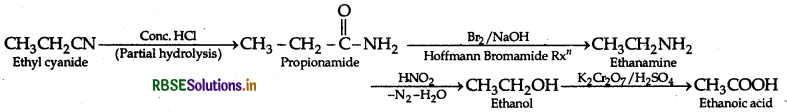
(ii) Butan-1-ol to butanoic acid
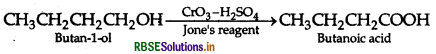
(iii) Methylbenzene to benzoic acid
Question 10.
(a) Illustrate the following name reactions giving suitable example in each case:
(i) Clemmensen reduction
(ii) Hell-Volhard-Zelinsky reaction
(b) How are the following conversions carried out?
(i) Ethylcyanide to ethanoic acid
(ii) Butan-1-ol to butanoic acid
(iii) Benzoic acid to m-bromobenzoic acid.
Answer:
(a) (i) Clemmenson's reduction is most commonly used to convert acyl benzenes to alkyl benzenes. - Benzaldehyde undergoes Clemmenson's reduction in the presence of zinc amalgam and concentrated hydrochloric acid to form toluene.

(ii) Hell-Volhard-Zelinsky reaction: Carboxylic acid reacts with chlorine or bromine in presence of small quantities of red phosphorus to give exclusively a-chloro or a-bromo acids.
Example:

(b) (i) Ethylcyanide to ethanoic acid
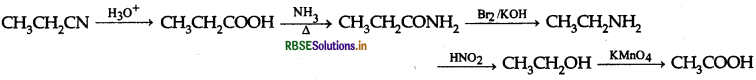
(ii) Butan-1-ol to butanoic acid
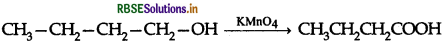
(iii) Benzoic acid to m-bromobenzoic acid
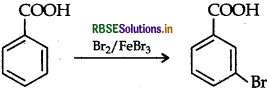
Question 11.
(a) Illustrate the following reactions giving a suitable example for each.
(i) Cross aldol condensation
(ii) Decarboxylation.
(b) Give simple tests to distinguish between the following pairs of compounds :
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
Answer:
(a) (i) Cross aldol condensation: When aldol condensation is carried out between two different aldehydes and or ketones, it is called cross aldol condensation.
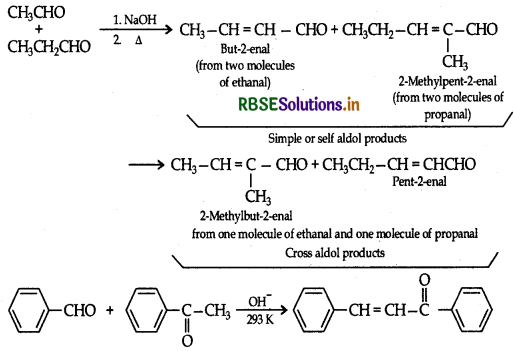
(ii) Decarboxylation : Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with soda lime. The reaction is known as
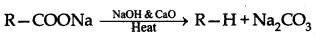
(b) (i) Pentan-2-one and Pentan-3-one
By Iodoform test:
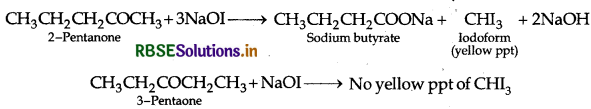
Add I2 and NaOH in both the solutions Pentan-2-one gives yellow coloured precipitate, but pentan-3-one does not.
(ii) Benzaldehyde and Acetophenone lodoform test: Warm each organic compound with I, and NaOH solution. Acetophenone forms yellow precipitate of iodoform. Benzaldehyde does not respond to this test.
(iii) Phenol and Benzoic acid: On addition of NaHCO3 to both with solutions, carbon dioxide gas is evolved benzoic acid while phenol does not form CO2.
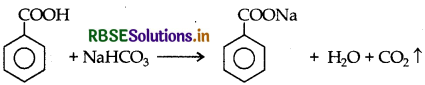

Question 12.
(a) Give a plausible explanation for each one of the following:
(i) There are two -NH2 groups in semicarbazide. However, only one such group is involved in the formation of semicarbazones.
(ii) Cyclohexanone forms cyanohydrin in good yield but 2 4, 6-trimethylcyclohexanone does not.
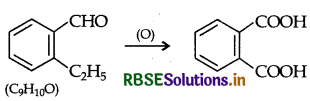
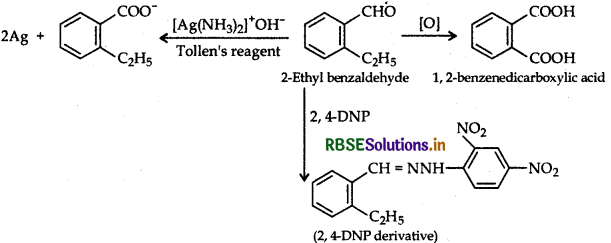
(b) An organic compound with molecular formula C9H1O forms 2, 4, - DNP derivative, reduces Tollens reagent and undergoes Cannizzaro reaction. On vigorous oxidation it gives 1, 2-benzene dicarboxylic acid. Identify the compound.
Answer:
(a) (i) Because one of the -NH, in semicarbazide is involved in the resonance with group.
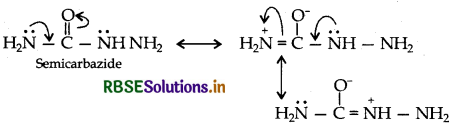
(ii) Due to steric hinderance in 2. 4. 6-trimethylcyclohexanone, it doesn't react with HCN easily.
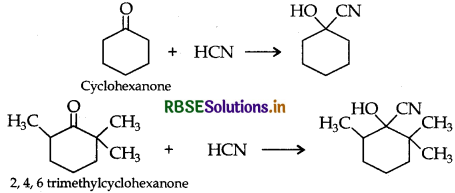
But the yield of the product is very less.
(b) Compound C9H10O, forms 2, 4-DNP derivative, so it contains a carbonyl group. Also it reduces Tollens reagent therefore carbonyl group is an aldehyde group. Since it undergoes Cannizzaro's reaction, aldehyde has no a-hydrogen atom, so compound is C8H9 CHO. On vigorous oxidation, compound gives 1, 2-benzene dicarboxylic acid hence the compound is
Question 13.
(a) Give chemical tests to distinguish between
(i) Phenol and Benzoic acid
(b) Write the structures of the main products of following reactions:
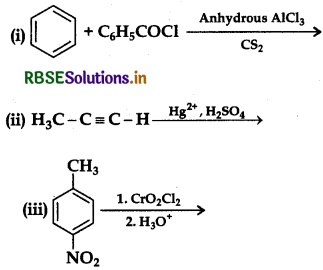
Answer:
(i) Phenol and Benzoic acid : On addition of NaHCO3 to both solutions carbon dioxide gas is evolved with benzoic acid while phenol does not form CO2.

(ii) Acetophenone and Benzophenone: They can be distinguished by iodoform test which is given by only acetophenone with the formation of yellow ppt. while benzophenone does not respond to iodoform test.

Question 14.
(a) Write a suitable chemical equation to complete each of the following transformations:
(i) Butan-1-ol to butanoic acid
(ii) 4-Methylacetophenone to benzene-1, 4-dicarboxylic acid.
(b) An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen's reagent and undergoes Cannizzaro reaction. On vigorous oxidation it gives 1, 2-benzenedicarboxylic acid. Identify the compound.
Answer:
(a) (i) Butan-1-ol to butanoic acid:
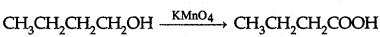
(ii) 4-Methylacetophenone to benzene-1, 4-dicarboxylic acid :
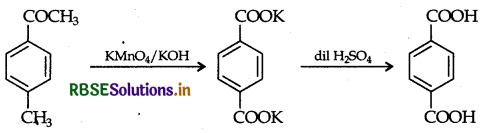
(b) C9H10O, forms 2, 4-DNP derivative, so it contains a carbonyl group. Also it reduces Tollens reagent therefore carbonyl group is an aldehyde group. Since it undergoes Cannizzaro's reaction, aldehyde has no a-hydrogen atom, so compound is C8H9 CHO. On vigorous oxidation, compound gives 1, 2-benzene dicarboxylic acid hence the compound is

Question 15.
(a) Give chemical tests to distinguish between:
(i) Propanol and propanone
(ii) Benzaldehyde and acetophenone.
(b) Arrange the following compounds in an increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) Benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
(iii) CH3CH2CO(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH (acid strength)
Answer:
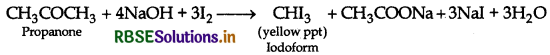
(a) (i) Propanone and Propanol:
It can be done by lodoform test where propanone gives yellow ppt. of iodoform while propanol does not respond to test.
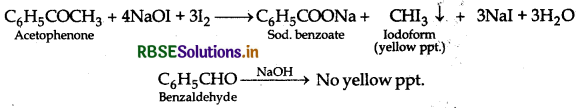
(ii) Benzaldehyde and acetophenone:
By Iodoform test: Acetophenone being a methyl ketone on treatment with I2/NaOH acetophenone undergoes iodoform test to give yellow ppt. of lodoform but benzaldehyde does not.
(b) (i) Methyl tert-butyl ketone< Acetone < Acetaldehyde
(ii) 4-Methoxybenzoic acid < Benzoic acid < 3, 4-Dinitro benzoic acid
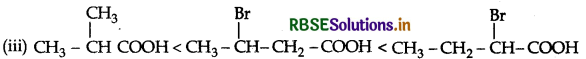
Question 16.
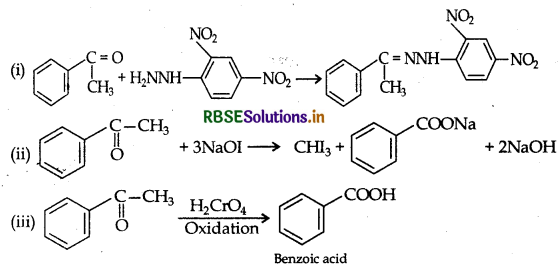
(a) An organic compound A' with molecular formula C8H8O forms an, orange red precipitate with 2, 4-DNP reagent and given yellow precipitate on heating with I2, and NaOH. It neither reduces Tollen's reagent nor Fehling's reagent nor does it decolourizes bromine water or Baeyer's reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid "having molecular formula C7H6O2. Identity the compounds A' and 'B' and explain the reactions involved.
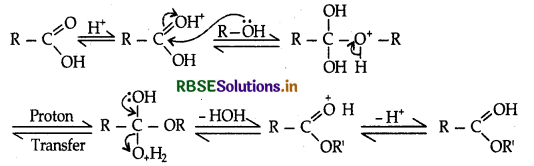
(b) Write the mechanism of esterification of carboxylic acids.
Answer:
(a) (i) Molecular formula: C8H8O.
(ii) Since it gives orange red ppt with 2, 4-DNP reagent. Therefore it must be either aldehyde or a ketone.
(iii) Since, it does not reduce Tollen's reagent nor Fehling's reagent, solution “A must be a ketone. Therefore, it must › be a methyl ketone.
(iv) 'A' on treatment with I2/NaOH gives yellow ppt. Therefore, it must be a methyl ketone 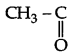
(v) It does not decolorise Br2, water or Baeyer's reagent so the unsaturation of 'A' is due to benzene ring. Therefore the structure of A should be as 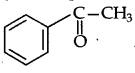
Reactions:
(b) Mechanism of esterification of carboxylic acids :

Question 17.
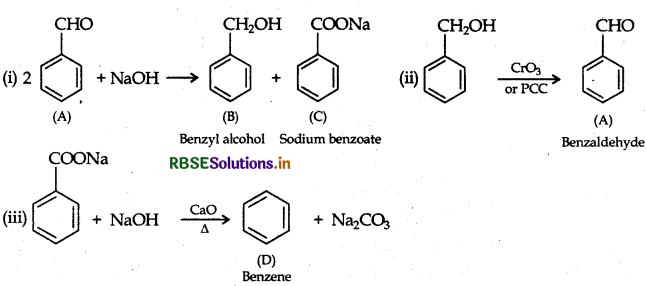
(a) An organic compound A' which has characteristic odour, on treatment with NaOH forms two compounds B' and 'C. Compound 'B' has the molecular formula C7H8O which on oxidation with CrO3, gives back compound ‘A. Compound 'C is the sodium salt of the acid. 'C when heated with soda lime yields an aromatic hydrocarbon D. Deduce the structures of 'A', 'B', 'C' and 'D'.
(b) Give reasons:
(i) Electrophilic substitution in Benzoic acid takes place at meta position.
(ii) Carboxylic acids do not give characteristic reactions of carbonyl group.
Answer:
(a) (A) gives characteristic odour which on treatment with NaOH and forms two compounds B and C
(A) should be benzaldehyde 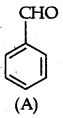
Reactions:
(b) (i) The benzene ring of benzoic acid undergoes electrophilic substitution reaction such as nitration, sulphonation etc. Since the -COOH group in benzene is an electron withdrawing group, therefore it is meta directing group.
(ii) The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure.
Question 18.
(a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Benzene amide and 4-aminobenzoic acid
(ii) Methyl acetate and Ethyl acetate
(b) An organic compound with molecular formula C9H10O forms 2. 4-DNP derivative and reduces Tolien's reagent and undergoes Cannizzaro's reaction. On vigorous oxidation, it gives 1.2 benzenedicarboxylic acid. Identify the compound and write chemical equations for the reactions.
Answer:
(a) (i) NaHCO3, test: p-Aminobenzoic acid is an acid which decomposes NaHCO3, solution to produce effervescence due to evolution of CO2 gas while benzene amide does not give this test.
(ii) Ethyl acetate when boiled with exces of NaOH gives ethyl alcohol and if it is heated with I2, and NaOH solution, it gives yellow precipitate of iodoform. Methyl acetate on hydrolysis with NaOH gives methyl alcohol which does not respond to iodoform test.
(b) (i) Molecular formula of compound: C9H10O
(ii) It forms a 2,4-DNP derivative and reduces Tollen's reagent. It must be an aldehyde.
(iii) Since it undergoes Cannizzaro reaction, therefore CHO group is directly attached to the benzene ring.
(iv) On oxidation it gives 1,2-benzenedicarboxylic acid, therefore it must be an ortho-substituted benzaldehyde, so the structure should be
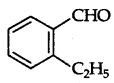
Reaction:
Question 19.
(a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Benzoic acid and Phenol
(ii) Benzaldehyde and Acetophenone
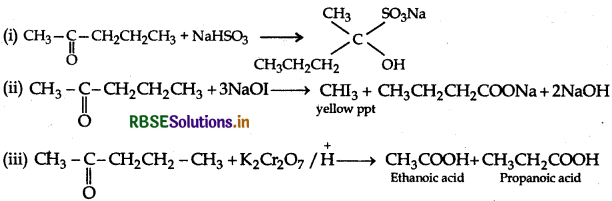
(b) An organic compound with molecular formula C5H10O does not reduce Tollen's reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid, identify the compound and write all chemical equations for the reactions.
Answer:
(a) Phenol and Benzoic acid: On addition of NaHCO3 to both with solutions, carbon dioxide gas is evolved benzoic acid while phenol does not form CO2.

(ii) Benzaldehyde and Acetophenone lodoform test: Warm each organic compound with I, and NaOH solution. Acetophenone forms yellow precipitate of iodoform. Benzaldehyde does not respond to this test.
(b) (i) Molecular formula of compound: C5H12O.
(ii) It gives an addition product with NaHSO3, so it must be either an aldehyde or methyl ketone.
(iii) It does not reduce Tollen's reagent so it is not an aldehyde.
(iv) It gives + ve iodoform test, therefore it is methyl ketone.
(v) On oxidation it gives a mixture of ethanoic acid and propanoic acid. Therefore, possible structure is pentan-2-one.
Reactions:
Question 20.
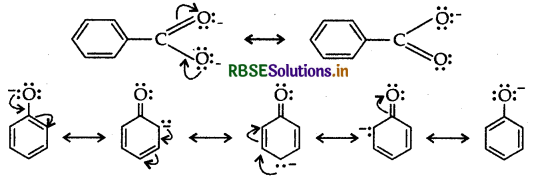
(a) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.
(b) How will you bring about the following converstions?
(i) Propanone to propane
(iii) Ethanal to but-2-enal
(ii) Benzoyl chloride to benzaldehyde
Answer:
(a) Carboxylic acid is a stronger acid than phenol because:
(i) In the resonating structure of phenol and carboxylic acid, the negative charge on the carboxylate ion is delocalised over two oxygen atoms while they are localized on oxygen atom.
(ii) The release of a proton from carboxylic acids is much easier than from phenols.
(b) (i) Propanone to Propane
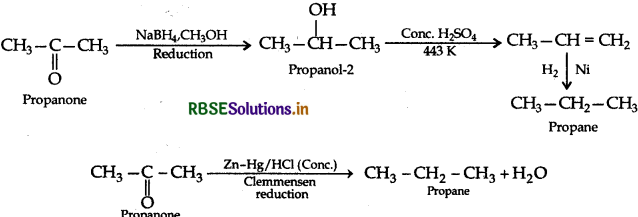
(ii) Benzoyl chloride to Benzaldehyde (Rosenmund reduction)
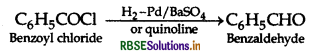
(iii) (i) The benzene ring of benzoic acid undergoes electrophilic substitution reaction such as nitration, sulphonation etc. Since the -COOH group in benzene is an electron withdrawing group, therefore it is meta directing group.
(ii) The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure.

Question 21.
(a) Complete the following reactions:
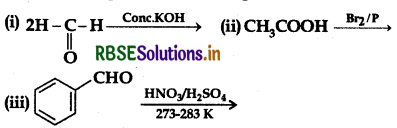
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Benzoic acid and phenol
Answer:
(b) (i) Ethanal and Propanal: Ethanal and propanal can be distinguished by iodoform test. Warm each compound with iodine and sodium hydroxide solution in water. Ethanal gives yellow crystal of iodoform while propanal does not respond to iodoform test.

(ii) Phenol and Benzoic acid: On addition of NaHCO3 to both with solutions, carbon dioxide gas is evolved benzoic acid while phenol does not form CO2.

Question 22.
(a) How will you convert the following:
(i) Propanone to Propan-2-ol
(ii) Ethanal to 2-hydroxy propanoic acid
(iii) Toluene to benzoic acid
(b) Give simple chemical test to distinguish between:
(i) Pentan-2-one and Penton-3-one
(ii) Ethanal and Propanal
Answer:
(a) (i) Propanone to Propan-2-ol

(ii) Ethanal to 2-hydroxy propanoic acid
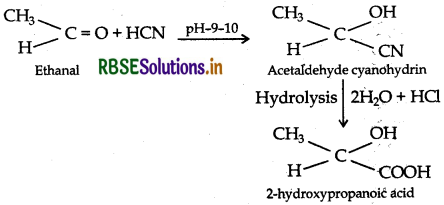
(iii) Toluene to Benzoic acid
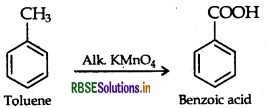
(b) (i) Pentan-2-one and Pentan-3-one By iodoform test:
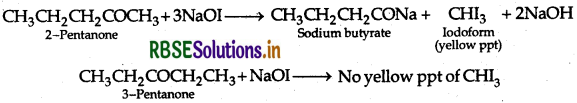
Add I2, and NaOH in both the solutions. Pentan 2-one gives yellow coloured precipitate, but pentan 3-one does not.
(ii) Ethanal and Propanal : Add I, and NaOH in both the solutions. Ethanal gives yellow coloured precipitate but propanal does not.

Question 23.
(a) Write the products of the following reactions:
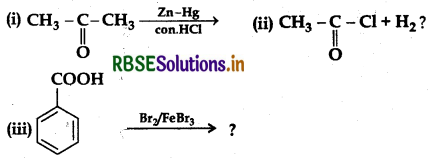
(b) Which acid of each pair shown here would you expect to be stronger?

Answer:
(a) (i) Clemmensen Reduction:
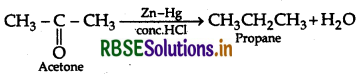
(ii) Rosenmund Reaction:
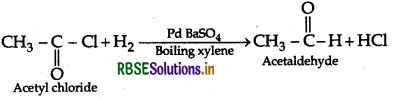
(iii) Bromination
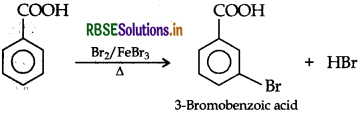
(b) (i) Due to much stronger l-effect of F over CI, the FCH2COO" ion is much more stable than ClCH2COO ̄ ion and hence FCH2COOH is a stronger acid than ClCH2COOH.
(ii) Ethanoic acid is more stronger acid than phenol due to less pk, than that of phenol and the carboxylate ion is much more resonance stabilized than phenoxide ion.
Question 24.
Two moles of organic compound 'A' on treatment with a strong base gives two compounds 'B' and C. Compound B' on dehydrogenation with Cu gives 'A' while acidification of 'C' yields carboxylic acid D with molecular formula of CH2O2. Identify the compounds A, B, C and D and write all chemical reactions involved.

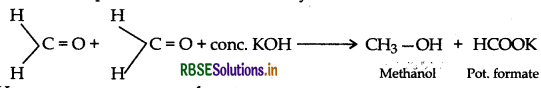
Answer:
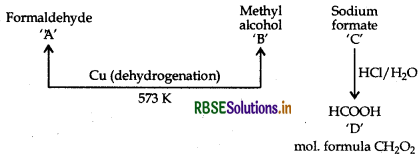
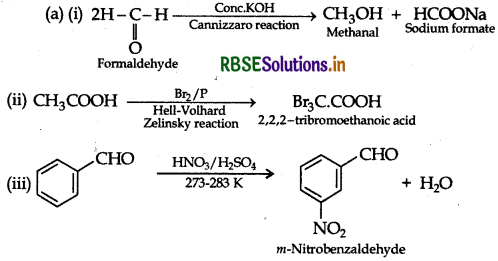
∴ A = Formaldehyde,
B = Methyl alcohol,
C = Sodium formate,
D = Formic acid
Question 25.
(a) How will you carry out the following conversions?
(i) Acetylene to Acetic acid
(ii) Toluene to m-nitrobenzoic acid
(iii) Ethanol to Acetone
(b) Give reasons:
(i) Chloroacetic acid is stronger than acetic acid.
(ii) pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl compounds.
Answer:
(a) (i) Acetylene to Acetic acid
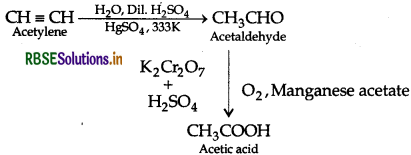
(ii) Toluene to m-nitrobenzoic acid
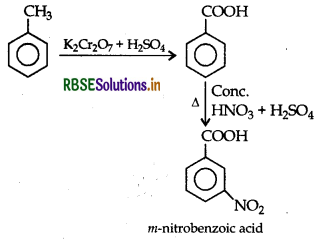
(iii) Ethanol to acetone
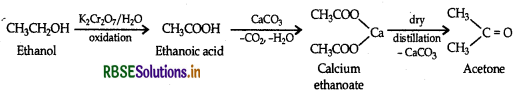
(b) (i) Because - I effect of CI decrease the electron density in the O-H bond therby making the release of a proton easier.

(ii) In strongly acidic medium, ammonia derivatives being will react with acid and will not react with carbonyl compound. In basic medium, OH will attack carbonyl group.
Question 26.
(a) Write the products of the following reactions:
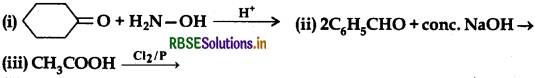
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzaldehyde and Benzoic acid
(ii) Propanal and Propanone
Answer:
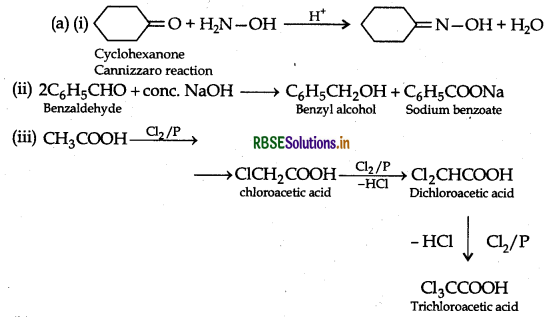
(b) (i) Benzaldehyde and Benzoic acid
(ii) Propanal and Propanone:
Question 27.
(a) Account for the following:
(i) CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
(ii) Carboxylic acid is a stronger acid than phenol.
(b) Write the chemical equations to illustrate the following name reactions:
(i) Wolff-Kishner reduction
(ii) Aldol condensation
(iii) Cannizzaro reaction
Answer:
(a) (i) Aldehydes are more reactive than ketones due to the following two reasons:
1. Due to smaller +1 effect of one alkyl group in aldehydes as compared to larger + effect of two alkyl groups, the magnitude of positive charge in the carbonyl carbon is more in aldehydes than in ketones. As a result, nucleophilic addition reactions occur more readily in aldehydes than in ketones.
2. Due to presence of a H-atom on the carbonyl group, aldehydes can be more easily oxidised than ketones.
(ii) The release of a proton from carboxylic acid is much easier than phenols because carboxylate ion much more resonance stabilized than phenoxide ion.
(b) (i) Wolff-Kishner reduction: The reduction of aldehydes and ketones to the corresponding hydrocarbons by heating them with hydrazine and KOH or potassium tert-butoxide in a high boiling solvent like ethylene glycol is called Wolff- Kishner reduction.

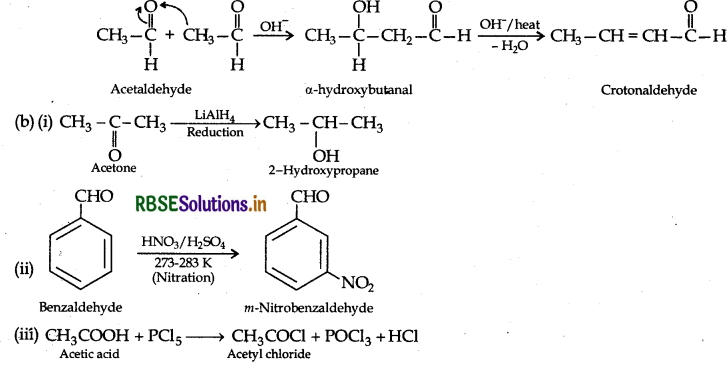
(ii) Aldol condensation : Those aldehydes and ketones with atleast one a-hydrogen atom condense together in presence of dil. alkali eg NaOH etc. to form a B-hydroxy aldehyde or α β-hydroxy ketone respectively which gets dehydrated in the presence of acid upon heating to form a, α β-unsaturated compound.
(iii) Cannizzaro reaction: Aldehydes, which do not have an a-hydrogen atom undergo self oxidation and reduction on treatment with conc. alkali and produce alcohol and carboxylic acid salt.
Question 28.
(a) Write the products formed when CH,CHO reacts with the following reagents:
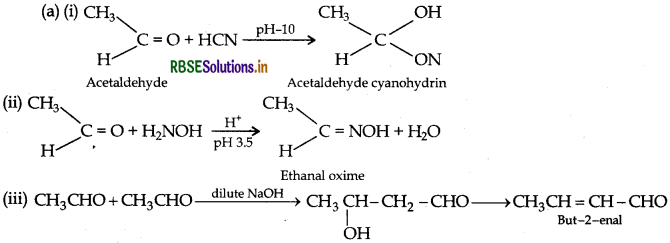
(i) HCN
(iii) CH3CHO in the presence of dilute NaOH
(ii) H2N - OH
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzoic acid and Phenol
(ii) Propanal and Propanone.
Answer:
(b) (i) Benzoic acid and Phenol: On addition of NaHCO3 to both solutions, carbondioxide gas is evolved with benzoic acid while phenol does not form CO2.
(ii) Propanal and Propanone: Propanal gives positive test with Fehling solution in which a red ppt. of cuprous oxide is obtained while propanone does not respond to test.


Question 29.
(a) Account for the following:
(i) Cl – CH2COOH is a stronger acid than CH3COOH.
(ii) Carboxylic acids do not give reactions of carbonyl group.
(b) Write the chemical equations to illustrate the following name reactions:
(i) Rosenmund reduction
(ii) Cannizzaro reaction
(c) Out of CH3CH2-CO-CH2-CH3 and CH3CH2- CH2- CO - CH3 which gives iodoform test?
Answer:
(a) (i) Because-I effect of Cl decreases the electron density in the O-H bond thereby making the release of a proton easier.

(ii) Carboxylic acids do not give the reactions of.carbonyl group as there is no carbonyl group present due to resonance.
(b) (i) Rosenmund reduction

(ii) Cannizzaro reaction: Aldehydes, which do not have an a-hydrogen atom undergo self oxidation and reduction on treatment with conc. alkali and produce alcohol and carboxylic acid salt.

Question 30.
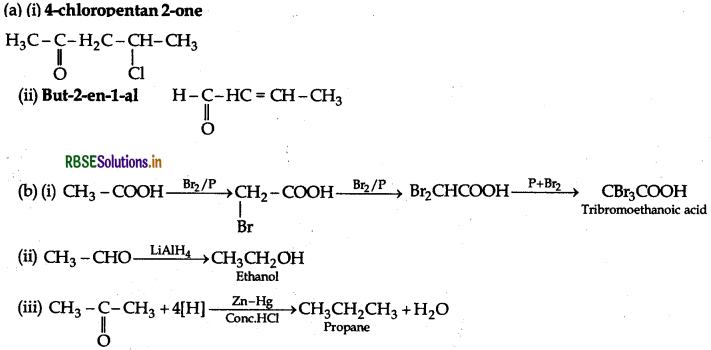
(a) Draw the structures of the following compounds:
(i) 4-chloropentan-2-one
(b) Write the product(s) in the following:
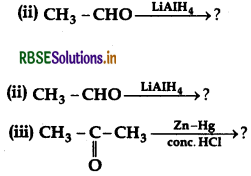
Answer:
Question 31.
(a) Write the IUPAC names of the following compounds:
(i) CH3CO(CH2)4CH3
(ii) Ph.- CH = CH– CHO
(b) Describe the following conversions in not more than two steps:
(i) Ethanol to 3-Hydroxybutanal
(ii) Benzoic acid to m-nitrobenzyl alcohol
(iii) Propanone to Propene
Answer:
(a) (i) CH3CO(CH2)4CH3
IUPAC name: Heptan-2-one
(ii) Ph- CH = CH - CHO
IUPAC name: 3-phenylprop-2-en-1-al
(b) (i) Ethanol to 3-hydroxybutanal:

(ii) Benzoic acid to m-nitrobenzyl alcohol:
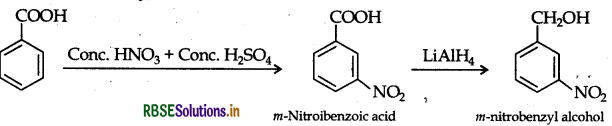
(iii) Propanone to Propene
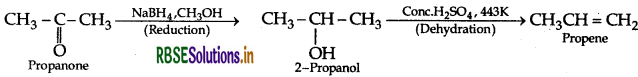
Question 32.
(a) Draw the structures of the following compounds:
(i) 4-Chloropentan-2-one
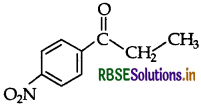
(ii) p-Nitropropiophenone
(b) Give tests to distinguish between the following pairs of compounds :
(i) Ethanal and Propanal
(ii) Phenol and Benzoic acid
(iii) Benzaldehyde and Acetophenone
Answer:
(a) (i) 4-Chloropentan-2-one
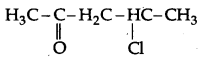
(ii) p-Nitropropiophenone
(b) (i) Ethanal and Propanal: Ethanal and propanal can be distinguished by iodoform test. Warm each compound with iodine and sodium hydroxide solution in water. Ethanal gives yellow crystal of iodoform while propanal does not respond to iodoform test.

(ii) Phenol and Benzoic acid : On addition of NaHCO3 to both solutions, carbon dioxide gas is evolved with benzoic acid while phenol does not form CO2.

(iii) Benzaldehyde and Acetophenone lodoform test: Warm each organic compound with I, and NaOH solution. Acetophenone forms yellow precipitate of iodoform. Benzaldehyde does not respond to this test.

Question 33.
(a) Describe the following giving chemical equations :
(i) Decarboxylation reaction
(ii) Friedel-Crafts reaction
(b) How will you bring about the following conversions?
(i) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(ii) Benzene to m-Nitroacetophenone
Answer:
(a) (i) Decarboxylation reaction: When sodium salt of carboxylic acid is heated with soda lime (NaOH + CaO), then corresponding alkane and CO2 will be evolved.
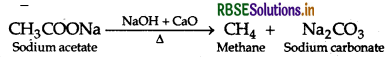
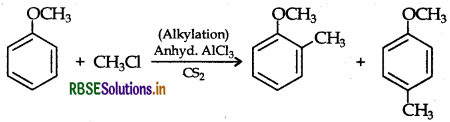
(ii) Friedel-Crafts reaction: The introduction of alkyl or acetyl group in the presence of anhydrous aluminium chloride (AICl3) as catalyst to ortho and para positions of an aromatic compound is called Friedel-Crafts reaction.
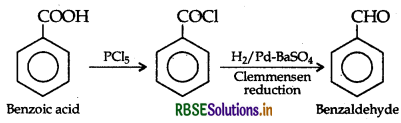
(b) (i) Benzoic acid to Benzaldehyde
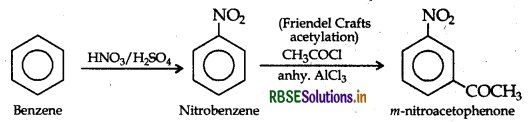
(ii) Benzene to m-Nitroacetophenone
(iii) Ethanol to 3-Hydroxybutanal

Question 34.
(a) Describe the following actions:
(i) Acetylation
(ii) Aldol condensation
(b) Write the main product in the following equations:
Answer:
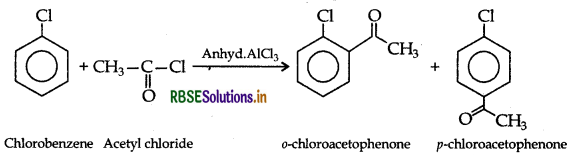
(a) (i) Acetylation: The addition of aryl (-COCH3) group on the ortho and para positions of acyl halide in the presence of a Lewis acid i.e. anhydrous aluminium chloride (AICl3) which acts as a catalyst.
(ii) Aldol condensation : Those aldehydes and ketones with atleast one a-hydrogen atom condense together in presence of dil. alkali eg NaOH etc. to form a B-hydroxy aldehyde or α β-hydroxy ketone respectively which gets dehydrated in the presence of acid upon heating to form a, α β-unsaturated compound.

Question 35.
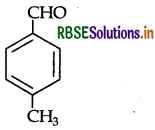
(a) Draw the structure of the following
(i) p-Methylbenzaldehyde
(b) Give chemical tests to distinguish between the following pairs of compounds:
(i) Benzoic acid and Ethyl benzoate.
(ii) Benzaldehyde and Acetophenone.
(iii) Phenol and Benzoic acid.
Answer:
(a) (i) p-Methylbenzaldehyde :
(ii) 4-Methylpent 3-en-2-one
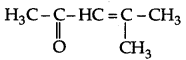
(b) (i) Distinction between Benzoic acid and Ethyl benzoate:
By Sodium bicarbonate test: Acids react with NaHCO3 to produce brisk effervescence due to the evolution of CO2 gas. Benzoic acid being an acid responds to this test, but ethyl benzoate does not.
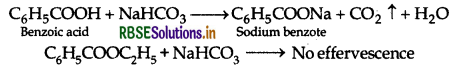
(ii) Benzaldehyde and Acetophenone
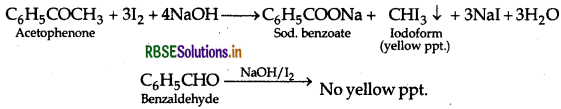
By lodoform test: Acetophenone being a methyl ketone on treatment with I2 and NaOH (NaO) undergoes iodoform test to give yellow ppt. of iodoform on heating whereas benzaldehyde does not.
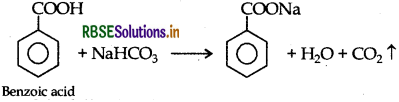
(iii) Phenol and Benzoic acid : On addition of NaHCO3 to both solutions, carbon dioxide is evolved with benzoic acid while phenol does not form CO2.
Question 36.
(a) Draw the structures of the following derivatives
(i) Propanone oxime
(ii) Semicarbazone of CH3CHO
(b) How will you convert ethanal into the following compounds? Give the chemical equations involved.
Answer:
(a) (i) Propanone oxime:
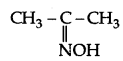
(ii) Semicarbazone of CH3CHO (Ethanal)

(b) (i) Ethanal to Ethane:

(ii) Ethanal to 3-hydroxy butanal (Aldol condensation)
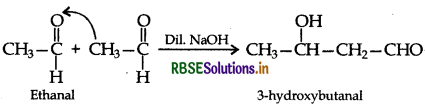
(iii) Ethanal to Ethanol (Partial Reduction)

Question 37.
(a) Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b) Draw the structure of the semicarbazone of ethanal.
(c) Why F-CH2-COOH is lower than that of Cl-CH2-COOH?
(d) Write the product in the following reaction:
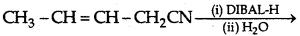
(e) How can you distinguish between propanal and propanone?
Answer:
(a) Cannizzaro reaction: Aldehydes which do not have an a-hydrogen atom undergo reduction on treatment with conc. alkali and produce alcohol and carboxylic acid salt.

(b) Semicarbazone of CH3CHO (Ethanal)

(c) In FCH2-COOH fluorine is more electron withdrawing than chlorine in CICH2-COOH so FCH2-COOH is more acidic than CICH2 - COOH, hence its pka value is lesser than CICH2-COOH.
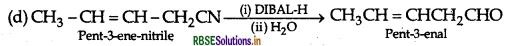
(e) Propanál and Propanone: Propanal being an aldehyde gives positive test with Fehling solution in which a red brown ppt. of cuprous oxide is obtained while propanone being a ketone does not respond to this test. 
Question 38.
(a) Write the structures of A and B in the following reactions:
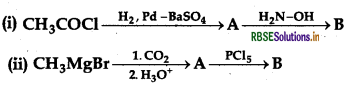
(b) Distinguish between:
(i) C6H5 - COCH3 and C6H5 - CHO
(ii) CH3COOH and HCOOH
(c) Arrange the following in the increasing order of their boiling points:
CH3CHO, CH3COOH, CH3CH2OH
Answer:
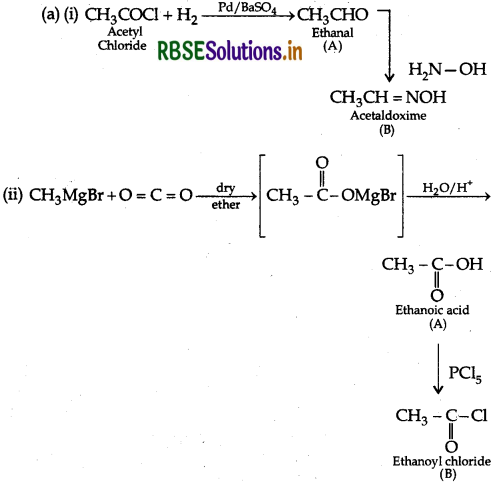
(b) (i) Benzaldehyde and Acetophenone
By lodoform test: Acetophenone being a methyl ketone on treatment with I2 and NaOH (NaO) undergoes iodoform test to give yellow ppt. of iodoform on heating whereas benzaldehyde does not.

(ii) CH3COOH (Acetic acid) and HCOOH (Formic acid). Formic acid is the only acid which contains aldehydic group and thus show reactions with Tollen's reagent (silver nitrate) and Fehling's solution while Acetic acid does not show.
Tollen's Test:
Add ammonical solution of silver nitrate to both the compounds, HCOOH gives silver mirror but CH3COOH does not.

(c) CH3CHO < CH3CH2OH < CHCOOH

Question 39.
(a) Write the chemical reaction involved in Wolff-Kishner reduction.
(b) Arrange the following in the increasing order of their reactivity towards nucleophilic reaction:
C6H5COCH3 CH3 − CHO,CH3COCH
(c) Why carboxylic acid does not give reactions of carbonyl group?
(d) Write the product in the following reaction
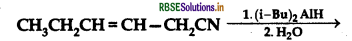
(e) A and B are two functional isomers of compound C3H6O. On heating with NaOH and I2 isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Answer:
(b) C6H5COCH3 < CH3COCH3 < CH3 CHO
(c) The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure.
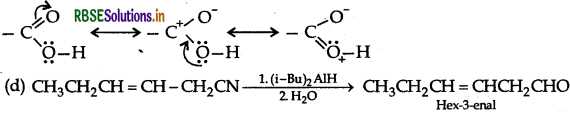
(e) The given compound has molecular formula C3H6O One of its functional isomer i. B shows iodo form test which can be only shown by compounds having methyl Ketone so the compound B will be Acetone or 2-propanone Its functional isomer A will be propanal.
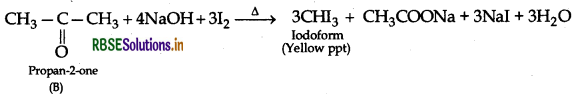
The formula of compound (A) will be 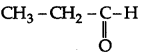 . It will not give iodoform test due to absence of methyl ketone group
. It will not give iodoform test due to absence of methyl ketone group 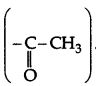
Question 40.
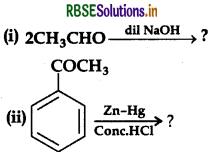
(a) Write the product(s) in the following
(b) Give simple tests to distinguish the following pairs of compounds:
(i) Ethanal and Propanal
(iii) Benzoic acid and Ethyl benzoate
(ii) Benzaldehyde and Acetophenone
Answer:
(a) (i) Aldol condensation:

(ii) Clemmensen reduction:
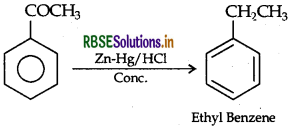
(b) (i) On heating with NaOH and I2, ethanal forms yellow ppt of CHI3, whereas propanal can not.
CH3CHO + 3I2 + 4NaOH → CHI3 + 3NaI + HCOONa + 3H2O
(ii) On heating with NaOH and I2, aceptophenone forms yellow ppt of CH3I, whereas benzaldehyde does not.
C6H5COCH3 + 3NaOI → C6H5COONa + CH3I ↓+ 2NaOH
(iii) On adding NaHCO3, benzoic acid produces brisk effervescence of CO2 gas whereas ethylbenzoate does not.
C6H5COOH + NaHCO3 → С6H5COONa + CO2↑ + H2O
Question 41.
(a) Give reasons:
(i) CH3-CHO is more reactive than CH3COCH3 towards HCN.
(ii) 4-nitrobenzoic acid is more acidic than benzoic acid.
(b) Describe the following
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
Answer:
(a) (i) Because carbonyl carbon of CH3-CHO is more electrophilic than CH3COCH3, due to only one electron donating CH-3 group.
(ii) Because of electron withdrawing nature of -NO2 group.
(b) (i) Acetylation : Introduction of an acetyl group/CH3COO- by heating an organic compound with acetyl chloride/ acetic anhydride.

(ii) Cannizzaro reaction: Aldehydes having no a-hydrogen atom when treated with conc. NaOH, undergoes self oxidation and self-reduction simultaneously.
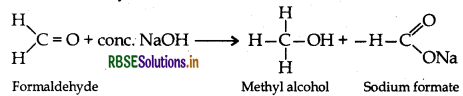
(iii) Cross Aldol Condensation: When aldol condensation is carried out between two different aldehydes or ketones, it is called cross aldol condensation.
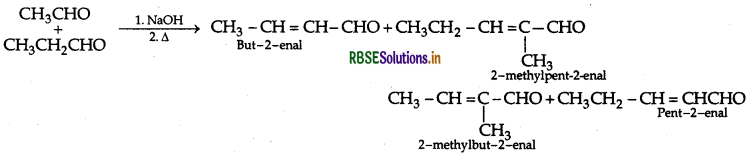
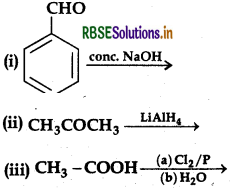
Question 42.
(a) Complete the following equations:
(b) Distinguish between:
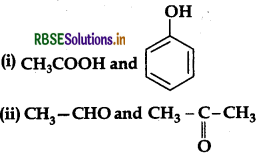
Answer:
(a) (i) Cannizzaro reacion:
(b) (i) On adding NaHCO3, CH3COOH produces brisk effervescence of CO2 gas whereas phenol does not.
(ii) On heating with Tollen's reagent, CH3CHO forms silver mirror whereas CH3COCH, does not.
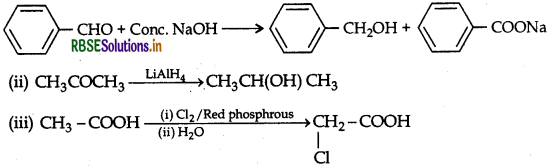
Question 43.
(a) What is meant by the following terms? Give an example of the reaction in each case.
(i) Aldol
(ii) Semicarbazone
(b) Complete the following:
Answer:
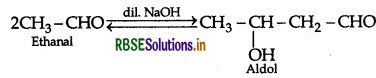
(a) (i) Two molecules of aldehyde and ketones containing a-hydrogen atom react in the presence of aqueous alkali giving product known as Aldol.
Example:
(ii) Aldehydes and ketones react with semicarbazide giving product called semicarbazone.
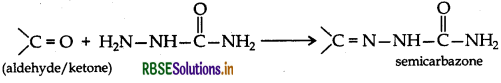
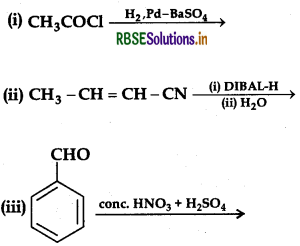
Question 44.
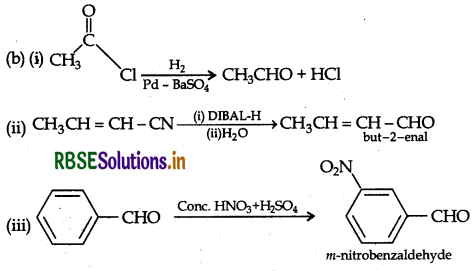
Write the product(s) in the following reactions.
Answer:
Question 45.
(a) Write the product(s) in the following reactions:
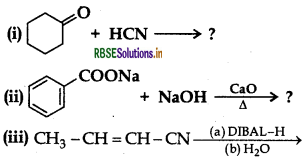
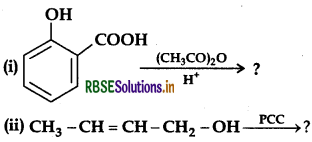
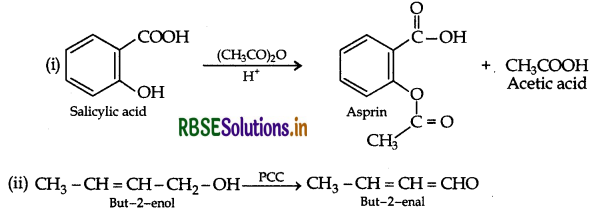
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Butanal and Butan-2-one
(ii) Benzoic acid and Phenol
Answer:
(b) (i) Tollen's reagent test. Add ammonical solution of silver nitrate (Tollen's Reagent) in both the solutions. Butanal gives silver mirror whereas Butan-2-one does not. Therefore Butanal gives Tollen's test.
(ii) Ferric chloride test. Add neutral FeCl3 in both the solutions phenol reacts with neutral FeCl3 to form an iron- phenol complex giving violet colour but benzoic acid does not.

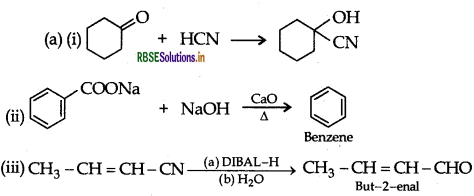
Question 46.
(a) Write the reactions involved in the following:
(i) Etard reaction
(ii) Stephen reduction
(b) How will you convert the following in not more than two steps:
(i) Benzoic acid to Benzaldehyde
(ii) Acetophenone to Benzoic acid
(iii) Ethanoic acid to 2-Hydroxyethanoic acid
Answer:
(a) (i) Etard reaction:

(ii) Stephen reduction:
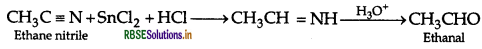
(b) (i) Benzoic acid to Benzaldehyde. Refer to Question 5. (b) (ii).
(ii) Acetophenone to Benzoic acid:
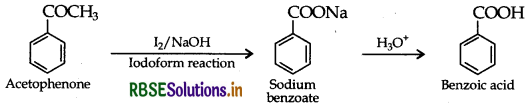
(iii) Ethanoic acid to 2-Hydroxyethanoic acid :
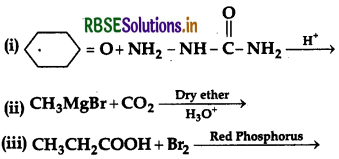
Question 47.
(a) How will you convert:
(i) Benzene to acetophenone
(ii) Propanone to 2-Methylpropan-2-ol
(b) Give reasons:
(i) Electrophilic substitution in benzoic acid takes place at meta position.
(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.
(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.
Answer:
(a) (i) Benzene to acetophenone

(ii) Propanone to 2-methylpropan-2-ol.
(b) (i) Because -COOH group is electron withdrawing group and deactivates the benzene ring. As a result of this ortho and para position acquires positive charge but only meta does not so electrophile can attack on metå position.
(ii) Because -COOH group of carboxylic acids is capable to do intermolecular hydrogen bonding forming a dimer while alcohols, aldehydes and ketones can not.

(iii) Because of smaller + I effect of one alkyl group in propanal as compared to larger - I effect of 2 alkyl groups of propanone, the magnitude of positive charge on the carbonyl carbon is more in propanal than propanone.
Question 48.
(a) Write the products of the following reactions
(b) Write simple chemical tests to distinguish between the following pairs of compounds:
(i) Propanal and propanone
(ii) Benzaldehyde and Benzoic acid
Answer:
(b) (i) Propanal and Propanone: Propanal gives positive test with Fehling solution in which a red ppt. of cuprous oxide is obtained while propanone does not respond to test.
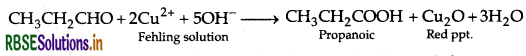
(ii) Distinction between Benzaldehyde and Benzoic acid: Benzaldehyde has no alpha hydrogen atom. Therefore, it undergoes Cannizzaro's reaction as follows:
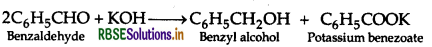
Where, Benzoic acid dose not undergo Cannizzaro's reaction.
COMPETITION CORNER:
Question 1.
Which of the following give nucleophilic reaction?
(a) Kolbe's reaction of phenol
(b) Williamson's synthesis of ether
(c) Reimer Tiemann reaction of phenol
(d) Koble's electrolytic synthesis of ethane from sodium acetate
(e) Aldol formation from ethanal.
Answer:
(e) Aldol formation from ethanal.

Question 2.
That reaction in which dichlorocarbene act as electrophile:
(a) Reimer-Tiemann Reaction
(b) Kolbe's reaction
(c) Friedal craft alkylation
(d) Fitting reaction
Answer:
(a) Reimer-Tiemann Reaction
Question 3.
Given Reaction
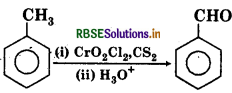
(a) Carbylamine reaction
(b) Hundsdiecker reaction
(c) Hoffmann reaction
(d) Etard reaction
Answer:
(d) Etard reaction
Question 4.
The product (B) in the following sequence of reaction is
Ethyl benzene 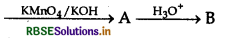
(a) Benzaldehyde
(b) Benzophenone
(c) Benzoic acid
(d) Acetophenone
Answer:
(c) Benzoic acid
Question 5.
Which one of the following compounds will not give Cannizzaro reaction with base?
(a) Cl3CCHO
(b) Me3CCHO
(c) C6H5CHO
(d) HCHO
Answer:
(a) Cl3CCHO
Question 6.
The reagent used in Etard reaction is:
(a) Chromyl chloride
(b) Ethanoyl chloride
(c) SnCl2 and HCl
(d) CdCl2
Answer:
(a) Chromyl chloride
Question 7.
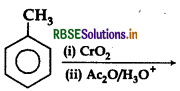
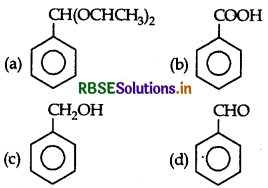
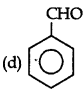
Answer:
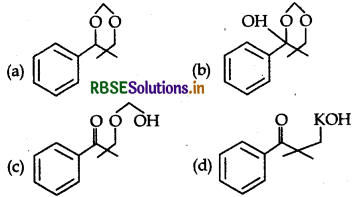
Question 8.
The main product of sequence of reaction is

Answer:
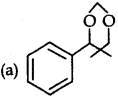
Question 9.
A organic compound X is oxidised by acidified K2Cr2O7 solution. The product formed reacts with phenyl hydrazine but does not give silver mirror test.
(a) 2-Propanol
(b) Ethanal
(c) Ethanol
(d) Propane
Answer:
(a) 2-Propanol
Question 10.
A Grignard reagent reacts with ketone, the product obtained is:
(a) 1 alcohol
(b) 2 alcohol
(c) 3 alcohol
(d) ethanol
Answer:
(c) 3 alcohol
Question 11.
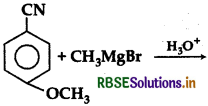
The product (p) in above reaction is:
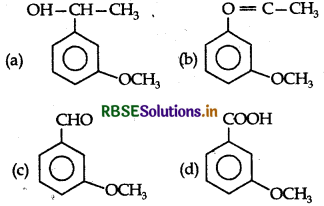
Answer:
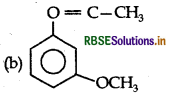
Question 12.
Which of the following is nucleophilic addition reaction?
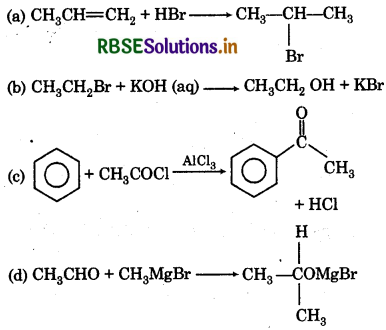
Answer:
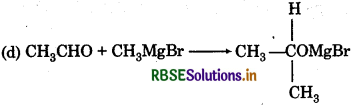
Question 13.
Tollen reagent is:
(a) Alkaline solution of copper nitrate
(b) Ammonical silver nitrate
(c) Ammonical copper nitrate
(d) None of these
Answer:
(b) Ammonical silver nitrate

Question 14.

The correct structure of product A In reaction is:
Answer:
Question 15.
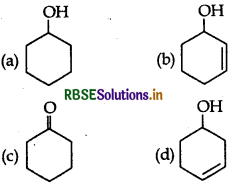
The correct order of acidity is:
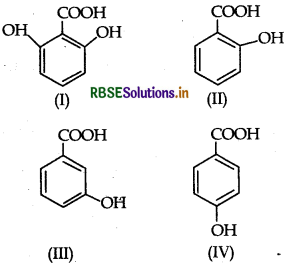
(a) I > II > III > IV
(b) III > I > II > IV
(c) III > IV > II > I
(d) I > III > IV > II
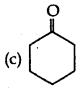
Answer:
(b) III > I > II > IV
Question 16.
The best representation of carboxylate ion structure is:
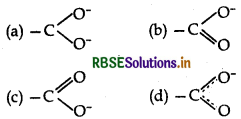
Answer:
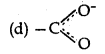

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम