RBSE Class 12 Chemistry Important Questions Chapter 10 Haloalkanes and Haloarenes
Rajasthan Board RBSE Class 12 Chemistry Important Questions Chapter 10 Haloalkanes and Haloarenes Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 10 Important Questions Haloalkanes and Haloarenes
Multi Choice Questions (MCQ):
Question 1.
Which of the following is gem-di halide.
(a) CH3CHBг2
(b) BrCH2CH2Br
(c) CH3CHBrCH2Br
(d) CH3CHBrCH2CH2Br
Answer:
(a) CH3CHBг2
Question 2.
If two halogen atoms are attached with adjacent carbon atoms, then they are known as.
(a) Polymethylene dihalide
(b) Gem-dehalide
(c) Vic-dihalide
(d) Alkylene halide
Answer:
(c) Vic-dihalide

Question 3.
If two halogen atoms are attached at a single carbon atom, then they are known as.
(a) Polymethylene dihalide
(b) Alkylene dihalide
(c) Vic-dihalide
(d) Alkylene halide
Answer:
(d) Alkylene halide
Question 4.
The reaction given below known as.
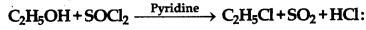
(a) Kharash effect
(b) Darjen reactions
(c) Williamson's synthesis
(d) Wurtz reaction
Answer:
(b) Darjen reactions
Question 5.
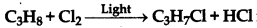 is an example of :
is an example of :
(a) replacement
(b) addition
(c) rearrangement
(d) elimination
Answer:
(a) replacement
Question 6.
The best conditions to get maximum yield of C2H5Cl.
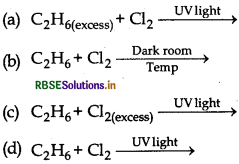
Answer:
\(\text { (a) } \mathrm{C}_2 \mathrm{H}_{6(\text { excess })}+\mathrm{Cl}_2 \stackrel{\text { UVlight }}{\longrightarrow}\)
Question 7.
Anti-markownikoff's rule of HBr is not shown by:
(a) Propane
(b) 1-butene
(c) but-2-ene
(d) Pent-2-ene
Answer:
(c) but-2-ene
Question 8.
Which of the following compound is not obtained during the reaction of methane and chlorine in the presence of sun light.
(a) CHCl3
(b) CH3Cl
(c) CH2Cl2
(d) CH3CH2CH3
Answer:
(d) CH3CH2CH3
Question 9.
Iodoform test is not given by
(a) Ethanol
(b) Ethanal
(c) Pentanone-3
(d) Pentanone-2
Answer:
(c) Pentanone-3

Question 10.
Grignard reagent is prepared from:
(a) methylaniline
(b) diethyl ether
(c) ethyl iodide
(d) ethyl alcohol
Answer:
(c) ethyl iodide
Question 11.
With which I2 + NaOH give CHI3 on heating:
(a) C2H5OH
(b) CH3OH
(c) HCOOH
(d) C6H6
Answer:
(a) C2H5OH
Question 12.
Which one of the following is not starting material for preparing CHI3 (iodoform):
(a) CH3OH
(b) C2H5OH
(c) CH3CHO
(d) (CH3)2CO
Answer:
(a) CH3OH
Question 13.
Which one of the following reagent is not used for preparing alkyl halide from alcohol?
(a) HCl + ZnCl2
(b) NaCl
(c) PCl5
(d) SOCl2
Answer:
(b) NaCl
Question 14.
Which is ethyl bromide.
(a) CH3CHBr2
(b) C2H5Br
(c) CHBr = CHBr
(d) CH2BrCH2Br
Answer:
(b) C2H5Br
Question 15.
Which of the following compound does not give yellow precipitate with alkaline solution of iodine.
(a) CH3CH(OH)CH3
(b) CH3OH
(c) CH3CH2CH(OH) CH3
(d) CH3CH2OH
Answer:
(b) CH3OH
Question 16.
Which of the following not form ethyl chloride from ethanol.
(a) Cl2
(b) P(red)/Cl2
(c) PCl5
(d) PCl3
Answer:
(a) Cl2

Question 17.
Which of the following will give only two monochloro derivative.
(a) n-butane
(b) 2,4-dimethyl pentane
(c) benzene
(d) 3-methylpentane
Answer:
(a) n-butane
Question 18.
Haloform is trihalo derivative of which compound.
(a) ethane
(b) methane
(c) propane
(d) benzene
Answer:
(b) methane
Question 19.
Haloform reaction is used to prepare.
(a) halogen
(b) CCl4
(c) CHCl3
(d) halide
Answer:
(c) CHCl3
Question 20.
Which of the following is freon.
(a) CCl2F2
(b) CHCl3
(c) CH2F2
(d) CF4
Answer:
(a) CCl2F2
Very Short Answer Questions:
Question 1.
A solution of KOH hydrolyses CH3CHCICH2CH3 and CH3CH2CH2CH2Cl.
Which one of these is more easily hydrolysed?
Answer:
In aq. KOH SN1 mechanism takes place and the carbocation is formed. As 2° carbocation is more stable carbocation than 1° therefore 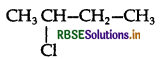 hydrolyses faster than CH3CH2CH2CH2Cl.
hydrolyses faster than CH3CH2CH2CH2Cl.
Question 2.
Give the IUPAC name of the following compund:
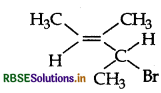
Answer:
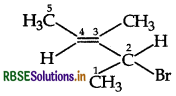
Question 3.
Write the IUPAC name of the following compound:
(CH3)3 CCH2Br
Answer:
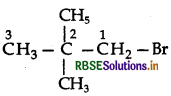
Question 4.
Write the IUPAC name of the following compound:
CH2 = CHCH2Br
Answer:
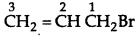
IUPAC name: 1-Bromo-prop-2-ene

Question 5.
Write the IUPAC name of the following compound:
Answer:
IUPAC name : 3 Bromo-2-methyl propene.
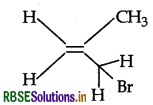
Question 6.
Give the IUPAC name of the following compound.
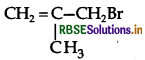
Answer:
IUPAC name: 3-bromo-2-methyl propene
Question 7.
What happens when bromine attacks
CH2 = CH - CH2 - C = CH?
Answer:
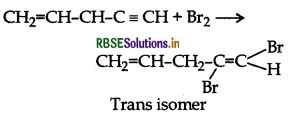
Question 8.
Write the IUPAC name of the following:
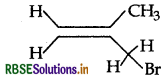
Answer:
IUPAC name: 3 Bromo-2-methyl propene.
Question 9.
Draw the structure of major monohalogen product formed in the following reaction:
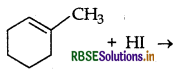
Answer:
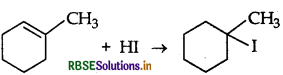
Question 10.
In the following pair of compounds, which will react faster by SN1 mechanism and why?

Answer:
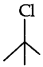
reacts faster by SN1 mechanism as it is a tertiary halide and it produces a stable tertiary carbocation.

Question 11.
Draw the structure of major monohalogen product in the following reaction:
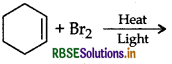
Answer:
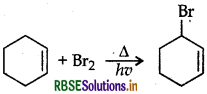
Question 12.
Predict the order of reactivity of four isomeric bromobutanes in SN1 reaction.
Answer:
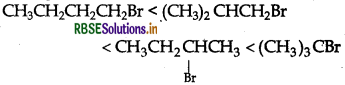
Question 13.
Predict the order of reactivity of the following compounds in SN1 reaction:
C6H5CH2Bг, C6H5C(CH3) (C6H5)Br,
C6H5CH(C6H5)Br, C6H5CH(CH3)Br
Answer:
C6H5C(CH3) (C6H5)Br > C6H5CH(C6H5)Br > C6H5CH(CH3)Br > C6H5CH2Br
Question 14.
Draw the structure of major monohalogen product in the following reaction:
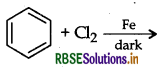
Answer:
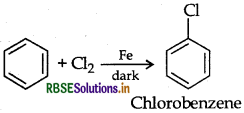
Question 15.
Give a chemical test to distinguish between H5Br and C6H5Bг.
Answer:
Both are heated with aqueous NaOH. C2H5Br ves ethanol and NaBr, which on reacting with AgNO3, ves yellow precipitate of AgBr. C6H5Br does not respond to this test.
Question 16.
Arrange the following in increasing order of Diling point:
(i) CH3CH2CH2CH2Br
(ii) (CH3)3C.Br
(iii) (CH3)2.CH.CH2.Br
Answer:
(CH3)3C.Br < (CH3)2.CH2CH2.Br < CH3CH2CH2CH2Br
Question 17.
Write the IUPAC name of the following:
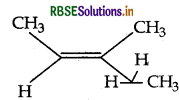
Answer:
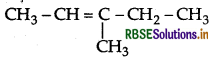
IUPAC name : 3-Methypent-2-ene.
Question 18.
Write the IUPAC name of
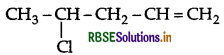
Answer:
IUPAC name : 4-chloropent-1-ene
Question 19.
What happens when CH3-Br is treated with KCN?
Answer:

Question 20.
Write the IUPAC name of
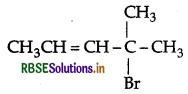
Answer:
IUPAC name: 4-bromo-4-methylpent-2-ene
Question 21.
What happens when ethyl chloride is treated ith aqueous KOH?
Answer:
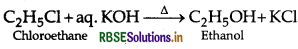
Question 22.
Write the IUPAC name of
(CH3)2CH.CH(Cl)CH3.
Answer:
IUPAC name: 2-chloro-3-methylbutane
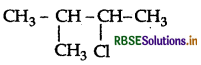
Question 23.
Which compound in the following pair undergoes faster SN1 reaction.

Answer:

reacts faster by SN1 mechanism as it is a tertiary halide and it produce a stable tertiary carbocation.

Question 24.
Write the IUPAC name of the following compound:
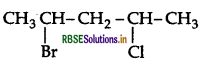
Answer:
IUPACE name: 2-Chloro-3, 3-dimethylbutane.
Question 25.
Write the IUPAC name of the following compound:
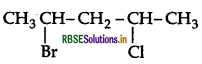
Answer:
IUPAC name: 2-Bromo-4-chloropentane.
Question 26.
Write the IUPAC name of the following compound:
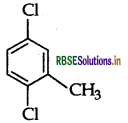
Answer:
IUPAC name: 2, 5-dichlorotoluene.
Question 27.
In the following pair of halogen compounds, which compound will react faster by SN1 mechanism?
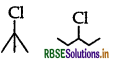
Answer:
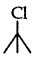 (3 alkyl halide) reacts faster than
(3 alkyl halide) reacts faster than 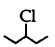 (2° alkyl halide) due to greater stability of 3° carbocations over 2° carbocation.
(2° alkyl halide) due to greater stability of 3° carbocations over 2° carbocation.
Question 28.
A hydrocarbon C5H12 gives only one mono-chlorination product. Identify the hydrocarbon.
Answer:
Since there is only one monochloroderivative, the compound contains 12 equivalent hydrogen in four equivalent CH3. The compound is 2,2-dimethyl propane.
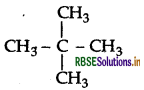
Question 29.
Identify the chiral molecule in the following pair:

Answer:
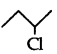
(2-Chlorobutane) is a chiral molecule.
Question 30.
Draw the structure of 2-bromopentane.
Answer:

Question 31.
Which would undergo SN2 reaction faster in the following pair and why?
CH3 - CH2 - Br and 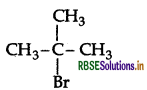
Answer:
CH3CH2Br reacts faster because it is a primary halide (1° halide).
Question 32.
Which would undergo SN1 reaction faster in the following pair:
CH3-CH2-CH2-Br and 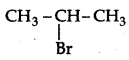
Answer:
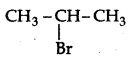 because the secondary carbocation formed is more stable than primary carbocation.
because the secondary carbocation formed is more stable than primary carbocation.
Question 33.
Out of  and
and 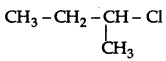 which is more reactive towards SN1 reaction and why?
which is more reactive towards SN1 reaction and why?
Answer:
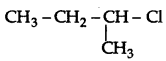 is more reactive than
is more reactive than 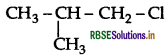 (1-Chloro-2- methylpropane) because 2-Chlorobutane forms a more stable 2° carbocation.
(1-Chloro-2- methylpropane) because 2-Chlorobutane forms a more stable 2° carbocation.
Question 34.
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction.
Answer:
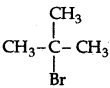 (2-Bromo-2-methyl propane) or tert-butyl bromide is most reactive towards SN1 reaction as it can form 3° carbocation.
(2-Bromo-2-methyl propane) or tert-butyl bromide is most reactive towards SN1 reaction as it can form 3° carbocation.
Question 35.
Write the structure of 1-Bromo-4-chlorobut-2-ene.
Answer:

Question 36.
Write the structure of 2,4-dinitrochlorobenzene.
Answer:
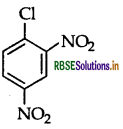
Question 37.
Out of 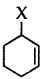 and
and 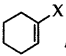 example of allylic halide?
example of allylic halide?
Answer:
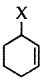 is an allylic halide.
is an allylic halide.
Question 38.
Out of 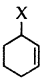 and
and 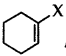 which is an example of vinylic halide?
which is an example of vinylic halide?
Answer:
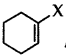
Question 39.
Out of 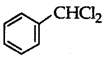 and
and 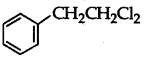 which is an example of a benzylic halide?
which is an example of a benzylic halide?
Answer:
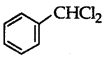 is a benzylic halide.
is a benzylic halide.
Short Answer Type Questions:
Question 1.
Which one in the following pairs of substances Indergoes SN2 substitution reaction faster and why?
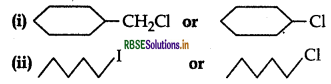
Answer:
(i) 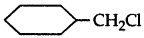 is a primary halide and therefore undergoes SN2 reaction faster.
is a primary halide and therefore undergoes SN2 reaction faster.
(ii)  AS iodine is a better leaving group because of its large size, therefore undergoes SN2 reaction faster.
AS iodine is a better leaving group because of its large size, therefore undergoes SN2 reaction faster.

Question 2.
Which one in the following pairs undergoes SN1 substitution reaction faster and why?
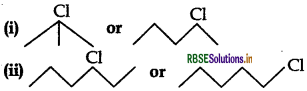
Answer:
(i) 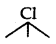 (3 alkyl halide) reacts faster than
(3 alkyl halide) reacts faster than 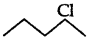 (2 alkyl halide) due to greater stability of 3 carbocations over 2 carbocation.
(2 alkyl halide) due to greater stability of 3 carbocations over 2 carbocation.
(ii) As 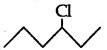 is a secondary alkyl halide which reacts faster in SN1 reaction than 1 alkyl halide
is a secondary alkyl halide which reacts faster in SN1 reaction than 1 alkyl halide 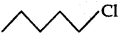 due to greater stability of 2 carbcations over 1 carbcations
due to greater stability of 2 carbcations over 1 carbcations
Question 3.
Complete the following reaction equations:
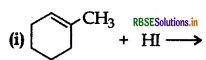
(ii) CH3CH2CH = CH2 + HBr →
Answer:
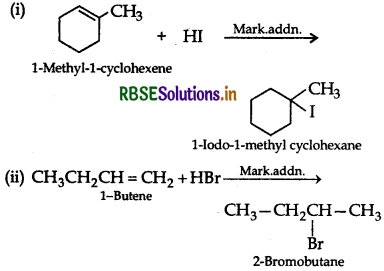
Question 4.
How are the following conversions carried out
(i) Benzyl chloride to benzyl alcohol.
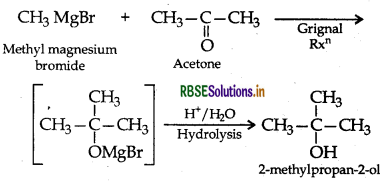
(ii) Methyl magnesium bromide to 2-methyl-propan
Answer:
(i) Benzyl chloride to benzyl alcohol

(ii) Methyl magnesium bromide to 2-methylpropan
Question 5.
Haloalkanes undergo nucleophilic substitution whereas haloarenes undergo electrophilic substitution.
Answer:
Haloarenes (say chlorobenzene) is a resonance hybrid of the following five structures:
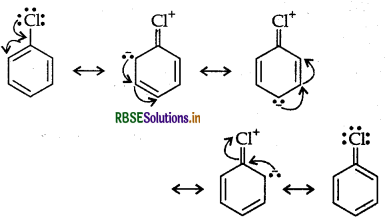
Resonance leads to lowering of energy and hence greater stability. On the other hand, no such resonance is possible in haloalkanes. Halogens directly attached to benzene ring are o, p-directing in electrophilic substitution reactions. This is due to greater electron density at these positions in resonance.
Question 6.
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same.
Answer:
The reasons are:
- Due to resonance/diagrammatic representation C-Cl bond acquires a partial double bond character. As a result, the C-Cl bond in chlorobenzene is shorter and hence stronger. Thus, cleavage of C-Cl bond in benzene becomes difficult which makes it less reactive towards nucleophilic substition.
- Due to repulsion between nucleophilic and electron rich arenes.
Question 7.
(a) Why does p-dichlorobenzene have a higher m.p. than its o-and m-isomers?
(b) Why is (±)-Butan-2-ol optically inactive?
Answer:
(a) p-isomers are comparetively more symmetrical and fit closely in the crystal lattice, thus require more heat to break these strong forces of attraction. Therefore higher melting point than o-and m-isomers.
(b) (±)-Butan-2-ol is optically inactive because in racemic mix one type of rotation is cancelled by other.
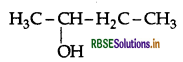
Question 8.
Account for the following:
(i) The C - Cl bond length in chlorobenzene is shorter than that in CH3-Cl.
(ii) Chloroform is stored in closed dark brown bottles.
Answer:
(i) In haloalkanes, the halogen atom is attached 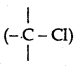 to sp3-hybridized carbon while in haloarenes it is attached to sp2-hybridized carbon whose size is smaller than sp3 orbital carbon. Therefore C - Cl bond in chloro-benzene is shorter than alkyl chloride.
to sp3-hybridized carbon while in haloarenes it is attached to sp2-hybridized carbon whose size is smaller than sp3 orbital carbon. Therefore C - Cl bond in chloro-benzene is shorter than alkyl chloride.
(ii) CHCl3 is stored in dark coloured bottles to cut off light because CHCl3 is slowly oxidised by air in presence of light to form an extremely poisonous gas, carbonyl chloride, popularly known as phosgene.
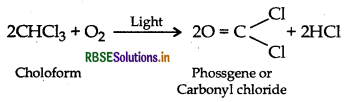
Question 9.
Give chemical tests to distinguish between the following pairs of compounds:
(a) Benzyl chloride and Chlorobenzene
(b) Chloroform and Carbon tetrachloride
Answer:
(a) Chlorobenzene and Benzyl chloride: Benzyl chloride is more reactive than chlorobenzene towards nucleophillic substitution reactions, therefore, benzyl chloride on boiling with aqueous KOH produces benzyl alcohol and KCl.
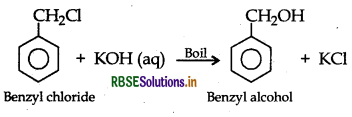
The reaction mixture on acidification with dil. HNO3 followed by treatment with AgNO3 solution produces white ppt. of AgCl due to formation of KCI
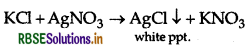
But chlorobenzene does not undergo hydrolysis under these mild conditions to give phenol and KCl.
(b) Chloroform and Carbon tetrachloride By Carbylamine test: CCl4 does not give this reaction but chloroform gives this reaction and produces offensive smell of phenyl isocyanide.
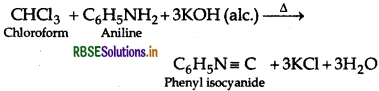
Question 10.
Explain why:
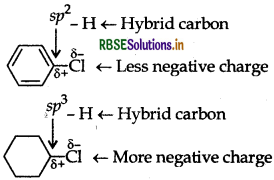
(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride water.
(b) Alkyl halides, though polar, are immiscible with water.
Answer:
(a) Chlorobenzene has lower dipole moment than cyclohexyl chloride due to lower magnitude of -ve charge on the Cl atom and shorter C - Cl distance. Due to greater S-character, a sp2-hybrid carbon is more electronegative than a sp3-hybrid carbon. Therefore, the sp2-hybrid carbon of C - Cl bond in chlorobenzene has less tendency to release electrons to Cl than a sp3 hybrid carbon of cyclohexyl chloride.
(b) Alkyl halides and polar molecules are held together by dipole-dipole interaction. The molecules of H2O are held together by H-bonds. Since the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide-alkyl halide molecules and water- water molecules, therefore alkyl halides are immiscible (not soluble) with water.

Question 11.
(i) Which alkyl halide from the following pair is chiral and undergoes faster SN2 reaction?
(ii) Out of S1 and S2, which reaction occurs with
(a) Inversion of configuration
(b) Racemisation
Answer:
(i) 2-bromobutane imm is a chiral compound and 1 Bromo Butane undergoes SN2 reaction faster.
(ii) (a) Inversion of configuration occurs with SN2 eaction.
(b) Racemisation occurs with SN1 reaction.
Question 12.
Draw the structure of major monohalo product in each of the following reactions:
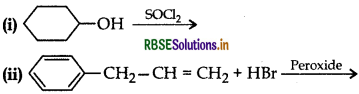
Answer:
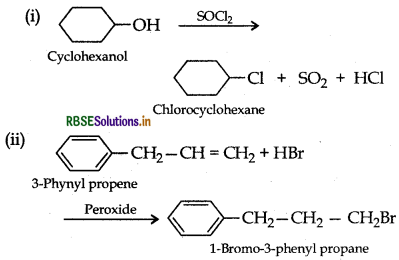
Question 13.
Write the IUPAC names of the following compounds:
(i) CH2 = CHCH2Br
(ii) (CCl3)3 CCI
Answer:
(i) CH2 = CHCH2 - Br
IUPAC name: 3-Bromopropene :
(ii) (CCl3)3 - C - Cl:
IUPAC name: 2-(Trichloromethyl) - 1, 1, 1, 2, 3, 3, 3- eptachloropropane
Question 14.
What are ambident nucleophiles? Explain with an example.
Answer:
Ambident nucleophile: A nucleophile that can orm new bonds at two or more spots in its structure, sually due to resonance contributors.
Example: S = C = N- can act as a nucleophile with ither the S or N attacking.
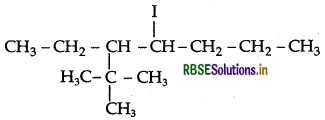
Question 15.
Write the structures of the following organic alogen compounds:
(i) 4-tert-Butyl-3-idodeptane
(ii) 4-Bromo-3-methylpent-2-ene
Answer:
(i) 4-tert-Butyl-3-iodoheptane
(ii) 4-Bromo-3-methylpent-2-ene
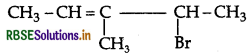
Question 16.
Write the structures of the following organic halogen compounds :
(i) p-Bromochlorobenzene
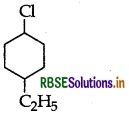
(ii) 1-Chloro-4-ethylcyclohexane
Answer:
(i) p-Bromochlorobenzene
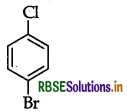
(ii) 1-Chloro-4-ethylcyclohexane
Question 17.
Write the equations for the preparation of 1- bromobutane from:
(i) 1-butanol
Answer:
(i) Preparation of 1-bromobutane from 1- butanol
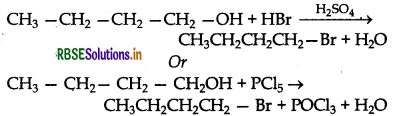
(ii) Preparation of 1-bromobutane from but-1-ene
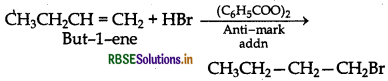
Question 18.
Which compound in each of the following pairs will react faster in SN2 reaction with -OH?
(i) CH3Br or CH,I
(ii) (CH3)3 CCl or CH3Cl
Answer:
(i) CH3I: Because Iodide is better leaving group than bromide.
(ii) CH3Cl: Carbon atom leaving group is less hindered.

Question 19.
Among the given pair of compounds alkyl halide (b) has a chiral centre.
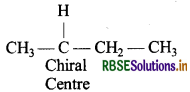
Answer:
The alkyl halide (a) does not contain a chiral centre and it also gives faster SN2 reaction as SN2 is more favourable in primary alkyl halides.
Question 20.
Which alkyl halide from the following pair is
(i) Chiral and
(ii) undergoes SN1 reaction faster?
(a) (CH3)3 CBr
(b) CH3CH2CHBrCH3
Answer:
(i) 2-Bromobutane is chiral as the central C atom has all 4-different groups.
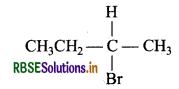
(ii) 3° Alkyl bromide i.e. (CH3)3 CBr undergoes SN1 reaction faster due to more stability of 3° carbocation.
Question 21.
(i) State one use each of DDT and iodoform.
(ii) Which compound in the following couples will react faster in SN2 displacement and why?
(iii) 1-bromopentane or 2-bromopentane
(iv) 1-bromo-2-methylbutane or 2-bromo-2- methylbutane.
Answer:
(i) Use of DDT (Dichlorodiphenyl Trichloroethane): As a powerful insecticide, it is widely used for sugarcane and fodder crops to kill mosquitoes, lice which carry pathogens.
Use of iodoform (CHI3): It is used as an antiseptic for dressing wounds. Its antiseptic action is due to liberation of iodine when iodoform comes in contact with skin but not due to iodoform itself.
(ii) In SN2 reactions, reactivity depends upon steric hindrance
(a) 1-Bromopentane (1° halogen) having less steric hindrance therefore is more reactive than 2-Bromopentane hence undergoes SN2 reactions faster.
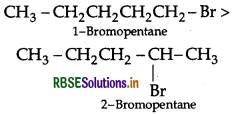
(b) 1-Bromo-2-methylbutane having less steric hindrance, is thus more reactive towards SN2 reaction than 2-bromo-2-methyl butane (more steric hindrance).
Question 22.
How would you differentiative between SN1 and example of each.
Answer:
|
(SN1 Substitution Nucleophilic Umimolecular) |
(SN2 Substitution Nucleophilic Biomolecular) |
|
1. It takes place in 2 steps. |
1. It takes place in single step. |
|
2. It follows first order Kinetics. |
2. It follows second order Kinetics. |
|
3. The rate of reaction depends upon the concentration of 3° alkyl halide only and is independent of the concentration of OH- ion Rate = K [3° Alkyl halide] |
3. The rate of reaction depends upon the concentration of both the reactants Rate = K [RX] [OH-] |
|
4. The Nu attacks from front side. |
4. The Nu attacks from back side. |
|
5. The reaction occurs occurs through a stable 3°carbocation. |
5. The reaction occurs through an unstable transition state. |
|
6. There is no Walden inversion. Example: Rx" of 3° Butyl bromide with aq KOH step 1: Step 1:
|
6. The inversion of configuration occurs which is known as Walden inversion.
|
Question 23.
Answer the following:
(i) Haloalkanes easily dissolve in organic solvents, why?
(ii) What is known as a recemic mixture? Give an example.
(iii) of the two bromoderivatives, C6H5CH (CH3) Br and C6H5CH (C6H5) Br, which one is more reactive in SN1 substitution reaction and why?
Answer:
(i) Because the new forces of attraction set up between haloalkanes and solvent molecules are of the same strength as the forces of attraction being broken.
(ii) A mixture which contains the equal proportions of two enantiomers of a compound in equal proportions is called recemiç mixture. Example: (+) butan-2-ol.
(iii) Since the reactivity of Sμl reactions increases as the stability of intermediate carbocation increases. Of the two 2° bromides, the carbocation intermediate derived from C6H5CH(C6H5) Br i.e. C6H5 CHC6H5 is more stable as compared to the carbocation C6H5 CH+ CH3 obtained from C6H5CH(CH3) Br because it is stabilized by two phenyl groups due to resonance.
Question 24.
Rearrange the compounds of each of the llowing sets in order of reactivity towards SN2 splacement:
1. 2-Bromo-2-methylbutane, 1-Bromopentane, 2- comopentae
2. 1-Bromo-3-methylbutane, 2-Bromo-2-methyl- atane, 3-Bromo-2-methylbutane
3.1-Bromobutane, 1-Bromo-2, 2-dimethyl-propane, Bromo-2-methylbutane
Answer:
- 1-Bromopentane > 2-Bromopentane > 2- omo-2-methylbutane
- 1-Bromo-3-methylbutane > 3-Bromo-2 ethylbutane
- 1-Bromobutane > 1-Bromo-2-methylbutane > 1- romo-2, 2-dimethylpropane.

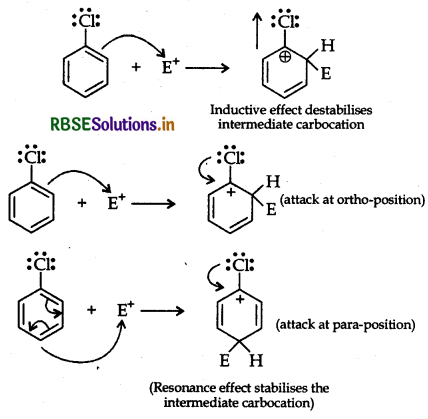
Question 25.
Although chlorine is an electron withdrawing oup, yet it is ortho-, para-directing in electrophilic omatic substitution reactions. Explain why it is so?
Answer:
Chlorine withdraws electrons through inductive fect and releases through resonance. Although Cl shows effect but through resonance, Cl tends to stablize the termediate carbocation and the effect is more pronounced ortho and para positions.
This can also be explained diagramatically as:
Question 26.
Answer the following questions :
(i) What is meant by chirality of a compound? Give an example.
(ii) Which one of the following compounds is more easily hydrolyzed by KOH and why?
CH3CHCICH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes SN2 substitution reaction faster and why?

Answer:
(i) Chirality: The objects which are non- superimposable on their mirror image are said to be chiral and this property is known as chirality for Butan-2-ol.
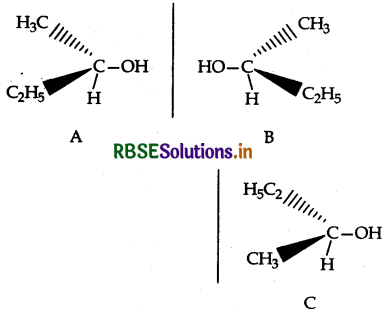
C is non-superimposable on its mirror image A.
(ii) 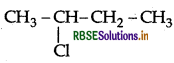 is more easily hydrolysed by KOH as it forms II° carbocation where CH3CH2CH2Cl will form 1° carbocation. II° carbocation is more stable than 1°.
is more easily hydrolysed by KOH as it forms II° carbocation where CH3CH2CH2Cl will form 1° carbocation. II° carbocation is more stable than 1°.
(iii) As I is a better leaving group because of its large size, it will be released at a faster rate in the presence of incoming nucleophile.
Therefore  substitution reaction faster.
substitution reaction faster.
Question 27.
Account for the following:
(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(b) Alkyl halides, though polar, are immiscible with water.
(c) Grignard's reagents should be prepared under anhydrous conditions.
Answer:
(a) Electron pairs of Cl atom are in conjugation with electrons of the benzene ring so C-Cl bond in chlorobenzene acquires some double bond character while C-Cl bond in cyclohexyl chloride. Since dipole moment is a product of charge and distance, so chlorobenzene has lower dipole moment than cyclohexyl chloride.
(b) Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attraction. The molecules of H2O are held together by H-bonds. Since the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide-alkyl halide molecules and water-water molecules, therefore, alkyl halides are immiscible with water.
(c) Grignard's reagents are very reactive. They react with alcohol, water, amines etc. to form corresponding hydrocarbon.
R-MgX + HOH → RH + Mg(OH)X
Therefore, Grignard's reagents must be prepared under anhydrous conditions.
Question 28.
Give reasons for the following:
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically inactive.
(iii) C-X bond length in halobenzene is smaller than C-X bond length in CH3-X.
Answer:
(i) I, is better leaving group/C-I bond is weaker than C-Br bond.
(ii) Butan-2-ol has assymmetric carbon (chiral center) with four different groups attached to the central carbon. As recemic mixture cancel each other optically. So they are optically inactive.
(iii) In halobenzene, halogen atom is attached to the sp2 hybrid carbon atom while in CH3-X halogen atom is attached to sp3 hybrid carbon atom. Hence C-X bond length in halo benzene is smaller than CH3-X.
Question 29.
(a) Draw the structures of major monohalo products in each of the following reactions:
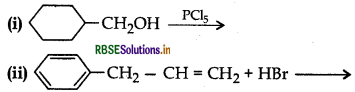
(b) Which halogen compound in each of the following pairs will react faster in SN2 reaction:
(i) CH3Br or CH3I
(ii) (CH3)3 C - Cl or CH3-Cl
Answer:
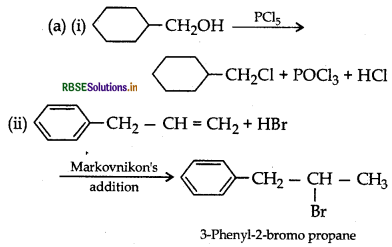
(b) (i) CH3Br will react faster in SN2 reaction.
(ii) CH3 - Cl will react faster in SN2 reaction.
Question 30.
(a) Which compound in each of the following pairs will react faster in SN2 reaction with -OH group?
(i) CH3Br or CH3I
(ii) (CH3)3 CCI or CH3Cl
(b) Write the product of the following reactions:
(i) CH3 - Cl + KCN → ?
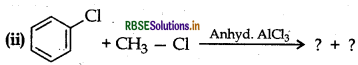
Answer:
(a) (i) As I- ion is a good leaning group than Br therefore reacts faster than CH3Br in SN2 reactions with -OH.
(ii) As 3° alkyl halides have more steric hindrance than 1° alkyl halide therefore CH3Cl undergoes SN2 reaction faster than 3° alkyl halide.
Question 31.
Give reasons:
(a) n-Butyl bromide has higher boiling point than t- butyl bromide.
(b) Racemic mixture is optically inactive.
(c) The presence of nitro (-NO2) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
Answer:
(a) n-Butyl bromide has higher boiling point than t-butyl bromide because it has larger surface area hence have more Van der Waals' forces.
(b) Rotation due to one enantiomer is cancelled by another enantiomer.
(c) The presence of nitro group (-NO2) at ortho and para positions withdraws the electron density from benzene ring and thus facilitating the attack of nucleophile out.
Question 32.
How can the following conversions be carried
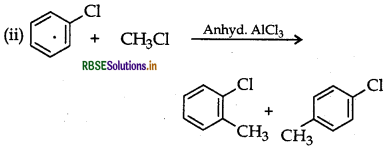
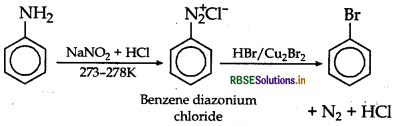
(i) Aniline to bromobenzene
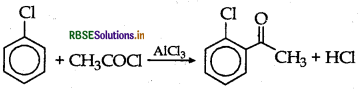
(ii) Chlorobenzene to 2-chloroacetophenone
(iii) Chloroethane to butane
Answer:
(i) Aniline to bromobenzene
(ii) Chlorobenzene to 2-chloroacetophenone
(iii) Chloroethane to butane
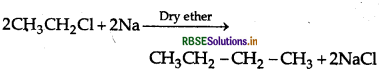
Question 33.
What happens when
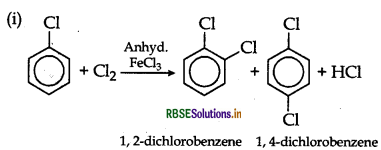
(i) Chlorobenzene is treated with Cl22/FeCl3,
(ii) Ethyl chloride is treated with AgNO2
(iii) 2-bromopentane is treated with alcoholic KOH?
Answer:
(ii) CH3CH2Cl + AgNO3 → CH3CH2NO2 + AgCl
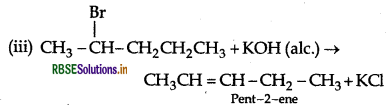
Question 34.
Name the following according to IUPA system:
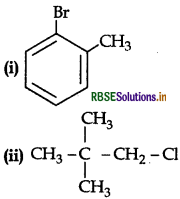
Answer:
(i) 2-Bromotoluene
(ii) 1 chloro, 2, 2 dimethyl propane
Question 35.
Give the IUPAC names of the following compounds:
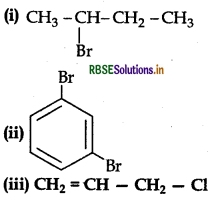
Answer:
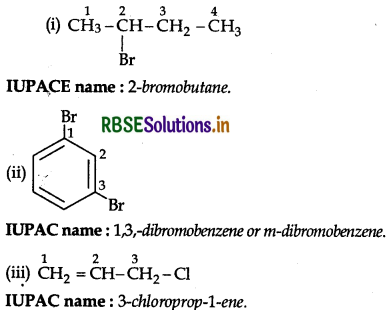
Question 36.
Give reasons:
(i) C - Cl bond length in chlorobenzene is shorter than C-Cl bond length in CH3-Cl.
(ii) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(iii) SN1 reactions are accompanied by racemization in optically active alkyl halides.
Answer:
(i) In C-Cl bond length in chlorobenzene is shorter than in CH3-Cl in chloromethane carbon holding chloride is sp3 hybridized, while in chlorobenzene is sp2 hybridized. The delocalization of electrons by resonance in chlorobenzene attribute for partial double bond character due to which bond length is shorter than a single bond.
(ii) Dipole moment is the product of charge and inter nuclear distance. In case of cyclohexyl chloride carbon- chlorine band length is longer than C-Cl band length in chlorobenzene. Hence dipole moments of cyclohexyl chloride is more.
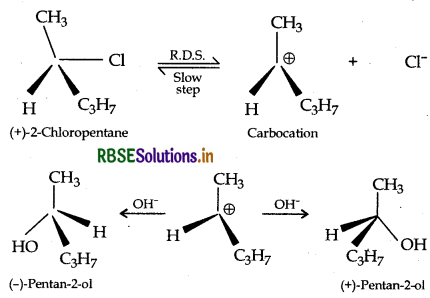
(iii) SN1 reactions are accompanied by racemization in optically active alkyl halides because the nucleophile will have an equal opportunity to attack on sp2 hybridised carbocation from either sides to give a racemic mixture.
Example:
Question 37.
How do you convert:
(i) Chlorobenzene to biphenyl
(ii) Propene to 1-iodopropane
(iii) 2-bromobutane to but-2-ene
Answer:
(i) Chlorobenzene to biphenyl (Fittig reaction)
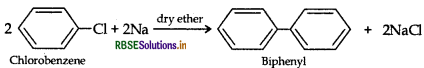
(ii) Propene to 1-iodopropane
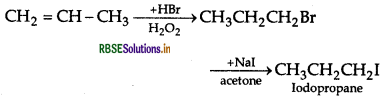
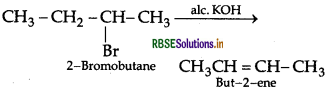
(iii) 2-bromobutane to but-2-ene
Question 38.
Write the major product(s) in the following:
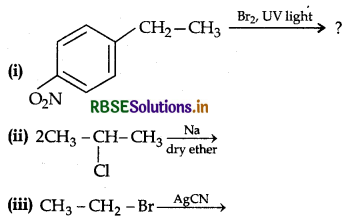
Answer:
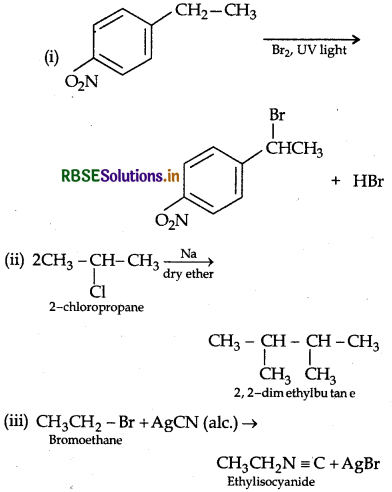

Question 39.
In the following pairs of the halogen compounds which compound undergoes faster SN1 reaction.
(i) CH3 - CH2 - CH2 - CH2 - Cl
(ii) 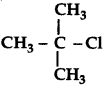
(iii) CH3 - CH2 - Br and CH3 - CH2 - I
Answer:
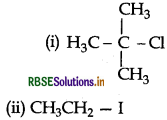
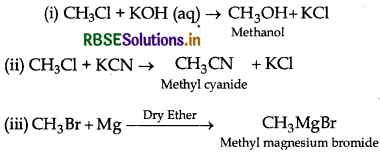
Question 40.
What happens when Cl is treated with aqueous KOH?
(i) CH3 - Cl is treated with aqueous KOH?
(ii) CH3 - Cl is treated with KCN?
(iii) CH3 - Br is treated with Mg in the presence of dry ether?
Answer:
Question 41.
Following compounds are given to you: 2-Bromopentane, 2-Bromo-2-methylbutane, 1-Bromopentane
(i) Write the compound which is most reactive towards SN2 reaction.
(ii) Write the compound which is optically active.
(iii) Write the compound which is most reactive towards β-elimination reaction.
Answer:
(i) 1-Bromopentane is most reactive towards SN2 reaction.
(ii) 2-Bromopentane is optically active.
(iii) 2-Bromo-2-methylbutane is most reactive towards β-elimination reaction.
Question 42.
Write structures of compounds A, B and C in each of the following reactions:
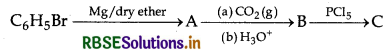
Answer:
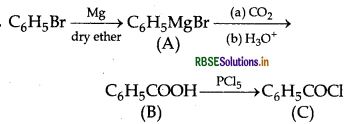
A = Phenylmagnesium bromide
B = Benzoic acid
C = Benzoyl chloride
Question 43.
The following compounds are given to you: 2-Bromopentane, 2-Bromo-2-methylbutane, 1- Bromopentane
(i) Write the compound which is most reactive towards SN2 reaction.
(ii) Write the compound which is optically active.
Answer:
(i) 1-Bromopentane CH3CH2CH2CH2Br is most reactive towards SN2 reaction.
(ii) 2-Bromopentane  is optically active.
is optically active.
(iii) 2-Bromo-2-methylbutane 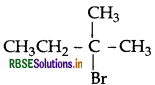 is most reactive towards β-elimination reaction.
is most reactive towards β-elimination reaction.
Question 44.
(a) Write the structural formula of A, B, C and D in the following sequence of reaction:
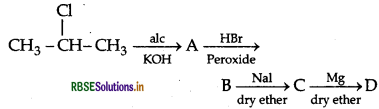
(b) Illustrate Sandmeyer's reaction with the help of a suitable example.
Answer:
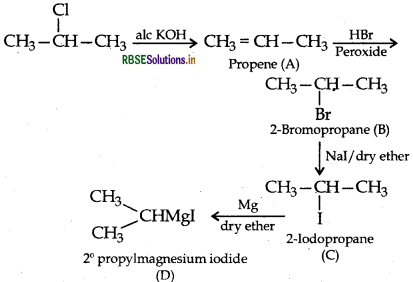
(b) Sandmeyer's reaction: The substitution of diazo group of benzene diazonium chloride by Chloro, Bromo and Cyano group with the help of solution of CuCl dissolved in HCl, CuBr/HBr and CuCN/KCN respectively is known as Sandmeyer's reaction.
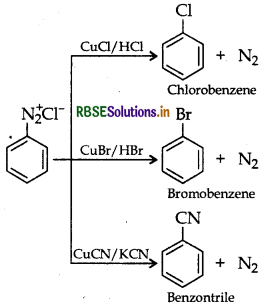
Question 45.
(a) Account for the following:
(i) Electrophilic substitution reactions in haloarenes occur slowly.
(ii) Haloalkanes, though polar, are insoluble in water.
(b) Arrange the following compounds in increasing order of reactivity towards SN2 displacement:
2-Bromo-2-Methylbutane, 1-Bromopentane, 2-Bromopentane.
Answer:
(a) (i) Due to -I effect of halogen atom, it withdraws electrons from the benzene ring and thus ring gets deactivated.
(ii) They fail to form hydrogen bonds with water. More energy is required to break hydrogen bonds in water and ess energy is released when new attractions are set up.
(b) 2-Bromo-2-Methylbutane < 2-Bromopentane < 1- Bromopentane.
Long Answer Type Questions:
Question 1.
(i) o-nitrophenol has lower b.p. than p-nitro-phenol. Explain.
(ii) Write IUPAC name of the following:
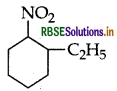
Answer:
(i) Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding whereas p- nitrophenol forms intermolecular H-bonding.
(ii) IUPAC name: 2-ethyl-1-nitrocyclohexane.
COMPETITION CORNER:
Question 1.
In a SN2 substitution reaction of the type:
Which one of the following has the highest reaction rate?
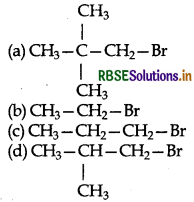
Answer:
(b) CH3 - CH2 - Br
Question 2.
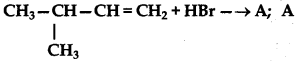
predominantly is:
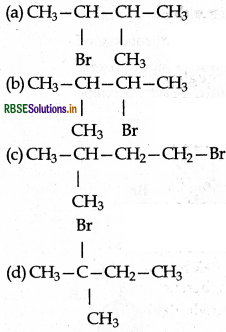
Answer:
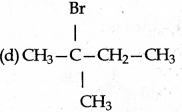
Question 3.
Which among the following is an example of nucleophilic substitution reaction?
Answer:
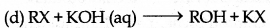

Question 4.
The order of rate of hydrolysis of alkyl halides 1°, 2°, 3° and CH3X by the SN2 pathway is:
(a) 1° > 2° > 3° > CH3X
(b) CH3X > 3° > 2° > 1°
(c) CH3X > 1°> 2° > 3°
(d) 3° > 2° > 1° > CH3X
Answer:
(c) CH3X > 1°> 2° > 3°
Question 5.
The increasing order of hydrolysis of the following compounds is:
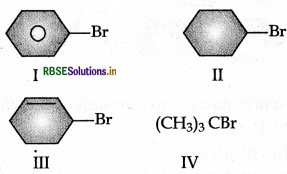
(a) I < IV < II < III
(b) I < II < III < IV
(c) I < II < IV < III
(d) IV < III < II < I
Answer:
(c) I < II < IV < III
Question 6.
The organic chloro compound which shows complete steriochemical inversion during a SN2 reaction is:
(a) CH3Cl
(b) (C2H5)2CHCl
(c) (CH3)3CCl
(d) (CH3)2CHCl
Answer:
(a) CH3Cl
Question 7.
The major product of the following reaction is:
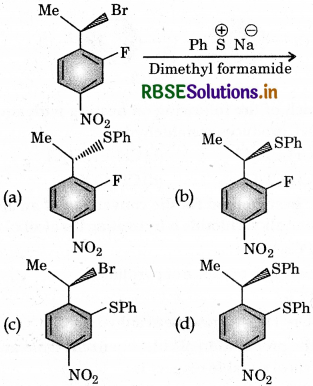
Answer:
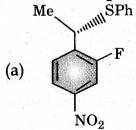
Question 8.
In a SN2 substitution reaction of the type
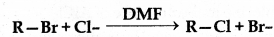
Which of the following has highest relative rate?
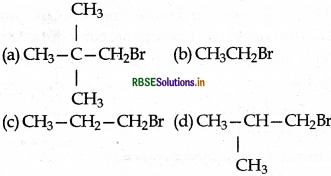
Answer:
(b) CH3CH2Br
Question 9.
Trichloroacetaldehyde, CC3CHO reacts with chloro-benzene in presence of sulphuric acid produces:
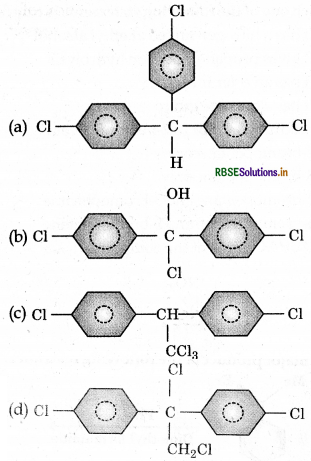
Answer:
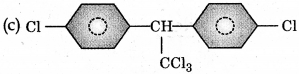
Question 10.
Which of the following on heating with aqueous KOH, produces acetaldehyde?
(a) CH3CH2Cl
(b) CH2ClCH2Cl
(c) CH3CHCl2
(d) CH3COCl
Answer:
(c) CH3CHCl2

Question 11.
The best method for the conversion of an alcohol into an alkyl chloride is by treating the alcohol with:
(a) PCl5
(b) SOCl2 in presence of pyridine
(c) PCl3
(d) Dry HCl in presence of anhyd. ZnCl2
Answer:
(b) SOCl2 in presence of pyridine
Question 12.
In the preparation of chlorobenzene from aniline, the most suitable reagent is:
(a) Chlorine is presence of ultraviolet light
(b) Chlorine in the presence of AlCl3
(c) Nitrous acid followed by heating with Cu2Cl2
(d) HCl and Cu2Cl2
Answer:
(c) Nitrous acid followed by heating with Cu2Cl2
Question 13.
An organic compound on heating with CuO produces CO2 but no water. The organic compound may be:
(a) carbon tetrachloride
(b) methane
(c) ethyl iodide
(d) chloroform
Answer:
(a) carbon tetrachloride
Question 14.
Consider the following reaction:

Which one of the following statements is true for X?
(a) It has a nitrogen linked at ethyl alcohol
(b) It gives propanoic acid on hydrolysis
(c) It has an ester function
(d) It has a cyanide group
Answer:
(a) It has a nitrogen linked at ethyl alcohol
Question 15.
The addition of HBr to 2-pentene gives:
(a) 2-bromopentane only
(b) 3-bromopentane only
(c) 2-bromopentane and 3-bromopenatne
(d) 1-bromopentane and 3-bromopentane
Answer:
(c) 2-bromopentane and 3-bromopenatne
Question 16.
The IUPAC name of the compound:
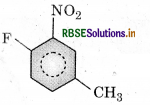
(a) 1-fluoro-4-methyl-2-nitrobenzene
(b) 4-fluoro-1-methyl-3-nitrobenzene
(c) 4-methyl-1-fluoro-2-nitrobenzene
(d) 2-fluoro-5-methyl-1-nitrobenzene
Answer:
(a) 1-fluoro-4-methyl-2-nitrobenzene
Question 17.
In the following reaction
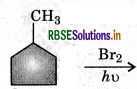
The major product obtained is:
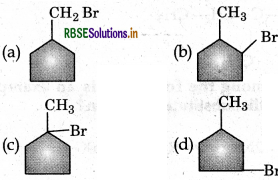
Answer:
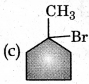
Question 18.
The reaction of toluene with Cl2 in presence of FeCl3 gives X and reaction in presence of light gives Y. Thus X and Y are:
(a) X = benzyl chloride, Y = m-chlorotoluene
(b) X = benzal chloride, Y = o-chlorotoluene
(c) X = m-chlorotoluene, Y = p-chlorotoluene
(d) X = o- and p-chlorotoluene, Y = trichloromethyl benzene
Answer:
(d) X = o- and p-chlorotoluene, Y = trichloromethyl benzene

Question 19.
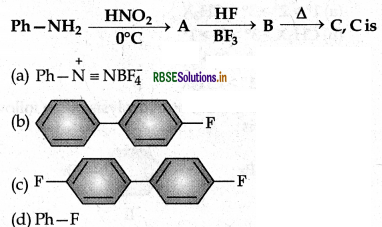
Answer:
(d) Ph - F
Question 20.
Which one is most reactive towards SN1 reaction?
(a) C6H5CH2Br
(b) C6H5CH(C6H5)Br
(c) C6H5CH(CH3)Br
(d) C6H5(CH3) (C6H5)Br
Answer:
(d) C6H5(CH3) (C6H5)Br

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम