RBSE Class 11 Biology Important Questions Chapter 9 Biomolecules
Rajasthan Board RBSE Class 11 Biology Important Questions Chapter 9 Biomolecules Important Questions and Answers.
Rajasthan Board RBSE Solutions for Class 11 Biology in Hindi Medium & English Medium are part of RBSE Solutions for Class 11. Students can also read RBSE Class 11 Biology Important Questions for exam preparation. Students can also go through RBSE Class 11 Biology Notes to understand and remember the concepts easily.
RBSE Class 11 Biology Chapter 9 Important Questions Biomolecules
Multiple Choice Questions
Question 1.
Which one of the following is not a constituent of cell membrane?
(a) Glycolipids
(b) Proline
(c) Phospholipids
(d) Cholesterol
Answer:
(b) Proline

Question 2.
Cytoskeleton is made up of:
(a) Callose deposits
(b) Cellulosic microfibrils
(c) Proteinaceous filament
(d) Calcium carbonate granules
Answer:
(c) Proteinaceous filament
Question 3.
Middle lamella is composed of:
(a) Muramic acid
(b) Calcium pectate
(c) Phosphoglyceride
(d) Hemicellulose
Answer:
(b) Calcium pectate
Question 4.
Peptide synthesis inside a cell take place in:
(a) Nucleus
(b) Mitochondria
(c) Chloroplast
(d) Ribosome
Answer:
(d) Ribosome
Question 5.
DNA is not present in:
(a) Nucleus
(b) Mitochondria
(c) Chloroplast
(d) Ribosome
Answer:
(d) Ribosome
Question 6.
An organic substance bound to an enzyme and essential for its activity is called:
(a) Isoenzyme
(b) Coenzyme
(c) Holoenzyme
(d) Apoenzyme
Answer:
(b) Coenzyme
Question 7.
About 98% of the mass of every living organisms is composed of just six elements including carbon, hydrogen, nitrogen, oxygen and:
(a) sulphur and magnesium
(b) magnesium and sodium
(c) calcium and phosphorus
(d) phosphorus and sulphur
Answer:
(d) phosphorus and sulphur
Question 8.
Modern detergents contain enzyme preparation of:
(a) Thermoacidophiles
(b) Thermophiles
(c) Acidophiles
(d) Alkaphiles
Answer:
(d) Alkaphiles
Question 9.
A competitive inhibitor of succinic dehydrogenase is:
(a) α - ketoglutarate
(b) Malate
(c) Malonate
(d) Oxaloacetate
Answer:
(c) Malonate

Question 10.
Which one is the most abundant protein in the animal world?
(a) Trypsin
(b) Haemoglobin
(c) Collagen
(d) Insulin
Answer:
(c) Collagen
Question 11.
Pyrimidinc of RNA are:
(a) adenine and cytosine
(b) adenine and guanine
(c) thymine and cytoeine
(d) uracil and cytoaine
Answer:
(d) uracil and cytoaine
Question 12.
Nitrogen base do not contain:
(a) carbon
(b) phoaphorus
(c) hydrogen
(d) nitrogen
Answer:
(b) phoaphorus
Question 13.
Framework elemente in plant are:
(a) N, Ca, Mg
(b) C, H, O
(c) Mg, Cu, Fe
(d) C, O, Cu
Answer:
(b) C, H, O
Question 14.
Lysine is an essential amino acid as it is:
(a) very rare
(b) importont constituent of all proteins
(c) with high nutrient value
(d) not formed in the body and has to be provided in diet
Answer:
(d) not formed in the body and has to be provided in diet
Question 15.
WhIch le a dlnucleoglde?
(a) ADP
(b) RNA
(c) NAD
(d) DNA
Answer:
(c) NAD
Question 16.
Nueleotide found free in the cell is:
(a) CAMP
(b) AMP
(c) ADP
(d) ATP
Answer:
(d) ATP
Question 17.
Which ja distributed more widely in a cell?
(a) DNA
(b) RNA
(c) Chloroplast
(d) Sphaeroeome
Answer:
(b) RNA
Question 18.
Most abundant RNA of the cell is:
(a) tRNA
(b) rRNA
(c) mRNA
(d) tRNA
Answer:
(b) rRNA
Question 19.
In vitro synthesis of DNA was carried out by:
(a) Nirenberg
(b) Watson and Crick
(c) Khorana
(d) Kornberg
Answer:
(d) Kornberg
Question 20.
Proteins were named by:
(a) Berzelius
(b) Sanger
(c) Fleming
(d) Dujardin
Answer:
(a) Berzelius

Quesiton 21.
Which of the following is the simplest amino acid?
(a) Glycine
(b) Tyrosine
(c) Alanine
(d) Aparagine
Answer:
(a) Glycine
Question 22.
Which of the following is not an amino acid?
(a) Lysine
(b) Arginine
(c) Thynume
(d) Tryptophan
Answer:
(c) Thynume
Question 23.
Identify the sulphur containing amino acid:
(a) Proline
(b) Tryptophan
(c) Methionine
(d) Aapartic acid
Answer:
(c) Methionine
Question 24.
Choose the correct non protein amino acid:
(a) Cysteine
(b) Hydroxylysine
(c) Hydroxyproline
(d) γ amino butyric acid
Answer:
(d) γ amino butyric acid
Question 25.
Molecules that bear charged groups of opposite polarity are known as:
(a) Cations
(b) Anions
(c) Zwitter ions
(d) Negative ions
Answer:
(c) Zwitter ions
Question 26.
Which of the following is an example of saturated fatty acids?
(a) Stearic acid
(b) Oleic acid
(c) Linoleic acid
(d) Linolenic acid
Answer:
(a) Stearic acid
Question 27.
An essential fatty acid for human is:
(a) Laurie acid
(b) Caproic acid
(c) Palmitic acid
(d) Arachidonic acid
Answer:
(d) Arachidonic acid
Quesiton 28.
The maximum number of double bonds present in essential fatty acids is:
(a) 4
(b) 2
(c) 1
(d) 5
Answer:
(a) 4
Question 29.
Arachidonic acid is:
(a) Non essential fatty acid
(b) Essential fatty acid
(c) Polyunsaturated fatty acid
(d) Both (b) and (c)
Answer:
(d) Both (b) and (c)
Question 30.
Which one is the sweetest sugar?
(a) Sucrose
(b) Glucose
(c) Fructose
(d) Maltose
Answer:
(c) Fructose
Question 31.
Glycogen is a homopolymer made of:
(a) Ribose units
(b) Amino acids
(c) Galactose units
(d) Glucose units
Answer:
(d) Glucose units
Question 32.
What is the substrate for lipase enzyme?
(a) Lipid
(b) Protein
(c) Nucleic acid
(d) Carbohydrate
Answer:
(a) Lipid
Question 33.
Enzymes are biocatalysts. They catalyse biochemical reactions. In general they reduce activation energy of reactions. Many physio-chemical processes are enzyme mediated. Some examples of enzyme mediated reactions are given below. Tick the wrong entry.
(a) Hydrolysis of sucrose
(b) Dissolving CO2 in water
(c) Formation of peptide bond
(d) Untwining the two strands of DNA
Answer:
(c) Formation of peptide bond
Question 34.
A phosphoglyceride is always made of:
(a) Only an unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
(b) A saturated and unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
(c) A saturated or unsaturated fatty acid esterified to a phosphate group which is also attached to a glycerol molecule
(d) Only a saturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached
Answer:
(b) A saturated and unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
Question 35.
The most abundant intracellular cation is:
(a) K+
(b) H+
(c) Na+
(d) Ca++
Answer:
(a) K+
Quesiton 36.
Which of the metabolites is common to respiration mediated breakdown of fats, carbohydrates and proteins:
(a) Pyruvic acid
(b) Acetyl Co - A
(c) Glucose - 6 - phosphate
(d) Fructose 1, 6 - biphosphate
Answer:
(b) Acetyl Co - A

Quesiton 37.
Macromolecule chitin is:
(a) Simple polysaccharide
(b) Sulphur containing polysaccharide
(c) Nitrogen containing polysaccharide
(d) Phosphorus containing polysaccharide
Answer:
(c) Nitrogen containing polysaccharide
Question 38.
Transition state structure of the substrate formed during an enzymatic reaction is:
(a) Transient but stable
(b) permanent and stable
(c) Transient and unstable
(d) Permanent but unstable
Answer:
(c) Transient and unstable
Question 39.
Which of the following hormone is a derivative of fatty acid?
(a) Gastrin
(b) Thyroxin
(c) Estrogen
(d) Prostaglandin
Answer:
(d) Prostaglandin
Question 40.
Which one of the following is enriched with non - reducing sugar?
(a) Grapes
(b) Table sugar
(c) Mother’s milk
(d) Germinating barley’s grains
Answer:
(b) Table sugar
Question 41.
Glutenin is an important protein in:
(a) Potato
(b) Wheat
(c) Soyabean
(d) Spinach
Answer:
(b) Wheat
Question 42.
One molecule of triglyceride is produced using:
(a) One fatty acid and one glycerol
(b) One fatty acid and three glycerols
(c) Three fatty acids and three glycerols
(d) Three fatty acids and one glycerol
Answer:
(d) Three fatty acids and one glycerol
Question 43.
Which of the following statement is wrong for sucrose:
(a) It is a disaccharide
(b) It is a non - reducing sugar
(c) It accumulates in the cytoplasm
(d) It is comprised of maltose and fructose
Answer:
(d) It is comprised of maltose and fructose
Question 44.
Which structural level enables the protein to function as enzymes?
(a) Primary
(b) Secondary
(c) Tertiary
(d) Quaternary
Answer:
(c) Tertiary
Question 45.
A typical fat molecule is made up of:
(a) One glycerol and one fatty acid molecule
(b) Three glycerol and three fatty acid molecules
(c) One glycerol and three fatty acid molecule
(d) Three glycerol molecules and one fatty acid molecule
Answer:
(c) One glycerol and three fatty acid molecule
Question 46.
The amino acid tryptophan is the precursor for the synthesis of:
(a) Cortisol and cortisone
(b) Melatonin and serotonin
(c) Estrogen and progesterone
(d) Thyroxine and triiodothyronine.
Answer:
(b) Melatonin and serotonin
Question 47.
Which one of the following statement is wrong?
(a) Uracil is a pyrimidine
(b) Sucrose is a disaccharide
(c) Cellulose is a polysaccharide
(d) Glycine is a sulphur containing amino acid
Answer:
(d) Glycine is a sulphur containing amino acid
Question 48.
A non proteinaceous enzyme is:
(a) Ligase
(b) Lysozyme
(c) Ribozyme
(d) Deoxyribonuclease
Answer:
(c) Ribozyme
Question 49.
Which of the following is the least likely to be involved in stabilizing the three dimensional folding of most proteins?
(a) Ester bonds
(b) Hydrogen bonds
(c) Electrostatic interaction
(d) Hydrophobic interaction
Answer:
(a) Ester bonds
Question 50.
Transition state structure of the substrate formed during an enzymatic reaction is:
(a) Permanent but unstable
(b) Transient and unstable
(c) Permanent and stable
(d) Transient but stable
Answer:
(b) Transient and unstable
Very Short Answer Type Questions
Question 1.
Which is the important energy carrier in the cell?
Answer:
Adenosine triphosphate (ATP).
Question 2.
Name the monomer subunits which form nucleic acids?
Answer:
Nucleotide.
Question 3.
What are micromolecules? Give example.
Answer:
Large complex organic compound present in living system formed by polymerisation of micromolecules, e.g., protein (polymer of amino acid).

Question 4.
What are unimeric protein molecules?
Answer:
Unimeric protein molecules contain only one polypeptide chain.
Question 5.
Who proposed lock and key hypothesis of enzyme action?
Answer:
Emil Fischer (1894).
Question 6.
Who proposed induced fit model of enzyme action?
Answer:
DanielKoshland (1960).
Question 7.
What is chitin?
Answer:
It is a structural polysaccharide like the cellulose.
Question 8.
Name two hexose sugars.
Answer:
Glucose, Fructose.
Question 9.
Name a muco polysaccharide obtained from marine algae.
Answer:
Agar - Agar.
Question 10.
What are conjugated lipids?
Answer:
These are esters of fatty acids with alcohol which contain a phosphate, carbohydrate or protein group also.
Question 11.
Name the polymer which form the exoskeleton of insects.
Answer:
Chitin.
Question 12.
Name the following:
(i) Sugar present in DNA
(ii) Base not found in DNA.
Answer:
(i) Deoxyribose
(ii) Uracil.
Question 13.
Name the abundant proteins in biosphere.
Answer:
RUBISCO.
Question 14.
Lipids are not biomacromolecules. Why?
Answer:
Because their molecular weight does not exceed 800.
Question 15.
Which lipid can cause heart ailment?
Answer:
Cholesterol.
Quesiton 16.
Why do oils generally remain in liquid state even in winters?
Answer:
Oils are unsaturated lipids, hence have lower melting points.
Question 17.
Name an element found in proteins but not in lipid and carbohydrates.
Answer:
Nitrogen.
Question 18.
What does an enzyme do in terms of energy requirement of a reaction?
Answer:
Enzyme lowers the activation energy of reaction.
Question 19.
What is the function of ATP in cell metabolism?
Answer:
ATP is the energy currency of the cell.
Question 20.
Name the protein which form intercellular ground substance.
Answer:
Collagen.
Question 21.
What is the difference between DNA and RNA in term of nitrogenous bases?
Answer:
RNA has uracil instead of thymine.
Question 22.
Who proposed double helix model of DNA?
Answer:
J.D. Watson and F.H.C. Crick.

Question 23.
What is Chargaff rule?
Answer:
A + G = T + C that means purines and pyrimidines are present in equal amount in DNA.
Short Answer Type Questions - I
Question 1.
How are glycosidic bonds formed?
Answer:
The glycosidic or ketone group of a monosaccharide can react and bind with an alcoholic group of another organic compound to join the two compounds together. This bond is known as glycosidic bond.
Question 2.
What do you mean by steady state?
Answer:
An open system always remains in steady state i. e., the rate of input of energy and matter is always equal to the output of energy and matter.
Question 3.
Why are enzymes called biocatalyst?
Answer:
The substances which changes the rate of chemical reaction without altering the equilibrium point of reaction is called catalyst. The catalysts of the organism are called enzyme and they are synthesized in the living cell, hence, called as biocatalysts.
Question 4.
Give the function of carbohydrates.
Answer:
- They play a role in metabolic reactions.
- Ribose and deoxyribose are found in nucleic acids.
- Glucose is oxidised during respiration to liberate energy.
- Glucose is used in synthesis of fats as well as proteins.
Question 5.
What do you meant by activation energy?
Answer:
It is the energy required to initiate a chemical or biochemical reaction. Activation energy overcomes the energy barriers of the reactants which occurs amongst the reactants due to
- presence of electrons over their surface
- absence of precise and forceful collision essential for bringing the reactive sites of the chemical together.
Short Answer Type Questions - II
Question 1.
Write the difference between nucleotides and nucleosides.
Answer:
|
Nucleotide |
Nucleoside |
|
1. Nucleotide is made up of nitrogenous bases sugar and phosphoric acid. |
1. Nucleoside is formed of nitrogenous base and pentose sugar only. |
|
2. These are acidic in nature. |
2. These are basic in nature. |
|
3. These are monomers of nucleic acids. |
3. These are precursors of nucleotides. |
Question 2.
Enumerate the functions of lipids.
Answer:
- Most of the plants and animals fats constitute storage compound. Fat is stored mainly in adipose cells in the animals.
- In oil seed plants, oil provides nourishment to developing embryo during seed germination. Oil extracted from these seeds is used in cooking.
- Fats provide energy to the body.
- Fats serve as insulator and protect body from cold. It gets deposited beneath the skin.
- Phospholipid form an structural component of all biomembranes in cell.
- Cholesterol acts as precursor for synthesis of various hormones, vitamins and bile salts.
- The lipid form the white matter, grey matter of brain and myelin sheath of neurons.

Question 3.
Describe the structure and function of ATP.
Answer:
- ATP is primary and universal carrier of chemical energy in the cell.
- The living cell capture, store and transport energy in the form of ATP.
- The ATP molecule consists of a nitrogenous base adenine, a pentose sugar (ribose) and three inorganic phosphate molecules.
- Two phosphate bonds are high energy bonds and one is relatively poor in energy.
- Energy released in living cell is thus stored in the chemical bonds of the ATP molecule which than serve as major energy yielding and energy requiring substance in the cell.
Quesiton 4.
Differentiate between cofactors, coenzymes and prosthetic groups.
Answer:
|
Cofactors |
Co - enzymes |
Prosthetic groups |
|
1. It is a non protein group which is attached to an enzyme. |
1. It is non protein organic cofactor loosely attached with enzyme. |
1. It is a non protein organic co - factor that is tightly held with enzyme. |
|
2. It may be organic or inorganic or metallic factor. |
2. NAD is co - enzyme for dehydrogenase. |
2. Some prosthetic group have porphyrin of the cytochrome. |
Question 5.
Discuss the lock and key hypothesis of enzyme action.
Answer:
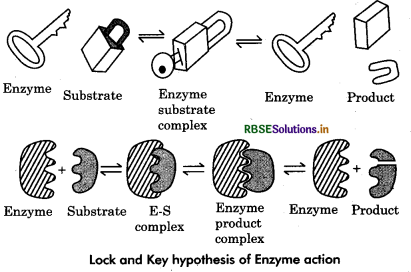
This hypothesis was given by Emil fischer in 1894. According to this hypothesis, both the substrate and an enzyme have specific geometric shapes that fit exactly into each other and form enzyme : substrate complex (E.S. complex). The substrate molecules undergo chemical change and form the products. A temporary enzyme product complex is formed. However as the products have a different configuration than the active site, the later does not hold them. Consequently the products are released. The empty active site of the enzyme attracts and binds new substrate molecules.
Question 6.
How do inorganic catalysts differ with that of enzymes.
Answer:
- Catalysts are simple, small mineral ions while the enzymes are made up of proteins that are highly complex having three dimensional shape.
- The activity of catalysts can not be controlled by regulators while enzyme activity can be controlled by use of certain regulators which can change the conformation of protein molecules.
- Catalysts are not affected much by factors like pH and temperature while the enzymes are highly sensitive to change in pH and temperature conditions.
- Catalysts can catalyse diverse reactions while enzymes act on specific reaction only.
Question 7.
Discuss in brief the chemical structure of DNA.
Answer:
- Deoxyribonucleic acid (DNA) is the polymer of deoxyribonucleotides.
- A deoxyribonucleotide molecule is made up of one molecule of deoxyribose sugar, a nitrogenous base (out of ATGC) and phosphoric acid component.
- Many deoxyribonucleotide join each other by phosphodiester bond to form a polynucleotide chain of DNA.
- A polynucleotide chain has 3' end and 5' end.
- In DNA two polynucleotide chain run antiparallel to form a helical structure.
- Both polynucleotide chains are complementary to each other as their nitrogenous bases are held together by hydrogen bonds.
- Adenine (A) pairs with Thymine (T) by double bond while Guanine (G) pairs with cytosine (C) with triple bond (A = T, G = C).
Question 8.
What are proenzymes? Give an example of proenzyme.
Answer:
Proenzymes are also called zymogens. These are inactive precursor of an enzyme. A zymogen requires a biochemical change to become in its active form. They are converted into their active form by certain factors like PH, substrate, enzyme primer etc.
Example: Pepsinogen is a proenzyme which is converted in its active form pepsin by HCl.
Long Answer Type Questions
Question 1.
What are primary and secondary metabolites? Discuss the different types of secondary metabolites useful to human welfare.
Answer:
Thousands of organic biomolecules of a cell like monosaccharides, disaccharides, oligosaccharides, fatty acids, glycerol, lipids, nitrogenous bases, nucleotides etc., are constantly utilized in various metabolic (anabolic and catabolic) reactions occuring in the cells. Therefore these are called as metabolites. In animal tissues, one notices the presence of all such categories of compounds. These are called as primary metabolites. However, when one analyses plant, fungal and microbial cells, one would see thousands of compounds other than these primary metabolites. Some prominent ones are alkaloids, flavonoids, rubber, essential oils, antibiotics, coloured pigments, scents, gums, spices. These are called secondary metabolites.
Primary metabolites have identifiable functions and these play known roles in normal physiological processes. On the other hand, role or functions of all the secondary metabolites in host organisms are not properly understood at present. Many of them are useful to human welfare, e.g., rubber, drugs, spices, scents and pigments. Some secondary metabolites have ecological importance.

Question 2.
Discuss the structure of maltose, lactose and sucrose given with suitable diagrams. Write their functions also.
Answer:
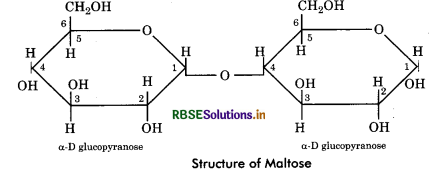
Maltose: It is simplest of all disaccharides. It is made up of two molecules of glucose held together 1 - 4 carbon atoms. It is formed during hydrolysis of starch (in
germinating starchy seeds).
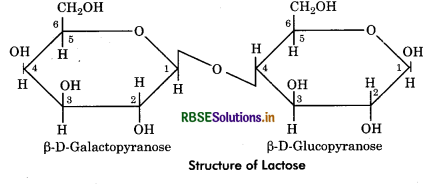
Lactose: It is composed of D - glucose and D - galactose units. It is also called milk sugar and constitute 5% of mammalian milk. The glycosidic bond is ß (1 → 4) linkage as galactose occurs in p state.
Sucrose: It is also called cane sugar or table sugar obtained from stems of sugarcane or beetroot. Sucrose is formed by condensation of one molecule of D - glucose (α - pyranose form) and D - fructose (ß - furanose form). The union is between aldehyde group of glucose (at carbon 1) and keto group of fructose (at carbon 2). Thus, sucrose does not possess free aldehyde or ketone group, hence it is a non reducing sugar.
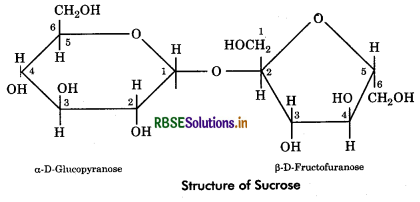
Quesiton 3.
Differentiate the structural polysaccharides and storage polysaccharides giving suitable examples. How they differ in their structures?
Answer:
Storage Polysaccharides: These types of polysaccharides may serve as food storage in plants (starch) and animals (glycogen).
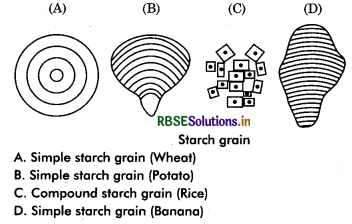
Starch: It is a polymer of glucose units linked together in α - 1, 4 glycosidic linkage. This bond can be broken by enzyme thus starch can be digested. Starch is formed during photosynthesis in green plants and stores energy. Starch occurs abundantly in rice, wheat, legume, potato, tapioca, banana etc. Molecular weight of starch may be 5000 to 106. A starch grain is made up of several layers. The shells arranged in concentric or ecentric fashion around a point called hilum. When a grain contains only one hilum, it is called simple starch grains e.g, Maize and in those grains where more than one hilum are present, called compound starch
grains, e.g., potato, rice.
Inulin: It is the polymer of fructose. It occurs as a reserve food present in roots, tubers and rhizomes of many composites e.g., Dahlia, Dandelion etc. Inulin is also called dahlia starch. Inulin is formed by ß (2 → 1) glycosidic linkages between fructofuranose units. It is soluble in water. Colour reaction to iodine in absent in human body, does not has enzyme to metabolise inulin. Inulin is filtered out through kidneys. It is used for determining the rate of glomerular filtration.
Glycogen: It is a branched polymer of glucose. It consists of α - D glucose units, mostly linked 1 - 4, but highly branched via frequent 1 - 6 linkages, each of which starts other α - 1, 4 chain of some 8 - 12 glucose units. Glycogen is
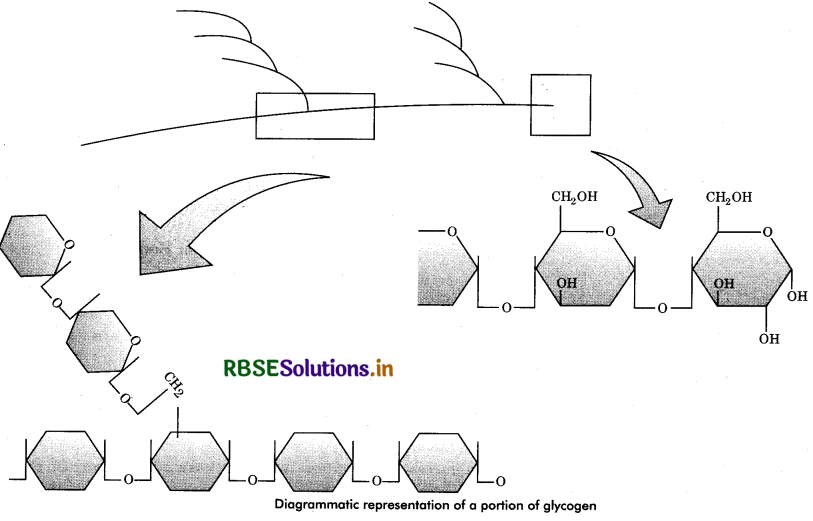
found mostly in muscles and livers of animals and it is also called animal starch. The glycogen molecule is long, much branched chain of about 30,000 glucose units with molecular weight of about 4,000,000. It gives red colour with iodine solution.
Advantages of Storing Carbohydrates
- Glycogen and starch serve as depot of glucose.
- During their synthesis, many molecules of water are removed from monosaccharides as condensation of bulk to stored occurs. So they give the cell a way of storing important food molecule in a form that is compact yet accessible to enzyme.
- Polysaccharides can be stored easily as compared to monosaccharides.
- Glycogen and starch are more flexible and soluble as compared to its cellulose counterpart.
Structural Polysaccharides: These are fibrous polysaccharides that are employed in the formation of cell walls of plants, fungi and exoskeleton of arthropods. e.g., cellulose, chitin etc.
Cellulose: Cellulose is linear polymer of glucose. Cellulose is ß - 1, 4 polymer of glucose whereas the starches are basically α - 1, 4 polymers. It is unbranched, linear and the molecules are folded in such a way so as to form long, highly tensile fibres that are aggregated into bundles called microfibrils. The molecules in a microfibril are held together by hydrogen bonds. Cellulose is made up of 5000 to 20000 glucose units with molecular weight 0.5 to 2.5 million. Cellulose is made up of 100 - 200 parallel chains with few of its hydroxyls exposed to the surrounding solvent. This type of structure is responsible for insolubility and rigidity of cellulose.
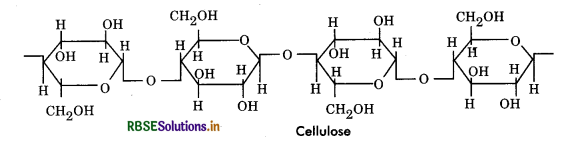
Importance of Cellulose
- Cellulose form more than 50% of organic carbon of plants.
- Cotton fibres contain more than 95% cellulose.
- Wood has 25 to 50% cellulose. It also contains hemicellulose and lignin.
- Cotton and linen fibres are almost pure cellulose. They are being used in preparation of textiles since prehistoric times.
- Cellulose is important component of diet of ruminants and other herbivores.
Chitin: It is a structural heteropolysaccharide (considered homopolysaccharide by most biochemists) found in cell walls of fungi. It is a long chain linear, unbranched polymer consisting of repeated units of N - acetyl - glucosamine, which is monosaccharide derivative of glucose. These units form covalent ß - 1, 4 linkages. Chitin with the chemical formula (C8H1305N)n is considered as a complex carbohydrate whose structure resembles that of cellulose, with one hydroxyl group on each monomer replaced with an acetyl amine group.
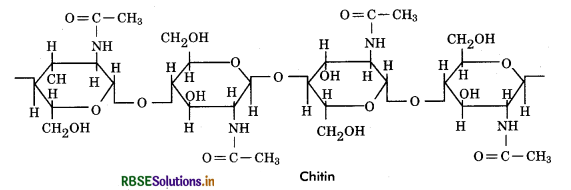
Functions
- Chitin forms hard exoskeleton of insects and protect the inner soft tissues from injury.
- The fungal cell wall that protects the microorganisms from the outside environment is also made up of chitin.
- Chitin is also used to make fertilizer. Such fertilizers are organic, non toxic and have shown to increase crop productivity.
- Chitin is also used for manufacturing strong and flexible surgical threads. Quite a few dissolvable stitches used to close wounds are made from chitin.

Question 4.
What are lipids? Why are they not considered as macromolecule? Discuss the simple and compound lipids giving the examples.
Answer:
Fats and their derivatives are together known as lipids. These are heterogenous group. Generally they are made up of carbon, hydrogen and very less amount of oxygen compared than two other elements. The term lipid was first used by Bloor (1943). They form about 3.5 percent of cell content. They are insoluble in water but soluble in organic solvents such as chloroform, acetone, alcohol etc. These include fat (true fats or glycerides), oils and waxes. Many substances of daily use such as cooking oil, rubber, cholesterol, waxes are either lipids or rich in lipids. Other common substances where lipids present are plasma membrane, lycopene (plant pigment), vitamin A, E, K, menthol and eucalyptus oil.
Classification of Lipids: Broadly lipids can be classified into following three categories:
- Simple lipids
- Compound or conjugated lipids
- Derived lipids
These are esters of fatty acids with various alcohols. They are of two subtypes:
- Neutral or true fats
- Waxes.
1. Neutral or True Fats: They are esters of fatty acids with glycerol.
Fatty Acids: Fatty acids are organic molecules made up of unbranched long hydrocarbon chain which contain a terminal carboxyl group (—COOH). Acetic acid (CH3COOH) is a simple fatty acid and stearic acid CH3(CH2)16 COOH is more complex.
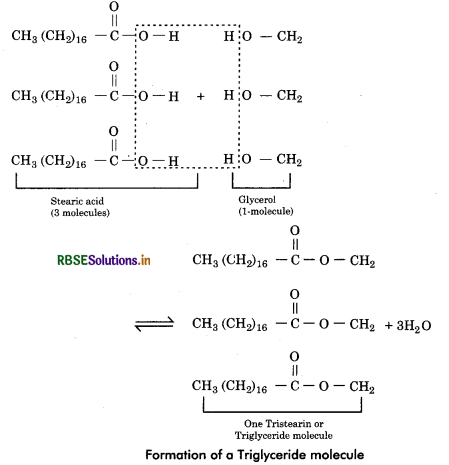
There are two types of fatty acids - saturated and unsaturated.
Saturated fatty acids: The fatty acids in which all carbon atoms are interlinked by single bonds. They do not have double bond between any carbon atoms of the molecular chain. They can not take up more hydrogen, hence are saturated. They have straight chain structure. They have higher melting points and are solid at normal temperature. They include palmitic acid (C16H32O2) and stearic acid (C18H36O2).
Unsaturated fatty acids: The fatty acids in which two adjacent carbon atoms at one or more places lack hydrogen atoms and are interlinked by double bonds (C = C). They can take up additional hydrogen hence are unsaturated. They have higher melting points and are liquid at normal temperature. They include oleic acid (C18H34O2), arachidonic acid (C2O H32O2).
Glycerol: A glycerol molecule has 3 carbons, each having a hydroxyl group (—OH).
Formation of fat: When the hydroxyl group of glycerol molecule join with the carboxyl group of 1 or 2 fatty acid molecules, these are called monoglycerides and diglycerides respectively. But these are not common fats. Generally, three hydroxyl groups of a glycerol molecules to form a fat or triglyceride molecule. The chemical linkage between the glycerol and fatty acids is called the ester bond. As a result three molecules of water get eliminated (condensation or dehydration).
Conjugated or compound lipids are the esters of glycerol with two saturated or unsaturated fatty acids along with some other compound like amino acid, protein, carbohydrate etc. These include phospholipids, glycolipids, sulpholipids, lipoprotein etc.
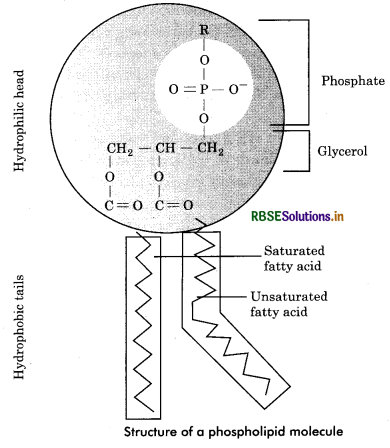
Phospholipids : The major phospholipids are esters of glycerol and a mixture of fatty acids and phosphoric acid. They usually take part in the formation of structural components of cell. In such cases, phosphate group is joined to one of glycerol’s outer —OH groups and two fatty acids are linked with other two —OH groups. A nitrogen containing base such as choline may be bound form to phosphate group. A phospholipid molecule is bipolar. Its two long fatty acid (tails) represent the hydrophobic end while the phosphate containing end is hydrophilic end. (Fig. 9.15). The hydrophilic represents the polar groups and hydrophobic represents the non - polar groups. Phospholipids Contain a phosphorus atom in addition to glycerol, fatty acid and a nitrogenous base. They are of following types:
(a) Phosphatldic acids: These are lipids which on hydrolysis give rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids of which one is saturated and other one is unsaturated.
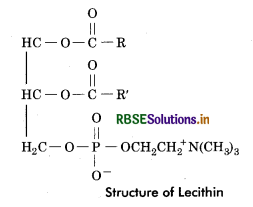
(b) Lecithin: These lipids on hydrolysis give rise to fatty acids, phosphoric acid, glycerol and nitrogenous base choline.
(c) Cephalins: Cephalins are the lipids which on hydrolysis give rise to fatty acids, glycerol, phosphoric acid and either the base ethanolamine (choline) or the amino acid serine.
(d) Plasma logens: These lipids on hydrolysis give rise to one molecule each of aliphatic aldehyde, fatty acid, glycerol, phosphoric acid and nitrogen containing base (choline).
(e) Sphingomyelins: These on hydrolysis give rise to one molecule of fatty acid, phosphoric acid, a nitrogenous base sphinosine and choline but no glycerol.
Question 5.
Discuss the primary, secondary, tertiary and quaternary structure of proteins.
Answer:
The proteins have four different levels of organisation: primary, secondary, tertiary and quaternary.
1. Primary Structure: The primary structure (1°) of protein refers to the linear sequence of amino acids present in the polypeptide chain. Amino acids are covalently linked by peptide bonds or covalent bonds. Each component amino acid in a polypeptide is called a ‘residue’ or ‘moity’. e.g., an enzyme ribonuclease is a protein with primary structure.
2. Secondary Structure: When hydrogen bonds are formed between the amino acids of polypeptide chains for the protection of their peptide bond, it gives a secondary structure (2°). Pauling and Corey studied the secondary structure and proposed two conformations - a - helix and p sheets.
Alpha helix: It is a right handed spiral structure in which the amino acids are located in such a manner that their side chains extend outward from the spiral. The helical structure is stabilized by a series of regularly spaced intramolecular hydrogen bonds formed between the carboxyl (—CO) and amino (—NH) groups of the amino acid units in the successive turns of the spiral. It is found in keratin protein (hair) and myosin proteins (muscles).
Beta pleated sheet: It is formed when two or more polypeptides line up side by side. Straight hydrogen bonds occur between —NH— group of one polypeptide and carbonyl (—CO—) group of adjacent polypeptide. Cross linkage help in stabilisation of ß - pleated sheet. The fibroin, a protein in silk fibres produced by insects and spider, has pleated structure. The protein lysozyme occurs in both helical and pleated forms.
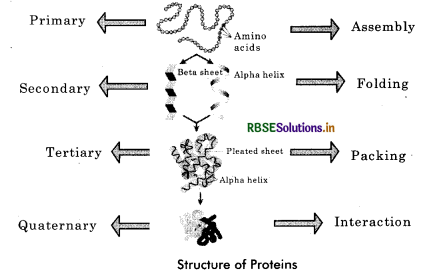
3. Tertiary Structure: Tertiary structure (3°) is based on various types of interactions between the side chains of the polypeptide. Thus, tertiary structure is bending and folding of secondary strand of polypeptide (α - helix or ß pleated sheet) in such a way as to form a compact structure with functional sites being established over its surface by coming together of polar regions of specific amino acids. Hydrophilic parts of amino acids generally pass into the interior of the protein. The tertiary structure is maintained by weak bonds such as hydrogen, ionic, disulphide and hydrophilic - hydrophobic bonds formed between one part of a polypeptide and another. The biological activity of a protein molecule depends largely on the specific tertiary structure. Tertiary structure is found in globular proteins. Tertiary structure is absolutely necessary for the many biological activities of proteins.
4. Quaternary Structure: The quaternary structure (4°) of protein involves the clustering of several individual peptide or protein chain into a final specific shape. A variety of bonding interactions including hydrogen bonding, salt bridges and disulphide bonds hold the various chains into a particular geometry. There are two kinds of quaternary structure occuring in proteins.
Homodimer: When association between identical polypeptide chains takes place.
Heterodimer: When interaction between subunits of very different structure occurs. The interaction between multisubunits are the same as that found in tertiary and secondary structures. Example: Collagen has three helical polypeptides supercoiled to form a rope - like structure of great strength. Haemoglobin has four helical polypeptide chains, two α - chains and two ß - chains.

Question 6.
Discuss the structure of DNA giving labelled diagram.
Answer:
DNA is the genetic material. It has many genetic information for the protein synthesis of a cell in coded form. However some viruses have RNA as genetic material (called retroviruses).
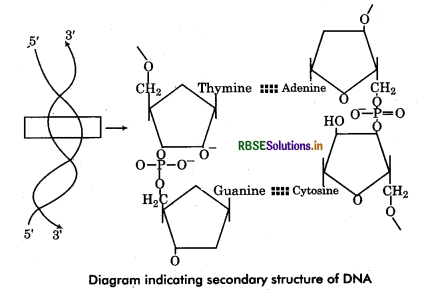
Chemical Structure of DNA: DNA has two long chains of deoxyribonucleotides (some viruses has single chain DNA). Both chains are twisted around a common axis to form a right handed double helix (spiral) structure. Arrangement of Deoxyribonucleotides in DNA : In each chain of DNA, the phosphate group of one nucleotide (at carbon 5' of pentose sugar) is joined with hydroxyl component of next nucleotide (at carbon 3” of pentose sugar). Thus the alternating sugar-phosphate- sugar components form the ‘back bones’ of the DNA double helix. The phosphate group provide acidity to the nucleic acid. The nitrogenous base molecules are joined to the pentose sugar at carbon V by glycosidic bond and project into the space enclosed in the helix. Both chains are held together by hydrogen bonds between nitrogen bases of respective chains.
Complementary base pairing : The polynucleotide chains show polarity (direction). In each chain, the last deoxyribose unit has a free 5' carbon atom so termed as 5' end while at another end the 3' carbon atom remains free so called 3 end. Both the chains are complementary to each other and run antiparallel (opposite) direction that means if a chain has 5' end at one terminal, its complementary chain will has 3' end at same terminal. The complementarity of nucleotide chains is due to the restriction of specific base pairing of both chains. The adenine of one chain is always joined to thymine of the other chain by 2 hydrogen bonds (A = T). Cytosine of one chain is always linked to guanine of the other chain by 3 hydrogen bonds (C = G). Since each base pair has joining of a pyrmidine base and a purine base therefore.
A-T base pairs equal in number to the G-C base pairs in molecule i. e. ,(A + G = T + C). It is called Chargaff rule a DNA molecules. In other words purines and of base pairing, pyrmidines are always in equal amount in a DNA.
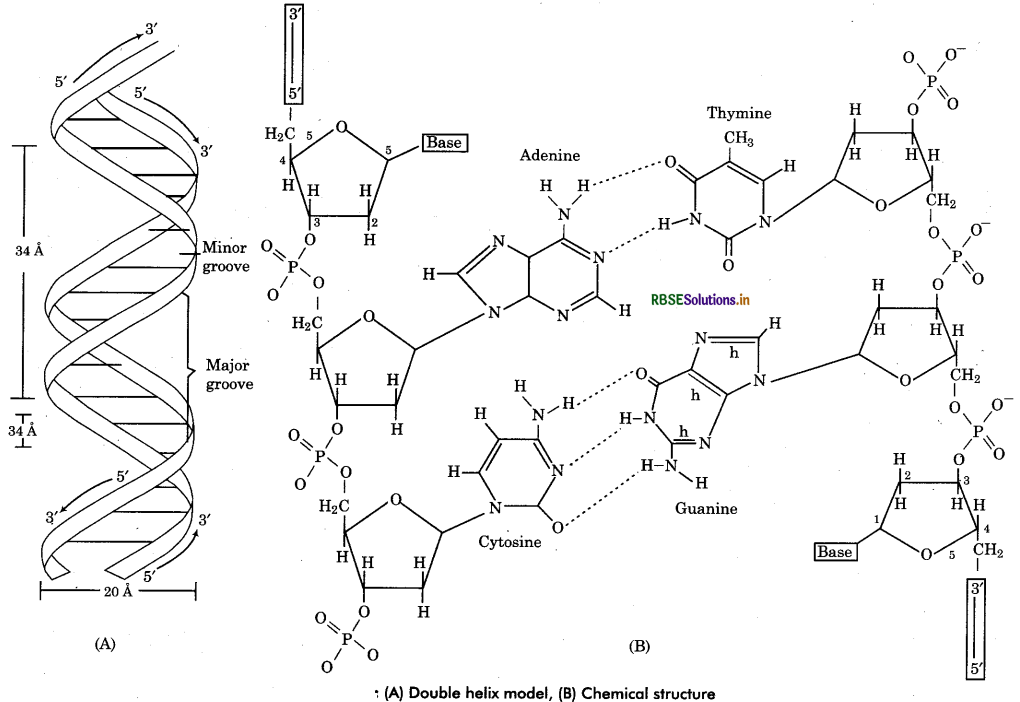
Question 7.
What are enzymes? How do enzymes speed up the rate of a chemical reaction?
Answer:
In 1878, German physiologist “Wilhelm Kunne’ first used the term enzyme. Edvard Buchner extract the enzyme from yeast cell and named it zymase. They are organic catalyst produced in plants and animals. So they are capable to increasing the rate of chemical reaction. In a chemical process, the rate refers to the amount of product formed per unit time. It can be expressed as
\(\text { rate }=\frac{\delta P}{\delta t}\)
A catalysed reaction proceeds at a great higher rate than that of uncatalysed reaction.
For example :
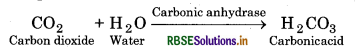
In the absence of any enzyme this reaction is very slow, with about 200 molecules of H 2 C03 being formed in an hour. However, by using the enzyme present within the cytoplasm called carbonic anhydrase, the reaction speeds dramatically with about 6,00,000 molecules being formed every second. Thus, the enzyme has accelerated the rate of reaction by about 10 million times. There are thousand of types of enzymes each catalysing a unique chemical or metabolic reaction. Thus, we can define the enzyme as under:
“An enzyme is a protein that enhances the rate of biochemical reaction in living beings.”
The enzymes speed up the rate of reaction by lowering the activation energy of substrate. They form enzyme substrate complex. This binding lowers the activation energy. (The energy which is required to overcome energy barrier of the reactants and make them reactive to start a chemical reaction). Enzymes bring down the energy barrier between a substrate and the product making the reaction easy and fast. Lower the activation energy higher will be the rate of reaction and higher the activation energy lower will be the rate of reaction. Enzymes generally lower the requirement of activation energy by:
- Bring the reactants together so they don’t have to expend energy moving about until they collide at random.
- Stabilizing the transition state (substrate + enzyme complex) of a reaction.
- Providing an alternative reaction pathway for a biological reaction.
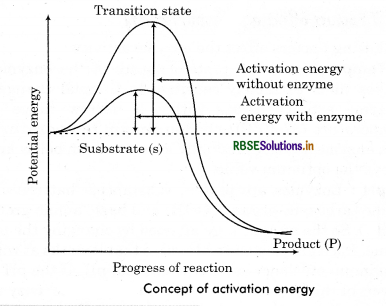
Question 8.
Discuss the various types of mechanisms of inhibition of enzyme actions.
Answer:
The reduction of an enzyme process due to some chemical substances that can check the enzyme activity by blocking or change their active sites. Any substance that can diminish the velocity of an enzyme catalyzed reaction is called an inhibitor and the process is called inhibition. Inhibition of enzyme may be of following types :
1. Competitive Inhibition: In this mechanism, the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme. Due to its close structural similarity with the substrate, the inhibitor competes with the substrate for substrate binding site of the enzyme. Consequently, the substrate cannot bind and as a result, the enzyme action declines. Example: Inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure. Such competitive inhibitors are often used in the control of bacterial pathogens.
2. Non - competitive inhibition: Sometimes, there is no competition for active sites of enzyme by inhibitor and it fixes itself with some other site of enzyme. As a result, the physical structure of enzyme is altered. This fails to form enzyme substrate complex and reaction fails to occur. For example inhibition of cytochrome oxidase by cyanide poison.
3. Allosteric Inhibition: It is reversible non-competitive inhibition that occurs in case of allosteric enzymes. The inhibitors are generally products or intermediates of reactions catalysed by the enzymes. Therefore allosteric inhibition is also called end product, feed back or retro inhibition. The product inhibitors function as negative modulators, combine with inhibitor sites and cause allosteric change in the active sites that prevents binding of substrate molecule.
Example: The hexokinase enzyme converting the glucose into glucose 6 - phosphate in following reaction:

Excess formation of glucose 6-phosphate inhibits the enzyme and stops its further production from glucose.

- RBSE Solutions for Class 11 Biology Chapter 10 Cell Cycle and Cell Division
- RBSE Solutions for Class 11 Biology Chapter 9 Biomolecules
- RBSE Solutions for Class 11 Biology Chapter 8 Cell: The Unit of Life
- RBSE Solutions for Class 11 Biology Chapter 7 Structural Organisation in Animals
- RBSE Solutions for Class 11 Biology Chapter 6 Anatomy of Flowering Plants
- RBSE Solutions for Class 11 Biology Chapter 5 Morphology of Flowering Plants
- RBSE Solutions for Class 11 Biology Chapter 4 Animal Kingdom
- RBSE Solutions for Class 11 Biology Chapter 3 Plant Kingdom
- RBSE Solutions for Class 11 Biology Chapter 2 Biological Classification
- RBSE Solutions for Class 11 Biology Chapter 1 The Living World
- RBSE Solutions for Class 11 Biology Chapter 5 पुष्पी पादपों की आकारिकी