RBSE Class 12 Physics Important Questions Chapter 13 Nuclei
Rajasthan Board RBSE Class 12 Physics Important Questions Chapter 13 Nuclei Important Questions and Answers.
RBSE Class 12 Physics Chapter 13 Important Questions Nuclei
Multiple Choice Questions
Question 1.
The energy released in a typical nuclear fusion reaction is approximately:
(A) 25 MeV
(B) 200 MeV
(C) 800 MeV
(D) 1050 MeV.
Answer:
(A) 25 MeV

Question 2.
A radioactive element has half life period 800 years. After 6400 years what amount will remain?
(A) \(\frac{1}{2}\)
(B) \(\frac{1}{16}\)
(C) \(\frac{1}{8}\)
(D) \(\frac{1}{256}.\)
Answer:
(D) \(\frac{1}{256}.\)
Question 3.
A positron has same mass as:
(A) proton
(B) α-particle
(C) α-neutron
(D) electron.
Answer:
(D) electron.
Question 4.
1H1 + 1H1 + 1H2 → X + 1e0 + energy.
The emitted particle is:
(A) Neutron
(B) Proton
(C) α-particle
(D) Neutrion.
Answer:
(C) α-particle
Question 5.
Which of the following isotopes is used for treatment of cancer?
(A) K40
(B) Co60
(C) Sr90
(D) I131.
Answer:
(B) Co60

Question 6.
Electron net (eV) is a unit of:
(A) Energy
(B) Potential
(C) Current
(D) Charge.
Answer:
(A) Energy
Question 7.
The Binding energy per nucleon is maximum in case of:
(A) 2He4
(B) 26Fe56
(C) 56Ba141
(D) 95U235.
Answer:
(B) 26Fe56
Question 8.
N14 is bombarded with 2He4. The resulting nucleus is 8O17 with the emission of:
(A) Neutrino
(B) Antineutrino
(C) Proton
(D) Neutron
Answer:
(C) Proton
Question 9.
A radioactive element 90X238 decays into 91Y238. The number of ß-particles emitted are:
(A) 4
(B) 6
(C) 2
(D) 1.
Answer:
(D) 1.
Question 10.
In the following nuclear reaction:
6C11 → 5B11 + ß+ + X
what does X stands for:
(A) A neutron
(B) A neutrino
(C) An electron
(D) A proton.
Answer:
(B) A neutrino

Question 11.
The volume occupied by an atom is greater than the volume of the nucleus by a factor of about:
(A) 101
(B) 105
(C) 1011
(D) 1015.
Answer:
(D) 1015.
Question 12.
What percentage of original radioactive atom is left after four half lives:
(A) 20%
(B) 10%
(C) 5%
(D) 6%.
Answer:
(D) 6%.
Question 13.
In fusion, the percentage of mass converted into energy is about:
(A) 0.01%
(B) 0.1%
(C) 1%
(D) 10%.
Answer:
(C) 1%
Question 14.
Half life of radium is 1600 years. Its average life is:
(A) 3200 years
(B) 4800 years
(C) 2309 years
(D) 4217 years.
Answer:
(C) 2309 years
Question 15.
Which of the following is used as modulator in nuclear reactor?
(A) Uranium
(B) Heavy Water
(C) Cadmium
(D) Plutonium.
Answer:
(B) Heavy Water
Question 16.
Hydrogen bomb is based on the principle of:
(A) Nuclear fusion
(B) Nuclear fission
(C) ß-decay
(D) None of these.
Answer:
(A) Nuclear fusion

Question 17.
Energy generated in stars is mainly due to:
(A) Nuclear fusion
(B) Nuclear fission
(C) Gravitational pull
(D) Chemical reaction.
Answer:
(A) Nuclear fusion
Question 18.
A chain reaction is continuous due to:
(A) Large mass defect
(B) Large energy
(C) Production of more neutrons in fission reaction
(D) None of these.
Answer:
(C) Production of more neutrons in fission reaction
Question 19.
The energy equivalent of one atomic mass unit is:
(A) 1.6 x 10-19 J
(B) 6.02 x 1023 J
(C) 9.31 MeV
(D) 931 MeV.
Answer:
(D) 931 MeV.
Question 20.
The average binding energy per nucleon of a nuclear inside an atomic nucleus is about:
(A) 8 MeV
(B) 8 MeV
(C) 8 joule
(D) 8 erg.
Answer:
(C) 8 joule
Question 21.
An atom bomb works on the principle of:
(A) Alpha-decay
(B) Beta-decay
(C) Nuclear fission
(D) Nuclear fusion.
Answer:
(C) Nuclear fission

Question 22.
The penetrating power is maximum for:
(A) α-rays
(B) ß-rays
(C) γ-rays
(D) None of these.
Answer:
(C) γ-rays
Question 23.
A nucleus of 11N23 contains:
(A) 12 electrons
(B) 12 protons
(C) 12 neutrons
(D) 11 neutrons.
Answer:
(C) 12 neutrons
Question 24.
A nucleus nXm emit one α-particle and two ß-particles the resulting nucleus is:
(A) n-2Ym-4
(B) n-4Zm-6
(C) nZm-6
(D) nXm-4
Answer:
(D) nXm-4
Question 25.
In a nuclear reactor, there is conservation of:
(A) Momentum
(B) Charge
(C) Energy
(D) All of these.
Answer:
(B) Charge

Question 26.
When 3Li7 nuclei are bombarded by protons, the resultant nuclei are 4Be8. The particles emitted will be:
(A) alpha particles
(B) beta particles
(C) gamma particles
(D) neutron.
Answer:
(C) gamma particles
Fill in the blanks
Question 1.
Electron volt is the unit of ...........................
Answer:
energy.
Question 2.
Atoms having same number of neutrons are called ...........................
Answer:
isotones.
Question 3.
The average binding energy required to extract one nucleon from the nucleus is called ...........................
Answer:
binding energy per nucleon.

Question 4.
The ........................... decay law can be expressed as N = N0 e-λt.
Answer:
radioactive.
Question 5.
Both neutrino (v) and antineutrino (\(\bar{v}\)) have almost ........................... mass and are ........................... particles.
Answer:
zero, neutral.
Question 6.
One atomic mass unit = ...........................
Answer:
1.67 x 10-31 kg.
Question 7.
The relation between nuclear radius and mass number ...........................
Answer:
R = R0 A1/3.
Question 8.
The ratio of size of atom and size of the nucleus is ...........................
Answer:
≃ 105.
Question 9.
During radioactivity, the electrons are emitted from the ........................... of atom.
Answer:
nucleus.

Question 10.
Half life of radioactive element depends upon ...........................
Answer:
nature of element.
Very Short Answer Type Questions
Question 1.
Give the value of nuclear density.
Answer:
Nuclear density = 2.9 x 1017 kg m-3.
Question 2.
What is impact parameters for scattering by 180°?
Answer:
Impact parameter is zero.
Question 3.
Define impact parameter.
Answer:
It is the perpendicular distance of velocity vector of α-particles from the central line of nucleus when the particle is far away from the nucleus of the atom.
Question 4.
Define atomic mass unit.
Answer:
It is defined as 1/12th of the mass of carbon atom C12.
Question 5.
Give value of atomic mass unit.
Answer:
1 atomic mass unit = 1.66 x 10-27 kg.

Question 6.
Define electron volt.
Answer:
It is the energy gained by an electron when accelerated by one volt.
Question 7.
Give relation between a.m.u. and electron volt.
Answer:
1 a.m.u. = 931.5 x 106 eV = 931.5 MeV.
Question 8.
Give value of electron volt.
Answer:
1 electron volt = 1.6 x 10-19 J.
Question 9.
Define nucleons.
Answer:
Protons and neutrons constituting a nucleus are called nucleons.
Question 10.
Define atomic number.
Answer:
The number of protons in a nucleus, is called atomic number. It is represented by the symbol Z.
Question 11.
How many Joules are there in 1 MeV?
Answer:
1 MeV = 1.6 x 10-13 J.
Question 12.
Define isotones.
Answer:
Isotones are the atoms having the same number of neutrons.
Question 13.
Define isotopes.
Answer:
Isotopes. The atoms which have the same atomic number Z, but different mass number A, are called isotopes.

Question 14.
Define isobars.
Answer:
Isobars. The atoms which have the same mass number A, but different atomic number Z, are called isobars.
Question 15.
Define mass number.
Answer:
The number of nucleons in a nucleus, is called mass number. It is represented by the symbol A.
Question 16.
What is the approxinate density of nucleus?
Answer:
Of the order of 1017 kg m-3.
Question 17.
What is the ratio of gravitational, electric and nuclear interaction?
Answer:
Fg : Fe : Fn : 1 : 1036 : 1038.
Question 18.
What is mass defect?
Answer:
It is the difference between the masses of nucleons constituting a nucleus and the rest mass of the nucleus.
Question 19.
Write expression for mass defect.
Answer:
Dm = ZMp + (A - Z) Mn - Mnucleus
Question 20.
What is the relation between nuclear radius and mass number?
Answer:
R = R0 A1/3
or R ∝ A1/3
However volume ∝ A.
Question 21.
Give an equation representing the decay of a free electron.
Answer:
0n1 → 1p1 + -1e0 + \(\bar{v}\)
where \(\bar{v}\) is antineutrino having zero mass. Half life period of neutron is 10.8 minute.
Question 22.
Which one has higher binding energy per nucleon: 92U235 or 8O16?
Answer:
8O16.

Question 23.
Define half life of a radioactive substance.
Answer:
Half life of a radioactive substance is the time in which half of the radioactive substance disintegrates. If T is the half life period and λ is the disintegration constant, then T = \(\frac{0.693}{\lambda}\).
Question 24.
A certian radioacitve substance has a half life period of 30 days. What is disintegration constant?
Answer:
λ = \(\frac{0.693}{\mathrm{~T}}=\frac{0.693}{30 \times 24 \times 60 \times 60}\)
or λ = 2.6736 x 10-7 s-1
Question 25.
Give the relation between half life and disintegration constant of a radioactive material.
Answer:
Half life T and disintegration constant λ are related by the relation.
T = \(\frac{0.693}{\lambda}\)
Question 26.
Define radioactive decay constant.
Answer:
It is defined as reciprocal of the time after which the number of atoms of a radioactive substance decreases to (1/2.7183) or 36.8% of their number present initially.
Question 27.
Define curie.
Answer:
Curie. It is the radioactivity of 1 gram of radium. One gram of radium gives only 3.7 x 1010 disintegrations per second. Therefore, the radioactivity of a smaple is said to be 1 curie if it undergoes 3.7 X 1010 disintegrations per second.
1 ci = 3.7 x 1010 disintegrations/s and 1 µ ci = 3.7 X 104 disintegrations/s.
Question 28.
What are secondary neutrons?
Answer:
Neutrons produced in fission, are called secondary neutrons.
Question 29.
Write expression for the rate of decay.
Answer:
The required expression is, R = λN.
Question 30.
Give units of rate of decay.
Answer:
The unit are:
- curie = 3.7 x 1010 disintegrations per sec.
- rutherford = 106 disintegrations per sec.

Question 31.
Define rate of decay or activity.
Answer:
The number of nuclei disintegrating per second, is called rate of decay. It is also called activity. It is represented by symbol R.
Question 32.
In which atom model electrons in the ground state are in stable equilibrium?
Answer:
Thomson atom model.
Question 33.
In which atom model electrons in the ground state are not in stable equilibrium?
Answer:
Rutherford’s model of atom.
Question 34.
In Rutherford’s atom model an atom has a highly non-uniform mass distribution. Explain.
Answer:
In Rutherford’s atom model, all the positive charge and almost whole mass is concentrated at the centre of the atom called nucleus hence the distribution of mass is highly non-uniform.
Question 35.
Draw the graph showing the variation of binding energy per nucleon with mass number. Explain using the graph, why heavy nuclei can undergo fission?
Answer:
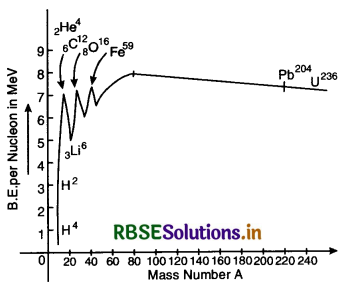
The binding energy per nucleon of heavy nuclei is quite low due to Coulomb’s repulsion between the protons and it is easy to break such nuclei.
Question 36.
Name the reaction which takes place when a slow neutron beam strikes 23592U nuclei. Write the nuclear reaction involved.
Answer:
The reaction is
10n + 23592U → 23692U → 9236Kr + 14156Ba + 3 10n
The reaction is nuclear fission.
Question 37.
In the series of radioactive disintegration of ZAX first an alpha-particle and then a beta particle is emitted. What is the atomic number and mass number of the new nucleus formed by these successive disintegrations?
Answer:
The reaction, during alpha decay is
ZAX → 42He + Z-4A-2Y
Here has A protons and (Z - A) neutrons and Z-4A-2Y has (A - Z) protons and (Z - 4) - (A - 2) = (Z - A - 2) neutrons.
The reaction during beta decay is
Z-4A-2Y → 0-1e + Z-4A-1Z

Question 38.
Does the ratio of neutrons to protons in a nucleus increase, decrease or remain the same after the emission of
(i) α-particle
(ii) ß-particle.
Answer:
(i) Increase, because only heavy elements emit α-particle.
(ii) Decreases, because only light elements emit ß- particle.
Question 39.
You are given two nuclides 3X7 and 3X4:
(i) Are they isotope of the same element? Why?
(ii) Which one of the two is likely to be more stable?
Answer:
(i) Yes, they are isotope of the same element because both have the same atomic number 3.
(ii) Since 3X4 has less number of neutrons, so it is more stable.
Question 40.
What is the ratio of the nuclear densities of the two nuelei having mass numbers in the ratio 1 : 4?
Answer:
The ratio is 1 : 1 as nuclear density is independent of mass number.
Question 41.
Which one of 73X and 43Y is likely to be more stable? Give reason.
Answer:
73X will be more stable than 43Y. Because the odd even pair is more stable than odd-odd pair.
Question 42.
Some scientists have predicted that a global nuclear war would be followed by nuclear winter. What would cause the nuclear winter?
Answer:
Because after the nuclear war the radioactive waste will hang in the atmosphere and will abstract the light radiation to reach the surface of earth and will cause the nuclear winter.
Question 43.
Why is the gold foil preferred as scattering object in Rutherford’s experiment?
Answer:
Because gold is a heavy substance having atomic number 79 and can be stretched into very thin foil.
Question 44.
What is self-sustained nuclear reaction?
Answer:
The nuclear reaction which once initiated keeps on ‘going on’, without any further need of any external ‘missiles’.

Question 45.
Why is heavy water used as moderator?
Answer:
It is used to slow down neutrons so that the fission reaction gets under control.
Question 46.
Complete the following nuclear reaction:
5B10 + (?)3 = Li7 + 2He4.
Answer:
Let the unknown particle be represented on
∴ 10 + A = 7 + 4 or A = 1
And 5 + Z = 3 + 2 or Z = 0
∴ The unknown particle is 0n1 i.e. neutron.
Question 47.
What is a nuclear fission reaction?
Answer:
The splitting of a higher atomic mass nucleus to lower atomic mass nuclei with emission of energy is called nuclear fission.
92U235 + 0n1 → 92U236 → 36Kr92 + 56Ba141 + 3 0n1 + energy is one of fission reaction.
Question 48.
Define the principle of hydrogen bomb.
Answer:
Nuclear fusion is the principle of hydrogen bomb. Atoms of lower atomic mass fuse into heavier atom with emission of energy. One of such reaction is
1H2 + 1H2 → 2He4 + 24 MeV.
Question 49.
What is the role of a moderator in a nuclear reactor?
Answer:
A moderator slows down the neutrons and hence the nuclear reaction.
Question 50.
Define critical mass of nuclear mass of nuclear chain reaction.
Answer:
When the chained nuclear reaction is sustained or steady, i.e., the rate of production of neutrons is equal to the rate of loss of neutrons, the size of the fissionable matter is said to be critical and its mass is called critical mass.
Question 51.
Define nuclear fission and fusion.
Answer:
Nuclear fission is a process in which heavy nucleus disintegrates into lighter nuclei. Nuclear fusion is a process in which nuclei of fighter atoms are fused together into heavy atom with the release of enormous energy.
Question 52.
Name the material used as ‘control rods’ in a nuclear reactor.
Answer:
Cadmium.
Question 53.
What is a thermonuclear reaction?
Answer:
The nuclear fusion of two fight nuclei takes place at very high temperature (≃107K). This is called thermonuclear reaction.
Question 54.
At what temperature the nuclear fusion is possible?
Answer:
About 5000 K to 6000 K.

Question 55.
Can fusion reaction be controlled in a sustained manner?
Answer:
No.
Question 56.
What are the types of damage caused by radiation?
Answer:
Two types of damage is caused by radiation:
1. Pathological damage
2. Genetical damage.
Question 57.
What is the source of solar energy?
Answer:
Solar energy is due to nuclear fusion taking place inside the sun.
4 1H1 → 2He4 + Q
where Q is the energy released when 4 protons fuse to a helium atom.
Question 58.
State the reason, why heavy water is generally used as a moderator in a nuclear reactor.
Answer:
The moderator helps to slow down the neutrons so that the fission reaction gets under control.
Question 59.
Name the absorbing material used to control the reaction rate of neutrons in a nuclear reactor.
Answer:
Cadmium and boron.
Question 60.
What is the nuclear radius of 125Fe, if that of 27Al is 3.6 fermi?
Answer:
Since R = R0A1/3
or R ∝ A1/3
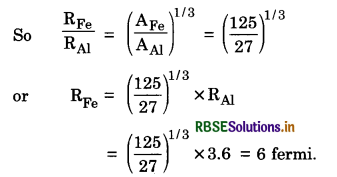
Question 61.
Out of the two characteristics the mass number (A) and the atomic number (Z) of a nucleus, which one does not change during ß- decay.
Answer:
Mass number (A) does not change during ß-decay, because during ß-decay, we get
AZX → AZ+1Y + -1e0.
Question 62.
Four nuclei of an element undergo fusion to form a heavier nucleus with release of energy, which of the two-the parent or the daughter nucleus would have-higher binding energy/nucleon?
Answer:
The daughter nuclei will have higher binding energy per nucleon as release in energy is accompanied by an increase of binding energy.

Question 63.
Name the reaction which takes place when a slow neutron beam strikes 23592U nuclei. Write the nuclear reaction involved.
Answer:
The reaction is
10n + 23592U → 23692U → 9236Kr + 14156Ba + 3 10n
The reaction is nuclear fission.
Question 64.
In the series of radioactive disintegration of ZAX first an alpha particle and then a beta particle is emitted. What is the atomic number and mass number of the new nucleus formed by these successive disintegrations?
Answer:
The reaction, during alpha decay is
ZAX → 42He + Z-4A-2Y
Here ZAX has A protons and (Z - A) neutrons and Z-4A-2Y has (A - Z) protons and (Z - 4) - (A - 2) = (Z - A - 2) neutrons. The reaction during beta decay is
ZAX → 42He + Z-4A-2Y → -1e0 + Z-4A-1Z.
Question 65.
Compare the radii of the two nuclei with mass number 1 and 27 respectively.
Answer:
Since r ∝ A1/3
∴ \(\frac{r_1}{r_2}=\left(\frac{\mathrm{A}_1}{\mathrm{A}_2}\right)^{\frac{1}{3}}=\left(\frac{1}{27}\right)^{\frac{1}{3}}=\frac{1}{3}.\)
Question 66.
You are given two nuclides 3X7 and 3X4:
(i) Are they isotopes of the same element? Why?
(ii) Which one of the two is likely to be more stable?
Answer:
(i) Yes, they are isotope of the same element because both have the same atomic number 3.
(ii) Since 3X4 has less number of neutrons, so it is more stable.

Question 67.
Which property of nuclear force explains the approximate constancy of binding energy per nucleon with mass number A for nuclei in the range 30 < A < 170?
Answer:
Short range nature of nuclear force.
Question 68.
What percentage of a given mass of radioactive substance will be left undecayed after four half periods?
Answer:
The percentage of mass undecayed
= \(\left(\frac{1}{2}\right)^4\) x 100 = \(\frac{100}{16}\) = 6.25%.
Question 69.
State the conditions for controlled chain reaction in a nuclear reactor.
Answer:
Since in a nuclear fission, three neutrons are realeased, so if on the average one neutron causes further fission, the chain reaction is said to be controlled.
Question 70.
Give the mass number and atomic number of elements on the right-hand side of the decay process 22086Ru → Po + He.
Answer:
The complete equation is 22086Ru → 21684Po + 42He
So for Po, Z = 84, A = 216
for He, Z = 2, A = 4.
Question 71.
Which has greater ionising power α-particle or ß-particle.
Answer:
α-particle has greater ionising power than ß-particle.
Question 72.
Why do α-particles have a high ionising power?
Answer:
Because a particle has large mass and large nuclear cross-section.
Question 73.
What will be the ratio of the radii of two nuclei of mass number A1 and A2?
Answer:
Since R ∝ A1/3
∴ \(\frac{\mathrm{R}_1}{\mathrm{R}_2}=\left(\frac{\mathrm{A}_1}{\mathrm{~A}_2}\right)^{1 / 3}\)
Question 74.
Name two radioactive elements, which are not found in observable quantity in nature, why is it so?
Answer:
Plutonium and tritium are not found in observable quantity in nature because of their very small values of half state.

Question 75.
A nucleus of mass number A, has a mass defect ∆m. Give the formula, for the binding energy per nucleon of this nucleus.
Answer:
B.E. Per nucleon = \(\frac{\text { Total binding energy }}{\text { Number of nucleons }}\)
= \(\frac{\Delta m c^2}{\mathrm{~A}}\).
Question 76.
What is the difference between an electron and a ß-particle?
Answer:
Basically there is no difference between an electron and a ß-particle. The electron of nuclear origin is called a ß-particle.
Question 77.
Write any one equation representing nuclear fusion reaction.
Answer:
The equation of a nuclear fission is
1H2 + 1H2 → 2He4 + 24MeV.
Question 78.
The radioactive isotope D decays according to the sequence 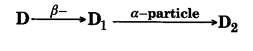 . If the mass number and atomic number of D2 are 176 and 71 respectively, what is (i) the mass number and (ii) atomic number of D?
. If the mass number and atomic number of D2 are 176 and 71 respectively, what is (i) the mass number and (ii) atomic number of D?
Answer:
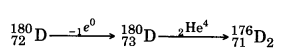
So Mass no = 180 and atomic number = 72.
Question 79.
Write the nuclear decay process for ß-decay of 3215P.
Answer:
3215P → 3216S + -1e0.

Question 80.
What is the source of solar energy?
Answer:
Nuclear fusion reaction taking place in the sun.
Question 81.
Two nuclei have mass numbers in the ratio 1 : 3. What is the ratio of their nuclear densities?
Answer:
The ratio is 1 : 1 as nuclear density is independent of mass number.
Short Answer Type Questions
Question 1.
What are isotopes? Give examples.
Answer:
Isotopes of an element are the atoms of the element which have the same atomic number but different atomic weights. For example 1H1, 1H2, 1H3 are the isotopes of hydrogen. As isotopes of an element have the same atomic number, they contain the same number of protons and the same number of electrons. But as their atomic weights are different they contain different number of neutrons. Isotopes of an element have identical chemical properties. Their physical properties, however, differ. All the known elements have one or more isotopes. The relative abundance of different isotopes differs from element to element.
Question 2.
What are isobars?
Answer:
Isobars are the atoms of different elements which have the same atomic weight, but different atomic numbers. Isobars contain different number of protons, different number of electrons and also different number of neutrons. Only the total number of nucleons in them is the same.
For example 11Na22 and 10Ne22 are isobars. Similarly, 20Ca40 and 18Ar40 are isobars 17C137 and 16S37 are also isobars. The chemical properties of isobars are widely different. Their physical properties may be identical. Isobars occupy different places in the periodic table.
Question 3.
What is meant by B.E. per nucleon and packing fraction?
Answer:
Average Binding-Energy per nucleon of a nucleus is the average energy we have to spend to remove a nucleon from the nucleus to infinite distance. It is given by the total binding energy divided by the mass number of the nucleus.
i.e. Average B.E./nucleon = \(\frac{\text { Total B.E. }}{\text { Mass number }}\)
Packing fraction of a nucleus is defined as the mass defect per nucleon of the nucleus. Thus
Packing friction = \(\frac{\text { Mass defect }}{\text { Mass number }}\)
= \(\frac{\Delta m}{\mathrm{~A}}\)

Question 4.
Is the rest mass of proton exactly equal to the rest mass of a neutron?
Answer:
No, the rest mass of proton and neutron are not exactly equal. They are only approximately equal.
mn = 1.00866 a.m.u.;
mp = 1.00782 a.m.u.
Question 5.
Is free neutron a stable particle?
Answer:
No, a free neutron is not a stable particle. It decays spontaneously into a proton, an electron and antineutrino
0n1 → 1H1 + -1e0 + \(\bar{v}\)
Question 6.
What is alpha decay, beta decay and gamma decay?
Answer:
During alpha decay, an alpha particle is emitted resulting in decrease of mass number by 4 and atomic number by 2.
92U238 → 2He4 + 90Th234
During beta decay, a neutron of the nucleus disintegrates to give rise to proton, electron and antineutrino.
0n1 → -1p1 + -1e0 + \(\bar{v}\)
Reaction for emission of beta particle results in increase of atomic number by 1 whereas the mass number remains constant.
90Th234 → -1ß0 + 91Pa234
Gamma decay usually follows alpha decay. During alpha particle emission, the nucleus is left in an excited state.
To return to normal stage, it emits a single γ-ray of large energy or γ-rays are emitted in steps. However, during γ-decay, atomic number and mass number remain the same.
Question 7.
A radioactive nucleus A decays as follows:
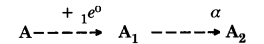
If the mass number and atomic number of A2 are 176 and 71 respectively, what are the mass number and atomic numbers of A1 and A? Which of these three elements are isobars?
Answer:
Writing atomic and mass numbers of A as x and y,
xAy → +1e0 + x-1A1y
x-1A1y → 2He4 + x-3A2y-4
Now in A2, x-3 = 71 or x = 74
and y-4 = 176
or y = 180
∴ Complete reaction is
74A180 → +1e0 + 73A1180
73A1180 → 2He4 + 71A2176
A and A1 are isobars since mass number of each is 180.

Question 8.
What are radio isotopes? What are their applications?
Answer:
The radioactive elements which are chemically identical but differ in their mass number are called radio isotopes. These are also called radio isotopes or radio-nuclides. Five most important radio-isotopes are iodine-131, phosphorus-33, sulphur-35, carbon-14 and sodium-24.
Applications
- In medicine, these are used for treatment of diseases. Malignant cells are more sensitive to radiations than a healthy cell and can be killed by controlled doses of radiation.
- Radioactive gold is used for treatment of syphilis.
- Radioactive bismuth is used in treatment of blood cancer.
- Radioactive iodine can be used to treat diabetes and heart trouble.
- Radio isotopes used as tracers are used for location of tumours.
- Radioactive chromium is used to study blood processes and blood diseases.
- Iron-59 helps us to determine the rate of formation of R.B.C. in the blood.
- Cobalt is used to detect flaws in metal structures.
Question 9.
Explain with the help of nuclear reaction in each of the following cases, how the neutron-to-proton ratio changes during (i) alpha decay; (ii) beta decay?
Answer:
For alpha decay, let us consider a nuclear reaction:
92U238 → 2He4 + 90Th234
Initial neutron-proton ratio
= \(\frac{238-92}{92}=\frac{146}{92}\) = 1.59
Final neutron-proton ratio
= \(\frac{238-90}{90}=\frac{144}{90}\) = 1.57
∴ Neutron-proton ratio decrease during ß-decay.
Question 10.
Define the term half life period and decay constant of a radioactive substance. Write their SI unit. Establish a relation between them.
Answer:
Half life period (T). It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Decay constant (λ). Decay constant of a radioactive element is the time after which the number of radioactive atoms reduces to \(\frac{1}{e}\) times the original number of atoms in the sample.
Relation between T and λ
Half life period. It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Let N0 = number of atoms at t = 0 (at the start of the observation)
N = number of atoms after time t
So, N = N0e-λt ..........................(1)
where λ is constant called disintegration constant
Let T = half life period
So, when t = T, N = N0/2.
Substituing in Eq.(1), we have
\(\frac{\mathrm{N}_0}{2}\) = N0e-λt
eλt = 2
λT = loge2 = 2.303 log102
= 0.693
or T = \(\frac{0.693}{\lambda}\)
Question 11.
A neutron is absorbed by 63Li nucleus with the subsequent emission of an α-particle.
(i) Write down the corresponding reaction.
(ii) Calculate the energy released in MeV in this reaction given 63Li = 6.01516 u; mass (neutron) = 1.0086654 u mass (α) = 4.0026044 u and mass (triton) = 3.0100000 u. Take 1u = 931 MeV/c2.
Answer:
(i) The nuclear reaction is:
10n + 63Li → 42α + 31T
(ii) Decrease in mass = m (neutron) + m (Li) - m (α) - m (T)
∴ BE = [m (neutron) + m (Li) - m (α) - m (T)]c2
= [1.0086654 + 6.015126 - 4.0026044 - 3.0100000] uc2
= 0.0111870 uc2
= 0.0111870 x 931
= 1.0415097 MeV.
Question 12.
(a) Show that decay rate R of a sample of a radio-nuclide is related to the number of radioactive nuclei N at that instant by the expression R = λN.
(b) The half life of 23892U against α-decay is 1.5 x 1017 s. What is the activity of a sample of 23892U having 25 x 1020 atoms?
Answer:
(a) The decay rate R is equal to the number of radioactive disintegration in the sample taking place per second.
i.e. R = \(-\frac{d \mathrm{~N}}{d t}\) ..........................(i)
From the law of radioactive disintegration, the rate of disintegration \(\left[-\frac{d \mathrm{~N}}{d t}\right]\) is proportional to number of atoms present at that instant i.e.
\(-\frac{d \mathrm{~N}}{d t}\) ∝ N
or \(-\frac{d \mathrm{~N}}{d t}\) = λN
Using Eq. (i), we get:
R = λN
(b) Here T = 1.5 x 1017 s
Since λ =\( \frac{0.693}{T}=\frac{0.693}{1.5 \times 10^{-17}}\) s-1
∴ R = λN
= \(\frac{0.693}{1.5 \times 10^{-17}}\) x 25 x 1020 dis s-1
= \(\frac{693 \times 25}{1.5}\) = 11550 atoms/s

Question 13.
(a) Draw the energy level diagram showing the emission of ß-particle followed by γ-rays by a 6027Co nucleus.
(b) Plot the distribution of kinetic energy of ß-particles and state why energy spectrum is continuous?
Answer:
(a) The energy spectrum of b-particles is continuous because an antineutrino is simultaneously emitted in β-decay: the total energy released in b-decay is shared by b-particle and the antineutrino so that momentum of the system may remain conserved.
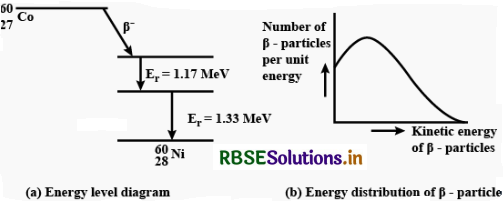
(b) Beta decay. The emission of electron ß-particle) from the nucleus of an atom is called ß-decay. It is believed that emission of an electron by the nucleus is due to transformation of a neutron into a proton and an electron. When a neutron changes into a proton, a ß-particle is ejected whereas a proton remains in the nucleus. The emission of ß-partic1e, therefore:
- increases the atomic number by one and
- keeps the mass number unaffected.
Question 14.
Half life of 23892U against α-decay is 4.5 x 109 years. Calculate activity of 1 g of sample 23892U.
Answer:
Number of atoms in 238 g of Uranium = 6.023 x 1023
∴ Number of atoms in 1 g
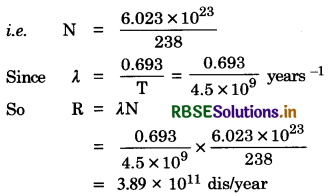
Question 15.
Write the nuclear reactions for the following:
(i) α-decay of 20484Po
(ii) ß-decay of 3215P
(iii) ß+ - decay to 116C
Answer:
(i) 20484Po → 42He + 20082Hg
(ii) 3215P → -1e0 + 3216S
(iii) 116C → -1e0 + 115B
Question 16.
Calculate the binding energy per nucleon (in MeV) of the nucleus 5626Fe.
[Given: mass of 11H = 1.00783 u, mass of 10n = 1.00867 u, mass of 5626Fe = 55.934939 u. 1u = 931 MeV/c2.
Answer:
B.E. of 5626Fe
= [ZmH+(A - Z)mN - m(AZX)] 931.5
= [{26 x 1.007825 + (56 - 26) x 1.008665} - 55.934939] 931.5
= [{26.20345 + 30.25995} - 55.934939] 931.5
= 492.26 MeV
B.E. of 20983Bi
= [{83 x 1.007825 + (209 - 83) x 1.008665} - 208.980388] 931.5
= [{83.64975 + 127.09179 - 208.980388] 931.5
= 1640.1 MeV.
B.E. per nucleon = \(\frac{1640.1}{209}\) = 7.84 MeV
Fe56 has greater binding energy per nucleon.
Question 17.
Give the mass number and atomic number of elements on the right hand side of the decay process:
22086Rn → Po + He.
The graph shows how the activity of a sample of radon-220 changes with time. Use the graph to determine its half-life. Calculate the value of decay constant of radon-220.
Answer:
The given reaction is
22086Rn → 21684Po + 42He
Mass number of Po = 216, He = 4
Atomic number of Po = 84, He = 2
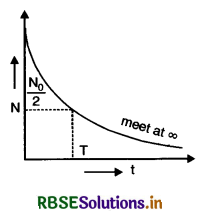
Half life
From the graph of decay of Rn - 220, we find that at t = 0, number of nuclei present = N0 and at t = ∞, N0 → 0
Since N = N0 e-λt ...................(i)
As at t = T, N = \(\frac{\mathrm{N}_0}{2}\)
∴ \(\frac{\mathrm{N}_0}{2}\) = N0e-λt
or λT = loge2 = 2.303 log10 2
or T = \(\frac{2.303 \times 0.3010}{\lambda}=\frac{0.693}{\lambda}\)
From Eq. (i)
It t = \(\frac{1}{\lambda}\), then
N = N0 e-λ.\(\frac{1}{\lambda}\) = \(\frac{\mathrm{N}_0}{e}\)
So decay constant is the time after which the number of radioactive elements reduces to \(\frac{1}{e}\) times the initial number of atoms.

Question 18.
With the help of an example, explain how the neutron to proton ratio changes during alpha decay of a nucleus.
Answer:
For alpha decay, let us consider a nuclear reaction:
92U238 → 2He4 + 90Th234
Initial neutron-proton ratio
= \(\frac{238-92}{92}=\frac{146}{92}\) = 1.59
Final neutron-proton ratio
= \(\frac{238-90}{90}=\frac{144}{90}\) = 1.57
∴ Neutron-proton ratio decrease during ß-decay.
Question 19.
When a deuteron of mass 2.0141u and negligible kinetic energy is absorbed by lithium (63Li) of nucleus of mass 6.0155 u, the compound nucleus disintegrates spontaneously into two alpha particles, each of mass 4.0026 u.
Calculate the energy in joules carried by each alpha particle. (1 u = 1.66 x 10-27 kg).
Answer:
The reaction is:
21H + 63Li → 84Be → 2[42He]
∴ Mass defect, ∆m
= m (21H) + m (63Li) - 2m (42He)
= [2.0141 + 6.0155 - 2 x 4.0026] u
= 0.0244 u
= 0.0244 x 1.66 x 10-27 kg
= 0.0405 x 10-27 kg
∴ Binding energy Q = ∆mc2
= 0.0405 x 10-27 x 9 x 1016
= 0.3645 x 10-11 J
= 36.45 x 10-13 J
This energy is equally shared by both the alpha particles.
∴ Energy carried by each alpha particle
= \(\frac{1}{2}\)[36.45 x 10-13] = 18.225 x 10-13 J
Question 20.
The ionising power of ß-particles is less compared to α-particles, but their penetrating power is more,why?
Answer:
The velocity of ß-particles emitted by a radioactive element is very large as compared to the velocity of a particles. They take very little time near the atoms of the medium. Thus the probability of ionising the atom is comparatively less. On the other hand, the loss of energy is very slow and hence they can penetrate the medium through a sufficient depth.

Question 21.
Natural radioactivity is found in heavy elements only. Explain.
Answer:
A heavy nucleus contains a large number of protons and neutrons. The protons inside the nucleus repel each other with coulomb's force, and in heavy nucleus the electrical repulsive forces become effective.
Question 22.
What is natural radioactivity? How does it vary with external parameters like temperature, pressure etc?
Answer:
The process of spontaneous disintegration of an unstable nucleus with the emission of high energy rays like α-rays, ß-rays and γ-rays is called natural radioactivity. This phenomenon is predominant in elements with high atomic number. Generally, trans-uranium elements (elements with atomic number Z > 92) exhibit this phenomenon. Natural radioactive decay does not depend on temperature, pressure etc.
Question 23.
A certain radioactive element disintegrates for an interval of time equal to its mean life. What fraction of the element has disintegrated?
Answer:
Here t = τ
Number of atoms left in the sample after time t = τ is given by
N = N0 e-λτ = N0 e-λ.\(\frac{1}{\lambda}\) [∵ τ = \(\frac{1}{\lambda}\)]
or N = N0 e-1 = \(\frac{\mathrm{N}_0}{e}\)
or N = \(\frac{\mathrm{N}_0}{2.718}\) = 0.368 N0
So fraction of element disintegrated
= N0 - 0.368 N0 = 0.632 N0.
Hence the fraction of the element disintegrated in time equal to its mean life is 0.632.

Question 24.
What are the characteristic properties of radioactive elements?
Answer:
Following are characteristic properties of radioactive elements:
- Radioacitive decay does not depend on temperature, light, pressure etc.
- Radioactive elements emit three types of radiations namely, α-rays, ß-rays and γ-rays
- Radioactivity is an irreversible process.
- Radioactive substances do not show any abnormality in their chemical and physical properties.
Question 25.
What is meant by electron capture?
Answer:
In case of an unstable nuclei having more number of protons, the coulomb barrier prevents any emission of positron to make the nucleus stable. So in that case, an orbital electron is captured by the nucleus which helps in transforming a proton into a neutron thereby reducing the atomic number of the nucleus.
Question 26.
What is the concept of compound nucleus?
Answer:
The theory of formation of a compound nucleus was put forward by Neil Bohr in 1936 and is a useful tool for explaining a nuclear reaction. According to this theory, when an incident particle hits target nucleus, it is captured by the target nucleus resulting in the formation of an unstable nucleus called compound nucleus, which in turn disintegrate to give a new particle and a new nucleus.
(i) Incident particle + target nucleus → compound nucleus.
(ii) Compound nucleus → product nucleus + emitted particle.
Question 27.
Uranium 92U238 is not suitable for chain reaction. Why?
Answer:
92U238 is found in the form of three isotopes i.e. U233, U235 and U238. But the percentage of U233 and U235 nuclei are very small. The incident slow neutron of energy 0.025 eV can cause fission in U235 but not in nuclei U238 because for fission of U238 neutron of energy more than 1 MeV is required. Also the secondary neutrons get slowed down on colliding with U238 nuclei and are unable to cause further fission of U238. Thus U238 is not suitable for chain reaction.
Question 28.
What is nuclear fission? Give an example to illustrate it. What is the importance of fission phenomenon?
Answer:
Nuclear fission. A process in which a heavy nucleus disintegrates into two lighter nuclei of nearly same masses is called nuclear fission.
e.g. When slow neutron is bombarded on a heavy nucleus of 92U235 yielding two daughter nuclei Ba and Kr and tremendous amount of energy, the nuclear reaction can be represented as:
92U235 + 0n1 → 92U236 → 56Ba141 + 36Kr92 + 30n1+ ∆Q
Importance of nuclear fission
1. Controlled nuclear fission is used in number of yields e.g.
(а) to prepare isotopes.
(b) to generate power for propulsion of ships, submarine and aircrafts.
(c) to produce plutonium for explosive purpose.
2. Uncontrolled nuclear fission is used in atom bomb.
Question 29.
Distinguish between controlled and uncontrolled chain reactions.
Answer:
Controlled chain reaction. In controlled chian reaction neutrons are absorbed by using cadmium rods and on an average one neutron remains available for exciting further fission.
Uncontrolled chain reaction. If the fissionable material is having mass greater than critical mass and the reaction is started it will accelerate at a rapid rate and whole material explodes and a tremendous amount of energy is released.
Question 30.
Distinguish between nuclear fission and nuclear fusion.
Answer:
Distinction between Nuclear fission and Nuclear fusion:
Nuclear fission
- A bigger nucleus is split into two or more smaller nuclei.
- It produces huge energy.
- Nuclear reaction can be controlled.
- It is the principle of atom or fission bomb.
Nuclear fusion
- Two light nuclei combine to form a heavier nucleus.
- It also produces huge energy.
- Nuclear reaction can be controlled.
- It is the principle of hydrogen or fusion bomb.

Question 31.
Explain nuclear holocaust. Describe its sources.
Answer:
Nuclear holocaust
Definition. Nucleus is a source of huge amount of energy. Uncontrolled release of large nuclear energy, is called atomic explosion. The situation caused by such atomic explosion, is called nuclear holocaust.
Sources. Its sources are
(1) atom bomb
(2) hydrogen bomb,
(i) Atom bomb. It works on the phenomenon of uncontrolled nuclear fission chain reaction. Uranium (U235) is used as fission material.
(ii) Hydrogen bomb. It works on phenomenon of fusion of hydrogen nuclei to form helium nucleus.The high temperature needed for fusion is produced by fission bomb.
Question 32.
Describe the nucleus as a source of nuclear energy.
Answer:
Nucleus as a source of Nuclear Energy.
Binding energy curve shows that average binding energy is 8.5 MeV per nucleon for moderate nuclei with mass number 40 to 120. It is less than this for lighter nuclei with A < 40 and also for heavy nuclei with A > 120. It means that light and heavy nuclei are less tightly bound. Whenever nuclei of less average binding energy per nucleon are converted into those of more average binding energy, some mass is converted into energy.
From this situation, it is expected that energy will be evolved if heavy nuclei be split into moderate nuclei or light nuclei be joined (fused) to form a less light nuclei. The former process is known as nuclear fission and the latter as nuclear fusion. Both these processes become source of nuclear energy.
Question 33.
A nucleus 2310Ne undergoes ß-decay and becomes 2311Ne. Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus anti-neutrino carry negligible kinetic energy.
(mass of 2310Ne = 22.994466 u, mass 2311Ne = 22.989770 u, 1u = 931.5 MeV/c2)
Answer:
The decay scheme is
2310Ne → 2311Ne + -1e0 + Q
So mass defect,
∆m = 22.994466 u - [22.989770 + Negligible mass]
= 0.004696 u
∴ Energy released = 0.004696 x 931.5
= 4.374 MeV.

Question 34.
State the law of radioactive decay. If N0 is the number of radioactive nuclei in the sample at same initial time, find out the relation to determine the number N present at a subsequent time. Draw a plot of N as a function of time.
Answer:
Law of radioactive decay
The number of atoms disintegrated per second is directly proportional to the number of radioactive atoms actually present at that instant and is independent of all physical conditions like temperature, pressure humidity, chemical composition etc.
Let at t = 0,
N0 = number of atoms present
N = number of atoms left after time t.
dN = number of atoms disintegrated in time dt.
i.e. -\(\frac{d \mathrm{~N}}{d t}\) ∝ N
or -\(\frac{d \mathrm{~N}}{d t}\) = λN, ..........................(i)
where λ is a constant called disintegration constant and depends upon the nature of the radioactive substance.
Now from, (i), we have
\(\frac{1}{N}\) dN = -λdt
or ∫\(\frac{1}{N}\) dN = λ∫dt
or logeN = λt + C ................(ii)
where C is constant of integration. To determine its value,
since N = N0 initially
i.e. when t = 0, N = N0
loge N0 = 0 + C
Substituting the value of C in (ii), we have
logeN = -λt + logeN0
or logeN - logeN0 = -λt
loge \(\frac{\mathrm{N}}{\mathrm{N}_0}\) = -λt
or \(\frac{\mathrm{N}}{\mathrm{N}_0}\) = e-λt
or N = N0e-λt ..................(iii)
which is the required equation.
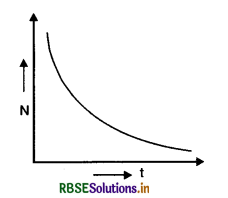
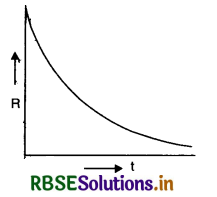
Eq. (iii) shows that the radioactive decay occurs according to exponential law and the number of radioactive atoms decreases exponentially. Since N shall become zero only when t becomes infinity, it shows that time taken by a radioactive element to disintegrate completely is infinitely long.
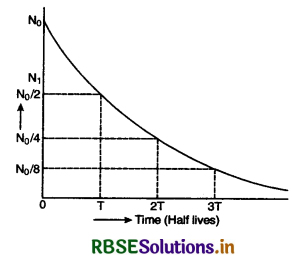
The curve in Fig. SAQ 34 shows the exponential decay of radioactive substance.
Question 35.
Draw a plot of the binding energy per nucleon as a function of mass number for a large number of nuclei. Explain the energy release in the process of nuclear fission from the above plot. Write a typical reaction in which a large amount of energy is released in the process of nuclear fission.
Or
Draw a graph showing the variation of binding energy per nucleon verses the mass number. Explain with the help of this graph the release of energy in the process of nuclear fission and fusion.
Or
Explain the processes of nuclear fission and nuclear fusion by using the plot of binding energy per nucleon (BE/A) versus the mass number A.
Answer:
Plot of binding energy per nucleon is shown in fig. SAQ 35.
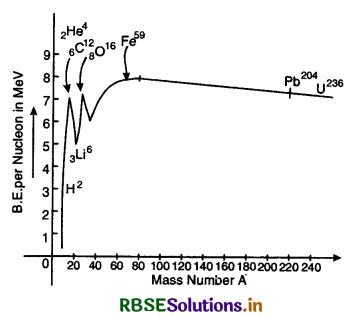
From the graph we find that
1. The binding energy per nucleon increases gradually from mass number A = 20 to mass number A = 40.
2. The curve is almost flat between mass number 40 and 120. This means that the variation in binding energy per nucleon in the range is small (about 10%). The average binding energy per nucleon in this region is about 8.5 MeV. It is maximum for iron (5926Fe) and has a value 8.8 MeV.
3. The binding energy per nucleon decreases slowly and continuously from A = 120 to A = 240. It reaches a value of 7.6 MeV at A = 238. Thus we find that if any nucleon with A = 240 breaks into two nuclei, nucleons get more lightly bound, so energy would be released in the process. This process is called nuclear fission.
4. When two light nuclei (A < 10) are join to form a heavier nucleus, the binding per nucleon of the fused heavier nuclei is more than the binding energy per nucleon of the lighter nuclei. Hence we find that the final system is more tightly bound than the initial system, and the energy released in this process is called nuclear fusion.
A typical nuclear fission reaction is
23592U + 10n → 23692U → 14456Ba + 8936Kr + 310n + 200 MeV

Question 36.
Distinguish between isotopes and isobars. Give one example for each of the species. A radioactive isotope has half life of 5 years. How long will it take the activity to reduce to 3.125%.
Answer:
Isotopes are elements having same atoms number but different mass number e.g. 23892U and 23592U are isotopes of U.
Isobars are elements having the same mass number but different atomic numbers, e.g. 4018Ar and 4020Ca.
Given \(\frac{\mathrm{A}}{\mathrm{A}_0}\) = 3.125% = \(\frac{1}{32}\),
t1/2 = 5 years, t = ?
Since \(\frac{\mathrm{A}}{\mathrm{A}_0} = (\frac{\mathrm{A}}{\mathrm{A}_0})\)n
So (\(\frac{1}{32}\)) = (\(\frac{1}{2}\))t/5 or (\(\frac{1}{2}\))5 = (\(\frac{1}{2}\))t/5
or \(\frac{t}{5}\) = 5 or t = 25 years.
Question 37.
Explain with the help of nuclear reaction in each of the following cases, how the neutron-to-proton ratio changes during (i) alpha decay; (ii) beta decay?
Answer:
For alpha decay, let us consider a nuclear reaction:
92U238 → 2He4 + 90Th234
Initial neutron-proton ratio
= \(\frac{238-92}{92}=\frac{146}{92}\) = 1.59
Final neutron-proton ratio
= \(\frac{238-90}{90}=\frac{144}{90}\) = 1.57
∴ Neutron-proton ratio decrease during ß-decay.
Question 38.
Define the term half life period and decay constant of a radioactive substance. Write their SI unit. Establish a relation between them.
Answer:
Half life period (T). It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Decay constant (λ). Decay constant of a radioactive element is the time after which the number of radioactive atoms reduces to \(\frac{1}{e}\) times the original number of atoms in the sample.
Relation between T and λ
Half life period. It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Let N0 = number of atoms at t = 0 (at the start of the observation)
N = number of atoms after time t
So, N = N0e-λt ..........................(1)
where λ is constant called disintegration constant
Let T = half life period
So, when t = T, N = N0/2.
Substituing in Eq.(1), we have
\(\frac{\mathrm{N}_0}{2}\) = N0e-λt
eλt = 2
λT = loge2 = 2.303 log102
= 0.693
or T = \(\frac{0.693}{\lambda}\)
Question 39.
A neutron is absorbed by 63Li nucleus with the subsequent emission of an α-particle.
(i) Write down the correspondihg reaction.
(ii) Calculate the energy relased in MeV in this reaction given 63Li = 6.01516 u; mass (neutron) = 1.0086654 u mass (α) = 4.0026044 u and mass (triton) = 3.0100000 u. Take 1u = 931 MeV/c2.
Answer:
(i) The nuclear reaction is:
10n + 63Li → 42α + 31T
(ii) Decrease in mass = m (neutron) + m (Li) - m (α) - m (T)
∴ BE = [m (neutron) + m (Li) - m (α) - m (T)]c2
= [1.0086654 + 6.015126 - 4.0026044 - 3.0100000] uc2
= 0.0111870 uc2
= 0.0111870 x 931
= 1.0415097 MeV.

Question 40.
(a) Show that decay rate R of a sample of a radio nuclide is related to the number of radioactive nuclei N at that instant by the expression R = λN.
(b) The half life of 23892U against α-decay is 1.5 x 1017 s. What is the activity of a sample of 23892U having 25 x 1020 atoms?
Answer:
(a) The decay rate R is equal to the number of radioactive disintegration in the sample taking place per second.
i.e. R = -\(\frac{d \mathrm{~N}}{d t}\) ..........................(i)
From the law of radioactive disintegration, the rate of disintegration \(\left[-\frac{d \mathrm{~N}}{d t}\right]\) is proportional to number of atoms present at that instant i.e.
\(-\frac{d \mathrm{~N}}{d t}\) ∝ N
or \(-\frac{d \mathrm{~N}}{d t}\) = λN
Using Eq. (i), we get:
R = λN
(b) Here T = 1.5 x 1017 s
Since λ = \(\frac{0.693}{T}=\frac{0.693}{1.5 \times 10^{-17}}\) s-1
∴ R = λN
= \(\frac{0.693}{1.5 \times 10^{-17}}\) x 25 x 1020 dis s-1
= \(\frac{693 \times 25}{1.5}\) = 11550 atoms/s
Question 41.
(a) Draw the energy level diagram showing the emission of ß-particle followed by γ-rays by a 6027Co nucleus.
(b) Plot the distribution of kinetic energy of ß-particles and state why energy spectrum is continuous?
Answer:
(a) The energy spectrum of b-particles is continuous because an antineutrino is simultaneously emitted in β-decay: the total energy released in b-decay is shared by b-particle and the antineutrino so that momentum of the system may remain conserved.
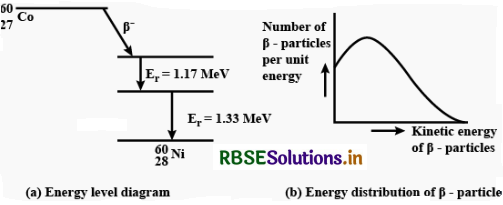
(b) Beta decay. The emission of electron ß-particle) from the nucleus of an atom is called ß-decay. It is believed that emission of an electron by the nucleus is due to transformation of a neutron into a proton and an electron. When a neutron changes into a proton, a ß-particle is ejected whereas a proton remains in the nucleus. The emission of ß-partic1e, therefore:
- increases the atomic number by one and
- keeps the mass number unaffected.
Question 42.
Estimate the ratio of the wavelength associated with the electron orbiting around the nucleus in the ground and first excited state of hydrogen atom.
Answer:
Since de-Broglie hypothesis is related to Bohr’s atomic model as
nλ = 2πr
Since r ∝ n2
∴ r = a0n2
So nλ = 2πa0n2
Hence λ = 2πa0n
∴ \(\frac{\lambda_1}{\lambda_2}=\frac{2 \pi a_0 1}{2 \pi a_0 2}=\frac{1}{2}\)
Question 43.
Write the nuclear reactions for the following:
(i) α-decay of 20484Po
(ii) ß-decay of 3215P
(iii) ß+- decay to
Answer:
(i) 20484Po → 42He + 20082Hg
(ii) 3215P → -1e0 + 3216S
(iii) 116C → -1e0 + 115B

Question 44.
Calculate the binding energy per nucleon (in MeV) of the nucleus 5626Fe.
[Given: mass of 11H = 1.00783 u, mass of 10n = 1.00867 u, mass of 5626Fe = 55.934939 u. 1u = 931 MeV/c2.
Answer:
B.E. of 5626Fe
= [ZmH+(A - Z)mN - m(AZX)] 931.5
= [{26 x 1.007825 + (56 - 26) x 1.008665} - 55.934939] 931.5
= [{26.20345 + 30.25995} - 55.934939] 931.5
= 492.26 MeV
B.E. of 20983Bi
= [{83 x 1.007825 + (209 - 83) x 1.008665} - 208.980388] 931.5
= [{83.64975 + 127.09179 - 208.980388] 931.5
= 1640.1 MeV.
B.E. per nucleon = \(\frac{1640.1}{209}\) = 7.84 MeV
Fe56 has greater binding energy per nucleon.
Question 45.
Give the mass number and atomic number of elements on the right hand side of the decay process:
22086Rn → Po + He.
The graph shows how the activity of a sample of radon-220 changes with time. Use the graph to determine its half-life. Calculate the value of decay constant of radon-220.
Answer:
The given reaction is
22086Rn → 21684Po + 42He
Mass number of Po = 216, He = 4
Atomic number of Po = 84, He = 2
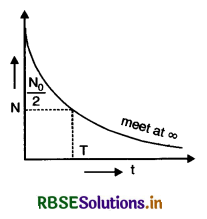
Half life
From the graph of decay of Rn - 220, we find that at t = 0, number of nuclei present = N0 and at t = ∞, N0 → 0
Since N = N0 e-λt ...................(i)
As at t = T, N = \(\frac{\mathrm{N}_0}{2}\)
∴ \(\frac{\mathrm{N}_0}{2}\) = N0e-λt
or λT = loge2 = 2.303 log102
or T = \(\frac{2.303 \times 0.3010}{\lambda}=\frac{0.693}{\lambda}\)
From Eq. (i)
It t = \(\frac{1}{\lambda}\), then
N = N0 e-λ.\(\frac{1}{\lambda}\) = \(\frac{\mathrm{N}_0}{e}\)
So decay constant is the time after which the number of radioactive elements reduces to \(\frac{1}{e}\) times the initial number of atoms.
Question 46.
With the help of an example, explain how the neutron to proton ratio changes during alpha decay of a nucleus.
Answer:
For alpha decay, let us consider a nuclear reaction:
92U238 → 2He4 + 90Th234
Initial neutron-proton ratio
= \(\frac{238-92}{92}=\frac{146}{92}\) = 1.59
Final neutron-proton ratio
= \(\frac{238-90}{90}=\frac{144}{90}\) = 1.57
∴ Neutron-proton ratio decrease during ß-decay.
Question 47.
When a deuteron of mass 2.0141 u and negligible kinetic energy is absorbed by lithium (63Li) of nucleus of mass 6.0155 u, the compound nucleus disintegrates spontaneously into two alpha particles, each of mass 4.0026 u. Calculate the energy in joules carried by each alpha particle. (1 u = 1.66 x 10-27 kg).
Answer:
The reaction is:
21H + 63Li → 84Be → 2[42He]
∴ Mass defect, ∆m
= m (21H) + m (63Li) - 2m (42He)
= [2.0141 + 6.0155 - 2 x 4.0026] u
= 0.0244 u
= 0.0244 x 1.66 x 10-27 kg
= 0.0405 x 10-27 kg
∴ Binding energy Q = ∆mc2
= 0.0405 x 10-27 x 9 x 1016
= 0.3645 x 10-11 J
= 36.45 x 10-13 J
This energy is equally shared by both the alpha particles.
∴ Energy carried by each alpha particle
= \(\frac{1}{2}\)[36.45 x 10-13] = 18.225 x 10-13 J

Question 48.
A radioactive nucleus ‘A’ undergoes a series of decays according to the following scheme:
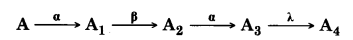
The mass number and atomic number of A are 180 and 72 respectively. What are these numbers for A4.
Answer:
An α-particle is 2He4, ß-particle is -1e0 and α-particle makes no change in Z and A. Hence the given reaction proceed as:
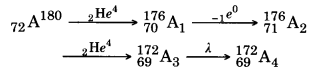
So the mass number A4 is 172 and atomic number is 69.
Question 49.
(i) Define ‘activity’ of a radioactive material and write its S.I. unit.
(ii) Plot a graph showing variation of activity of a given radioactive sample with time.
(iii) The sequence of stepwise decay of a radioactive nucleus is:
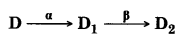
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values for D?
Answer:
(i) The activity of a radioactive substance is defined as the rate at which the nuclei of its atoms in the sample disintegrate.
i.e. R = \(-\frac{d \mathrm{~N}}{d t}\)
The S.I. unit of activity is curie (i)
(ii) Graph between variation of activity of a given radioactive sample with time is shown in Fig SAQ 49.
(iii) The sequence of stepwise decay is as follow
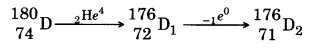
So atomic number and'mass number of D is 74 and 180 respectively.
Question 50.
What is the basic mechanism for the emission of ß- or ß+ particles in a nucleide? Give an example by writing explicitly a decay process for ß- emission. Is (a) the energy of the emitted ß-particles continuous or discrete; (b) the daughter nucleus obtained through ß-decay, an isotope or an isobar of the parent nucleus?
Answer:
Mechanism for emission of ß-
Beta decay. The emission of electron ß-particle) from the nucleus of an atom is called ß-decay. It is believed that emission of an electron by the nucleus is due to transformation of a neutron into a proton and an electron. When a neutron changes into a proton, a ß-particle is ejected whereas a proton remains in the nucleus. The emission of ß-particle, therefore:
(i) increases the atomic number by one and
(ii) keeps the mass number unaffected.
The energy of ß-disintegration is provided by the difference between the mass of the parent nucleus and the sum of the masses by the daughter nucleus and ß-particles. The ß-disintegration energy is thus given by:
Eß = ZMnA - [z+1MnA + me] ......................(1)
where
Mn = mass of nucleus
me = mass of electron
(A, Z) and (A, Z+ 1) mass number of atom i.e. number of parent and daughter nuclei.
i.e. n = p + \(\bar{e}\) + v (neutrino)
The ß+ emission
The atomic number decreases by one and the mass number remains unaffected
i.e. zXA → z-1YA + 1e0 + Q
i.e. p → n+ e+ +\(\bar{v}\) (antineutrino)
Example of ß- decay
zXA → z+1YA + -1e0 + Q
i.e. 90Th234 → 91Pa234 + -1e0. (ß- -particle)
(a) Energy emitted through ß-decay is continuous.
(b) The daughter nucleus is isobar of the parent nucleus.
Long Answer Type Questions
Question 1.
Define the terms nucleus, nucleons, atomic number, mass number, nuclide, isotopes, isobars, isotones and isomers.
Answer:
Nucleus. A nucleus consists of protons and neutrons and the size of nucleus is about 10-14 m. The protons and neutrons inside the nucleus go on interchanging to each other and hence called nucleons (i.e. protons or neutrons).
Nucleons. Protons and neutrons inside the nucleus of an atom are called nucleons.
Atomic number (Z). The number of protons in the nucleus is called atomic number of the element.
Mass number (A). Total number of nucleons in the nucleus is called mass number i.e. A = Z + N, so number of neutrons in the nucleus N = A - Z.
Nuclide. A nuclide is a specific nucleus of an atom characterised by its atomic number Z and mass number A Symbolically, nuclide is represented by ZXA
Isotopes. The atoms of an element which have same atomic number but different mass number are called isotopes, e.g. 8O16, 8O17,8O18 are three isotopes of oxygen and 17Cl35, 17Cl37 are two isotopes of chlorine.
Isobars. The atoms having the same mass number but different atomic number are called isobars e g. 18Ar40, 20Ca40 and 1H3, 2H3. Isobars have the same numbers of nucleons.
Isotones. Atoms whose nuclei have the same number of neutrons are called isotones e.g. 17Cl37 and 19Cl39.
Isomers. The nuclei have the same atomic number and same mass number but in different energy states are called isomers e.g. a nucleus in its ground state and the identical nucleus in metastable excited state are isomers.

Question 2.
Define electron volt and atomic mass unit and find relation between them. Express atomic mass unit in MeV (or eV).
Answer:
Electron volt. It is the unit of energy. It is defined as the energy acquired by an electron when it is accelerated through a potential difference of one volt.
∴ 1eV = 1.6 x 10-19 C x 1 V
1 eV = 1.6 x 10-19 J
A unit, called million electron volt (MeV) is often used in atomic physics.
1 MeV = 106 eV = 1.6 x 10-13 J
Atomic mass unit. The atomic mass unit (written as amu) is 1/12th the actual mass of carbon 6C12.
As we know, 1 gram atom of an element contains 6.023 x 1023 atom (Avogadro's number).
Thus 6.023 x 1023 atoms of carbon weigh = 12 g
1 atom of carbon weighs = \(\frac{12 \times 10^{-3}}{6.023 \times 10^{23}}\) kg
Hence 1 amu = \(\frac{1}{12} \times \frac{12 \times 10^{-3}}{6.023 \times 10^{23}}\) kg
= 1.66 x 10-27 kg
Relation between amu and MeV (or Energy equivalence of 1 amu.)
From Emstein's mass energy relation, we have E = m0c2.
where E is the energy equivalent of rest mass m0 and c is the velocity of light. Thus,
1 amu = 1.66 x 10-27 x (3 x 108)2 J
= \(\frac{1.66 \times 9 \times 10^{-11}}{1.6 \times 10^{-19}}\) (∵ 1 eV = 1.6 x 10-19 J)
= 931 x 106 eV
= 931 MeV.
Question 3.
(a) Write a note on the structure of the nucleus.
(b) Explain the properties of nucleus as regards nuclear charge, nuclear mass, nuclear size and density.
Answer:
(a) Strcture of nucleus. Rutherford's experiments on the scattering of α-particles by atoms led to the discovery of a positively charged heavy central core, called nucleus, of radius of the order of 10-15 m.
1. The entire positive charge and almost the entire mass of the atom is concentrated in the nucleus.
2. Nucleus contains protons and neutrons called nucleons. A proton carries a unit positive charge and mass 1836 times that of an electron. The neutron is electrically neutral and has mass 1836.6 times that of an electron. The number of protons in the nucleus is called atomic number (Z) whereas the total number of neutrons and protons is called mass number (A).
3. Negatively charged electrons round the nucleus revolve in various orbits. The negative charge on the electron is of the same magnitude as the positive charge on the proton. The number of electrons revolving round the nucleus is equal to that of protons so that an atom is normally electrically neutral.
(b) Nuclear charge. The positive charge of the nucleus is due to the presence of protons. The charge on a proton is equal to 1.6 x 10-19 C. If Z be the atomic number, then nuclear charge = Z x 1.6 x 10-19 C.
The mass of the nucleus is equal to the sum of the masses of all protons and neutrons minus the mass defect.
Let Z = atomic number
A = mass number
Mn = mass of neutron
and ∆m = mass defect
Then the number of neutrons = (A - Z)
∴ Mass of the nucleus = ZMp + (A - Z) Mn - ∆m.
Nuclear size. The distance of the closest approach of α-particle from. the nucleus is the measure of radius of nucleus or size of nucleus. For metals its value is about 10-14 m.
The dimensiohs of nucleus have been measured by several experiments involving the scattering of n,p,e, etc. Every time it is found that the volume of nucleus is directly proportional to the number of nucleons (A) in the nucleus.
If R is the radius of the nucleus having mass number A, then
\(\frac{4}{3}\)π R3 ∝ A
or R3 ∝ A
or R ∝ A1/3
or R = R0A1/3
where R0 = 1.2 x 10-15 m = 1.2 fm. It shows that greater the mass number, greater the nuclear radius.
Density of nucleus (ρ) It is defined as the nuclear mass per unit volume.
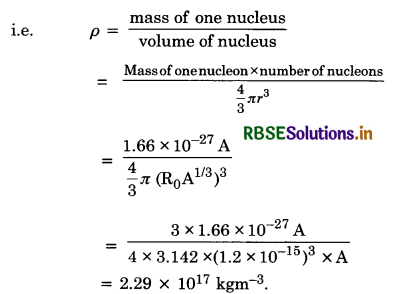
Thus the nuclear density is of the order of 1017 kgm-3 and is independent of its mass number. Therefore, all nuclei have the same approximate density.

Question 4.
State and explain mass defect and packing fraction.
Answer:
Mass defect. It is found that the mass of a stable nucleus is always less than the sum of masses of its constituent protons and neutrons in their free state. The difference between the sum of the masses of nucleons constituting it and the rest mass of the nucleus is called mass defect.
Let
Mp = mass of protons in the nucleus
Mn = mass of neutrons in the nucleus
M = mass of nucleus
N = number of neutrons
Z = number of protons
A = total number of nucleous
∴ The mass defect
∆m = ZMp + (A - Z) Mn - M.
Packing fraction (f). Though the atomic masses are considered to be whole numbers, yet they differ from the integers by small amount. This variation of atomic masses from whole numbers is considered to be due to some deviations of the mass of the nucleus from the mass of the constituents of the nucleus (i.e. protons and neutrons). The term packing fraction is defined as the mass defect per nucleon.
∴ f = \(\frac{\text { mass defect }}{\text { mass number }}=\frac{\Delta m}{\mathrm{~A}}=\frac{\mathrm{M}-\mathrm{A}}{\mathrm{A}}\)
where M is the atomic mass, A is the mass number (i.e. number of protons and neutrons in a.m.u.)
Thus f can be +ve, -ve or zero accordingly as M > A, M < A, M = A.
Packing fraction is a fundamental characteristic of the nucleus and is directly related to the availability of the energy of the nucleus and its stability. A negative packing fraction which will also correspond to a negative mass defect will mean that the atomic mass of the nucleus is less than the nearest whole number which will correspond to some conversion of mass into energy during the formation of the nucleus. Also, negative packing fraction will imply that the nucleus is exceptionally stable because we have to supply more energy to break the nucleus into its constituent nucleons while a positive packing fraction will indicate the nucleus to be less stable.

Question 5.
State and explain binding energy of a nucleus.
Answer:
Binding energy. The binding energy of a nucleus may be defined as the energy required to break up a nucleus into its constituent protons and neutrons at infinite distance apart.
Or
Binding energy in terms of mass defect can be defined as the energy equivalent to the mass defect, is called binding energy of the nucleus.
If ∆m is the mass defect, then
Binding energy, B.E. = ∆m c2 (in joule)
If mass defect is in a.m.u. then
B.E. = (∆m x 931.5) M eV.
Expression for binding energy. The mass defect (∆m) of a nucleus containing Z protons and (A - Z) neutrons is given by
Expression for binding energy. The mass defect (∆m) of a nucleus containing Z protons and (A - Z) neutrons is given by
∆m = Zmp + (A - Z) Mn - M ..........................(1)
where M is mass of the nucleus.
so M = (Zmp + (A - Z) Mn) - ∆m ..........................(2)
Since mass of atom m ZXA is given by
M = mN + ZMe
Using the value of mN from Eq. (2), we get
M = [ZMp + (A - Z) Mn] - ∆m + ZMe
or Mass defect,
∆m = Z (Mp + Me) + (A - Z) Mn - M
or ∆m = ZMH + (A - Z) Mn - M
∴ B.E. = ∆Mmc2
or B.E. = [ZMH + (A - Z) Mn - M] c2

Question 6.
Define binding energy per nucleon. Draw a curve between binding energy per nucleon and the mass number. From the curve state, which portion of the curve indicates possibilities of release of energy and by what process?
Answer:
Binding energy per nucleon. Binding energy per nucleon is the average energy required to extract one nucleon from the nucleus.
∴ B.E. per nucleon = \(\frac{\mathrm{B} \cdot \mathrm{E}}{\mathrm{A}}=\frac{\Delta m c^2}{\mathrm{~A}}\)
Binding Energy Curve. The graph between the binding energy per nucleon and the mass number A is called binding energy curve and is shown in Fig. LAQ 6.
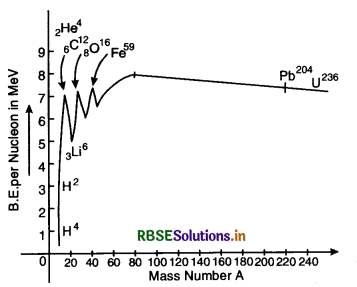
Conclusions
1. The binding energy per nucleon is very low for light nuclei such as deuteron (1H2), helium (2He3) etc.
2. The binding energy per nucleon rises rapidly with increasing volume of A upto A ≅ 20.
3. There exists peaks in the curve corresponding to mass numbers A = 4, 12, 16, 20. The nuclei corresponding to those mass numbers are 2He4, 6C12,8O16, 10N20. The peaks indicate that these nuclei have more binding energy per nuejeon than their immediate neighbours. So they are more stable nuclei.
4. The binding energy per nucleon increases gradually hum mass number A = 20 to mass number A = 40.
5. The curve is almost flat between mass number 40 and 120. This means that the variation in binding energy per nucleon in the range is small (about 10%). The average binding energy per nucleon in this region is about 8.5 MeV. It is maximum for iron (26Fe59) and has a value 8.8 MeV.
6. The binding energy per nucleon decreases slowly and continuously from A = 120 to A = 240. It reaches a value of 7.6 MeV at A = 238. This decrease is due to the increased coulomb potential energy of protons in the nucleus.
7. Binding energy per nucleon is smaller for light and heavier nuclei i.e. light and heavier nuclei are less stable than middle one. In ordier to attain higher value of binding energy per nucleon, the heavier nuclei may split into lighter nuclei (process of fission) and lighter nuclei may unite to form heavier nuclei (process of fusion). In both these nuclear processes, a large amount of energy is released.

Question 7.
Describe how neutron was discovered.
Answer:
Discovery of Neutron
Various experiments
1. In 1930, Bothe and Becker, found that when Beryllium was bombarded by α - particles, no protons were ejected as expected. Instead, a highly penetrating type of radiation was emitted. They assumed this radiation to be γ-rays and gave the following nuclear reaction:
4Be9 + 2He4 → (6C13) → (6C13) + hv (7MeV)
2. In 1932, Joliot and Curie found that these radiations could knock out protons from paraffin wax. Energy measurement showed that for ejection of protons incident radiation must have energy of 55 MeV and not just 7 MeV.
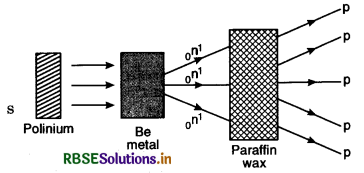
3. In 1932, Chadwick performed an experiment in which α-particle emitted from polinium source were made to bombard on Be metal. Highly penetrating radiations were found to come out of Be metal and when these radiations were made to fall on hydrocarban like 'paraffin wax', high energy protons were knocked out from the paraffin wax as shown in Fig. LAQ 7. Chadwick concluded that these penetrating radiations consisted of neutral particles having mass nearly that of protons. These particles were called neutrons.
The nuclear reaction may be written as
4Be9 + 2He4 → (6C13) → 6C12 + 0n1
where 0n1 represents the neutron with mass 1 u and no charge.
Properties of neutron.
- Neutron is elementary particle having mass equal to 1.6748 x 10-27 kg.
- It is an uncharged particle and hence the electric and magnetic fields have no influence on it.
- It possesses very high penetrating power due to its neutral character.
- It possesses very low ionising power (again due to its neutral character).
- The neutrons can be slowed down by passing them through heavy water, paraffin wax, graphite etc. This happens due to the scattering of the neutrons. The scatterers are commonly known as moderators.
- The slow neutrons, also known as thermal neutrons are found to be more efficient in causing nuclear reactions.
- Neutron is a stable particle within the nucleus. However, outside the nucleus, the neutron is an unstable particle. Its half life period is about 12 minutes. It decays according to the following scheme:
0n1 → 1H1 + -1e0 + \(\bar{v}\),
where \(\bar{v}\) is called antineutrino.

Question 8.
Describe forces which keep nucleons bound together giving their definitions, properties and theory.
Answer:
The forces that keep the nucleons bound together in a nucleus, are called nuclear forces.
Nuclear force. We know that inside the nucleus there are protons and neutrons and the coulomb's force of repulsion is about 1036 times as large as the gravitational force of attraction between two protons. Hence the nucleus should not be stable, but we find that the nucleus is very stable, it means that there must be a third type of force which is stronger than the coulomb's force and exist in the nucleus which is responsible for binding the nucleons together in the nucleus. This force is called nuclear force and it is about 100 times stronger than the coulomb's force.
Properties.
- They are strong attractive forces and exist between two protons, between two neutrons and between a proton and a neutron.
- They are charge independent. Force between two protons = force between two neutrons = force between a proton and neutron.
- They are spin dependent. It is more between nucleons having spin in the same direction and less between those having spin in opposite directions.
- They are short range forces. It is effective only upto a distance of 10 x 10-15 m (10 fermi) and zero beyond it. Hence it is not extended from one nucleon to all nucleons of the nucleus.
- They vary with distance. They start acting only when distance between nucleons becomes 10 fermi. As distance decreases, force increases. Increase is slow upto 5 fermi and then rapid. Force is maximum at distance 15 fermi (Fig. LAQ 8)
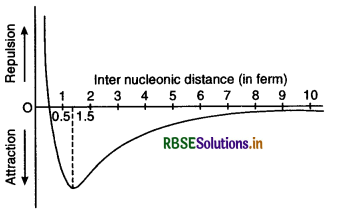
At lesser distances, repulsive forces start acting, decreasing the attractive force.

Question 9.
What is radioactivity? What is the difference between natural and induced radioactivities? Give the properties of different types of rays emitted by radioactive substances.
Answer:
Radioactivity. The phenomenon of spontaneous disintegration of the nucleus of an atom with the emission of some radiations is called radioactivity. There are a number of heavy elements occurring in nature whose nuclei are unstable and undergo spontaneous disintegration with emission of some radiations. The rate of emission or intensity of radiations is not influenced by any external agency such as the temperature or pressure. The substances having this property are called radioactive substances.
Natural and induced (or artificial) radioactivities. The radioactivity exhibited by some naturally occurring elements is called natural radioactivity. All elements with atomic number greater than 82 are radioactive. It is, however, possible to induce radioactivity in the elements with atomic number less that 83 by artificial means. This type of radioactivity is called induced or artificial radioactivity.
Properties of α-rays
- The α-rays are shot out from the radioactive material with large velocities ranging from 1.4 x 107 to 2.3 x 107 ms-1.
- They produce ionisation in the gas through which they pass. The ionising power is 100 times greater than that of ß-rays and 10,000 times greater than that of γ-rays.
- They affect a photographic plate. The effect is very-very feeble.
- They produce fluorescence in substances like zinc sulphide, barium platinocyanide etc.
- The α-rays are scattered when they pass through thin sheets of mica, gold foil etc. The angle of divergence of α-particles from its straight path is 2 to 3 degrees. Geiger and Marsaden found that a few particles, sometimes, were deflected by more than 20°. This was explained by Rutherford to be due to the repulsion between α-particle and the positively charged nucleus of the atom.
- The α-rays are deflected by electric and magnetic fields showing that they are charged particles as shown in in Fig. LAQ 9.
- They produce a heating effect. A quantity of radium always maintains itself at a temperature higher than that of the surroundings. The evolution of heat is due to the stoppage of α, ß and γ rays by the radioactive substance.
- The body suffers incurable burns when exposed to α-rays.
Properties of ß-rays
- ß-rays are shot out from radioactive elements with very high velocities ranging from 1% to 99% of the velocity of light. The velocity of all ß-particles given out by an element is not the same.
- They produce ionisation in air but the number of ions produced is very less than those of α-rays. Although their velocity is very large they possess a comparatively small mass than that of α-particle and hence they have small kinetic energy. As ß-particles are slowed down by collision with the atoms of the gas and change their path, their tracks in a Wilson Cloud Chamber are scattered and not continuous as those of α-particles.
- They affect a photographic plate and their effect is greater than those of α-rays.
- ß-rays produce fluorescence in barium, platinocyanide, calcium, tungsten, willemite etc.
- Because of their small mass, they can penetrate through large thickness of matter, e.g., they can easily pass through 1 cm of thick aluminium sheet.
- They are more easily scattered when they pass through matter, because their mass is very small as compared to the mass of the atomic nuclei.
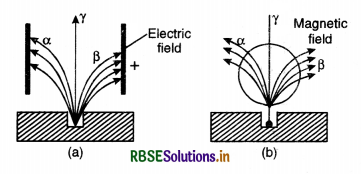
Properties of γ-rays
- The velocity of γ-rays is the same as that of , light i.e. 3 x 108 ms-1.
- They produce ionisation in gases through which they pass but their effect is very small as compared to that of α-rays and ß-rays. This is due to the fact that their mass is very small as compared to that of α and ß-ray particles.
- They affect photographic plate and their effect is greater than those for ß-rays.
- They produce fluorescence in barium, platinocyanide, etc.
- They are more penetrating that even ß-rays and can pass very easily through 30 cm thickness of iron.
- They are diffracted from crystals in a way similar to X-ray diffraction.
- They are not affected by electric and magnetic fields. This shows that they are neutral particles; further experiments have shown that they are similar to e.m. rays.
- γ-rays can be absorbed by the matter.

Question 10.
Write down the laws of radioactive disintegration and deduce an expression for the decay law. Hence define disintegration constant.
Or
State the laws of radioactive decay and hence derive the relation N = N0e-λt where the letters have their usual meanings.
Answer:
Laws of Radioactive disintegration
- Radioactivity is spontaneous process which does not depend upon external factors.
- During disintegration either α or ß-particle is emitted. Both are never emitted simultaneously.
- Emission of α-particle decreases atomic number by two and mass number by 4.
- Emission of ß-particle increases atomic number by one but mass number remains the same.
- Emission of γ-ray does not change atomic or mass number.
- The number of atoms disintegrated per second is directly proportional to the number of radioactive atoms actually present at that instant. This law is called radioactive decay law.
Let at t = 0
N0 = number of atoms present
N = number of atom left after time t.
dN = number of atoms disintegrated in time dt.
i.e. \(-\frac{d \mathrm{~N}}{d t}\) ∝ N
or \(-\frac{d \mathrm{~N}}{d t}\) = λN, ............................(i)
where λ is a constant called disintegration constant and depends upon the nature of the radioactive substance.
Now from, (i), we have
\(\frac{1}{N}\)dN = -λ dt
or ∫\(\frac{1}{N}\) dN = -λ∫ dt
or logeN = λt + C, ................................(ii)
where C is constant of integration. To determine its value,
since N = N0 initially,
i.e. when t = 0, N = N0
logeN0 = 0 + C
Substituting the value of C in (ii), we have
logeN = -λt + logeN0
or logeN - logeN0 = -λt
loge\(\frac{\mathrm{N}}{\mathrm{N}_0}\) = -λt
or \(\frac{\mathrm{N}}{\mathrm{N}_0}\) = e-λt
or N = N0 e-λt ...........................(iii)
which is the required equation.
Eq. (iii) shows that the radioactive decay occurs according to exponential law and the number of radioactive atoms decreases exponentially. Since N shall become zero only when t becomes infinity, it shows that time taken by a radioactive element to disintegrate completely is infinitely long.
The curve in Fig. LAQ 10. shows the exponential decay radioactive substance.
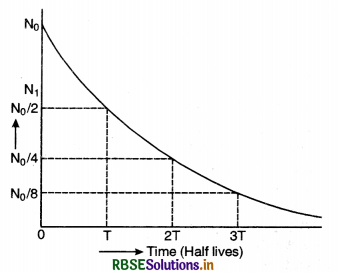
Disintegration constant
In Eq. (iii), if t = 1/λ
Then N = N0 e-λx\(\frac{1}{\lambda}\) = N0e-1 = \(\frac{\mathrm{N}_0}{e}\)
Thus disintegration constant is defined as the time after which the number of radioactive atoms reduce to 1/e times the original number of atoms.

Question 11.
What is meant by (i) half life Band (ii) average life of a radioactive substance? Derive expression for them. What is relation between average life and half life.
Answer:
(i) Half life period. It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Let N0 = number of atoms at t = 0 (at the start of the observation)
N = number of atoms after time t
So, N = N0e-λt ........................(1)
where λ is constant called disintegration constant
Let T = half life period
So, when t = T, N = -N0/2.
Substituting in Eq, (1), we have
\(\frac{\mathrm{N}_0}{2}\) = N0e-λt
eλt = 2
λt = loge2 = 2.303 log102
= 0.693
or T = \(\frac{0.693}{\lambda}\)
(ii) Average life. The atoms of a radioactive substance are constantly disintegrating and thus the life of every atom is different.
So average life of a radioactive substance is the ratio of sum of lives of all the atoms to the total number of atoms.
Average life =
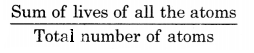
Suppose dN atoms disintegrate in a time dt, t seconds after the separation of the substance, when the actual number of atoms present is N.
Then \(\frac{d \mathrm{~N}}{d t}\) = -λN
dN = -λNdt = -λN0e-λt dt
where N0 is the number of atoms in the beginning of time. Each of these dN atoms disintegrate between the time t and t + dt i.e these atoms had a life of t seconds.
∴ Total life of dN atoms = t dN
Hence total life time of all the atoms
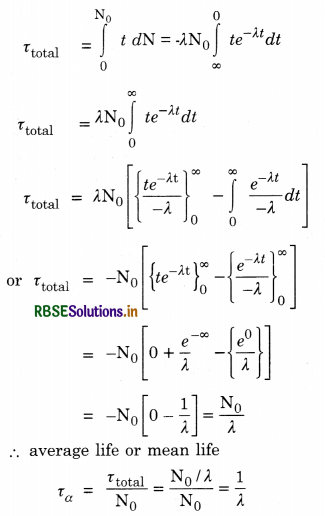
Hence average life τa of the atom is the reciprocal of the radioactive disintegration constant λ.
Relation between average life and half life
Since average life of a radioactive element is given by
τa = \(\frac{1}{\lambda}\) ...........................(i)
and half life of a radioactive element is given by
T = \(\frac{0.693}{\lambda}\)
Using Eq. (i), we get
T = 0.693 τa.

Question 12.
What is meant by the Alpha decay? Estimate the velocity or energy of α-particle.
Answer:
Alpha decay
Alpha decay is a process involving the emission of a fast-moving helium nucleus (an alpha-particle) by nuclei which generally contain 210 or more nucleons.
Since 2He4 contains two protons and two neutrons, after an alpha emission the parent nucleus is transformed into a daughter nucleus which has (i) an atomic number smaller by two and (ii) mass number smaller by four.
Transformation of the ZXA nucleus into the Z-2XA-4 nucleus by an alpha decay can be expressed by the equation.
ZXA = Z-2YA-4 + 2He4 ...........................(1)
Energy released. The energy Q, released in this process, can be obtained from Einstein's mass energy relation.
It is given by the expression
Q = (MX - MY - Mα) c2
This energy is shared by the daughter nucleus, Z-2YA-4 and the alpha-paraticle, 2He4.
As the parent nucleus ZXA is at rest before it undergoes alpha-decay, alpha-particles are emitted with fixed energy, which can be calculated by applying the principle of conservation of energy and momentum.
Let vα and vy be the velocities of the alpha-particles and the daughter nucleus, Z-2YA-4. According to the principle of conservation of momentum,
MY. vY = Mα. vα ..................(2)
By equating the sum of kinetic energies of the nucleus Y and the alpha particle of the released in the alpha-decay, we have another equation.
\(\frac{1}{2}\) Mα.vα2 + \(\frac{1}{2}\) MYv2 = Q .........................(3)
By substituting for vy from Eq. (2) in Eq. (3), we can easily obtain
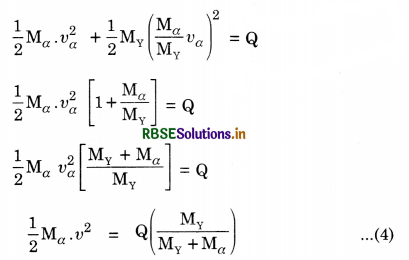
If we substitute My = A - 4 amu and Mα = 4 amu in Eq. (4) the kinetic energy carried by the alpha-particle can be approximated by the relation.
[K.E.]α = Q\(\frac{(\mathrm{A}-4)}{\mathrm{A}}\) .......................(5)

Question 13.
What is beta decay? Write a brief account of ß-particle spectrum.
Answer:
Beta decay. The emission of electron (ß-particle) from the nucleus of an atom is called ß-decay. It is believed that emission of an electron by the nucleus is due to transformation of a neutron into a proton and an electron. When a neutron changes into a proton, a ß-particle is ejected whereas a proton remains in the nucleus. The emission of ß-particle, therefore:
- increases the atomic number by one and
- keeps the mass number unaffected.
The energy of ß-disintegration is provided by the difference between the mass of the parent nucleus and the sum of the masses by the daughter nucleus and ß-particles. The ß-disintegration energy is thus given by:
Eß = ZMnA - [Z+1MnA + me] ........................(1)
where
Mn = mass of nucleus
me = mass of electron
(A, Z) and (A, Z + l) = mass number of atom i.e. number of parent and daughter nuclei.
Theory of ß-particle Spectra
According to Eq. (1) the same mass disappears during the emission of a ß-particle and all ß-particles must be emitted with the same energy. But actually it is found that
(1) ß-particle emitted by a radioactive substance has a continuous energy range, extending from zero to a certain maximum. The upper energy level is called the end point energy and is the characteristic of nature of ß-emitter Fig. LAQ 13 shows the spectrum of the ß-particles emitted with end point energy 1.17 MeV.
(2) Every ß-particle energy distribution curve has a well defined maximum whose height and position depend upon the nature of ß-emitter.
Pauli suggested that in ß-particle disintegration, another particle neutrino was simultaneously emitted along with ß-particle. The neutrino is supposed to be fundamental particle having:
(i) zero charge
(ii) zero rest mass, and
(iii) a spin of \(\frac{1}{2} \cdot \frac{h}{2 \pi}\)
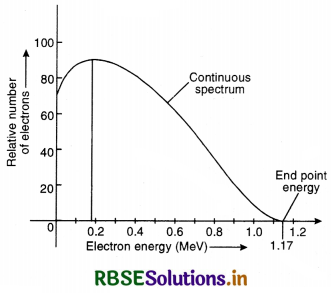
A ß-decay can thus be represented as
n → p + e- + v (neutrino)
Under neutrino hypothesis, each ß-decay is accompanied by discrete energy Eß which corresponds to the end point energy in the continuous ß-particles spectrum. The disintegration energy is shared by ß-particle, neutrino and the recoil nucleus in a continuous range of different ways. The neutrino may take any amount of the available ß-disintegration energy so that the energy left at the disposal of the ß-particle will be different for each disintegration. Thus ß-particles of all possible energies extending from zero to a certain maximum (end point energy) shall be emitted. Thus Pauli's neutrino hypothesis satisfactorily explains the continuous energy range of ß-particle spectrum.
It was found late that ß-decay is accompanied by two kinds of neutrinos, the neutrino (v) and antineutrino (\(\bar{v}\)). In ordinary ß-decay, it is an antineutrino that is emitted.
n → p + e- + \(\bar{v}\)

Question 14.
What is gamma decay? Write a short note on the energy distribution in gamma ray spectrum.
Answer:
Gamma decay. The emission of high energy photons, also called gamma rays, by a radioactive substance is called gamma decay.
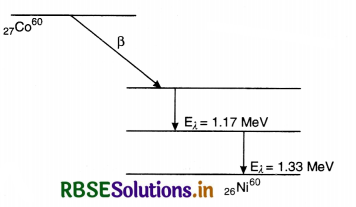
The emission of an alpha or beta particle by a radioactive nucleus often leaves the product nucleus in an excited state. The excited nuclei return to their ground state by emitting γ-rays whose energies correspond to the energy differences between the various excited states and the ground state. The γ-rays emitted by the nuclei have an energy range upto several MeV. The γ-rays emitted by a source thus possess discrete, though different energies and gamma ray energy spectrum consists of sharp lines of definite wavelength.
γ-rays are uncharged, they suffer weak interaction with matter and are not easily detected.
A well known example of such a process is decay of 27Co60. By beta-emission, the 27Co60 nucleus is first transformed into the excited 28Ni60 nucleus, which in turn reaches the ground state by emitting photons of energies 1.17 MeV and 1.33 MeV. In Fig. 13.20 this process has been shown through an energy-level diagram.

Question 15.
What is rate of decay? What are the units of measuring radioactivity?
Answer:
Rate of Decay (R). It is equal to the number of radioactive disintegrations in the sample taking place per second. Mathematically, it is represented as:
R (t) = \(\frac{d \mathrm{~N}}{d t}\) = λN (t) ....................(1)
Unit of measuring radioactivity
Radioactivity is measured by the number of radioactive nuclei that decay per second. The two units of radioactivity are curie (ci) and rutherford (rd).
(i) Curie. It is the radioactivity of 1 gram of radium. One gram of radium gives only 3.7 x 1010 disintegrations per second. Therefore, the radioactivity of a sample is said to be 1 curie if it undergoes 3.7 x 1010 disintegrations per second.
∴ 1 ci = 3.7 x 1010 disintegrations, and 1µ ci= 3.7 x 104 disintegrations.
(ii) Rutherford (rd). The radioactivity of a material is said to be 1 rutherford if it undergoes 106 disintegrations per second. Thus
1 rd = 106 disintegrations
∴ 1 µ ci = 3.7 x 104 rd and 1 m ci = 37 rd.

Question 16.
What do you mean by nuclear reaction? What quantities are conserved in nuclear reaction? What is Q-value of nuclear reaction?
Answer:
Nuclear Reaction
The conversion of the nucleus of an element into a nucleus of another element is called a nuclear reaction. This is generally caused by bombarding the target nucleus by high energy particle.
For example, if an incident projectile ‘a’ hits the target nucleus X, a nuclear reaction takes place and as a result there is a new nucleus Y and an outgoing particle ‘b’ are produced.
i.e. a + X → Y + b
In short form, this reaction can be written as X (a,b) Y.
In 1919, Rutherford observed that when α-particles, from a polonium source were made to hit nitrogen atom, protons were given off in the reaction. The nuclear reaction for this process can be written as
7N14 + 2He4 → 8O17 + H1
The above reaction can also be written as 7N14 (α,p) 8O17.
Following quantities remain conserved during a nuclear reaction.
- Conservation of charge. Total charge of the reactant is conserved.
- Conservation of nucleons. Total number of nucleons before and after the reaction remains constant.
- Mass and energy conservation. In a nuclear reaction mass and energy are not separately conserved. However, their total is always conserved i.e. the energy equivalent of mass is liberated as can be understood in the Q-value of the nuclear reactions.
- Conservation of linear momentum. It is a fundamental law and is always conserved in nuclear reactions also.
- Conservation of angular momentum. Total angular momentum J is composed of orbital angular momentum L and spin angular momentum S. Vector sum of the total angular momentum is conserved in a nuclear reaction.
Q-Value of a nuclear reaction
Consider a nuclear reaction represented by the equation
a + x → y + b
where target nucleus ‘x’ is supposed to be at rest initially. Since the total energy of the system is to be conserved. Let the mass of projectile ‘a’, target nucleus x, product nucleus y and product particle ‘b' be represented as ma, Mx, My and ma respectively. Then from the law of conservation of total energy, we have
(Ea + mac2) + Mxc2 = (Ey + Myc2) + (Eb + mp c2) .......................(1)
Where E’s correspond to the kinetic energies. Let us introduce a quantity called Q-value of the nuclear reaction, which represents the difference of energy of the products and the reactants.
i.e. Q = Ey + Eb - Ea
So Eq. (1) may take the form
(ma + Mx - My - mb) c2
= Ey + Eb - Ea = Q
or Q = [(ma +Mx) - (My + mb)] c2
Thus Q-value of a nuclear reaction is defined as the energy available from the difference in mass of the projectile plus the target and the product nucleus and the product particle.
If (ma + Mx) > (My + mb), then Q > 0 and the reaction releases energy called exoergic reaction. If (ma + Mx) < (my + mb), then Q < 0 and the reaction absorb energy and called endoergic reaction.

Question 17.
What is nuclear fission? Discuss the source of energy released during fission.
Answer:
Nuclear fission. A process in which a heavy nucleus disintegrates into two lighter nuclei of nearly equal masses, is called nuclear fission
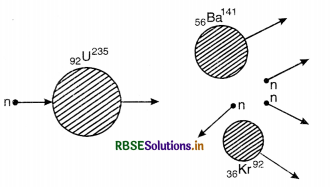
Slow neutrons were bombarded on a heavy nucleus of 92U235 yielding two lighter nuclei of barium (56Ba141) are krypton (36Kr92) along with three neutrons and tremendous amount of energy (Fig. LAQ 17). The nuclear reaction can be represented as follows:
92U235 + 0n1 → 56Ba141 + 36Kr92 + 3 0n1 + Q
Energy released in fission. This huge energy is provided by the difference of mass of the nuclei before and after fission.
Before fission:
Mass of 92U235 = 235.0439 a.m.u.
Mass of one 0n1 = 1.0087 a.m.u.
Total mass of reactants = 236.0526 a.m.u.
After fission. Mass of 56Ba141 = 140.9139 a.m.u.
Mass of 36Kr92 = 91.8973 a.m.u.
Mass of 3 neutrons = 3.0261 a.m.u
Total mass of reaction products = 235.8373 a.m.u.
Mass lost in fission = 236.0526 - 235.8373 = 0.2153 a.m.u.
This mass is converted into energy in the fission process. Thus, energy released in a single fission = 2153 x 931 = 200 MeV
This energy appears mainly in the form of kinetic energy of fission fragments and secondary neutrons. It may be subsequently converted into heat. The nuclear fission is important reaction for two reasons.
- A large amount of energy about 200 MeV is released in fission of each nucleus.
- The reaction is accompanied by emission of secondary neutrons.
Theory. Bohr and Wheeler gave the explanation of the nuclear fission based on the liquid drop model of the nucleus. The nucleus is considered to be spherical in shape and is acted upon by two kinds of forces.
- The surface tension force (maintaining its spherical shape)
- The coulomb's repulsive force (tending to deform the shape).
When these spherical nuclei are bombarded by slow neutrons, results in its capture and form a compound nucleus and sets oscillations in the nucleus tending to deform its shape and the surface tension forces try to keep it spherical, but if the effect of coulomb’s force is increased, the nucleus ultimately splits into two parts of comparable masses and few neutrons. The sequence of steps leading to fission is as shown in Fig LAQ 17 (a).
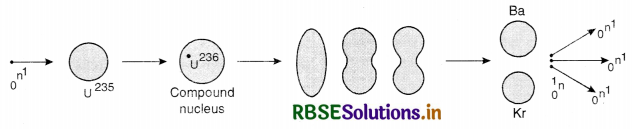
Question 18.
Explain the meaning of ‘chain reaction’ and controlled ‘chain reaction’.
Answer:
Nuclear chain reaction. In fission reaction, on the average three neutrons are emitted. These three neutrons can further produce fission of three more uranium nuclei and so on. This can lead to a chain reaction and within no time a large number of uranium can undergo fission resulting in the enormous amount of energy.
It is also possible that one or more neutrons produced during fission may escape out of uranium piece or may be absorbed and get lost. The chain reaction can be sustained only if, on the average, more than one neutron per fission are available to cause further fission as shown in Fig. LAQ 18. The escape of neutrons which do not cause further fission depends upon following factors.
1. Leakage of neutrons from the system. If the size of the fissionable material is small, then the incident neutrons escape and the chain reaction will not be sustained. So for a sustained nuclear chain reaction, the number of neutrons lost must be equal to the number of neutrons produced and the system is said to be critical. For a material to be of critical size, its linear dimension, should be of the order of mean free path of the neutrons.
2. Presence of non-fissionable material in the system. The non-fissionable material present in the sample of U235 can slow down the incident neutrons and they get absorbed without causing further fission. Thus to have sustained chain reaction, the fissionable material should be free from such impurities.
3. Absorption of neutrons by U238. In a sample of uranium, there are three isotopes namely U233, U235 and U238 having abundance of 0.006%, 0.714% and 99.28%. If neutrons are made to bombard on such a material, they are slowed down by collision with U238 and are thus absorbed. U238 is also fissionable but require the energy of incident neutrons more than 1 MeV, where as U235 is fissionable with neutrons having energy of the order of 0.025 eV. Thus for self-sustained chain reaction to take place either U238 is seprated from U235 (this is called enriched uranium) or moderators be used to slow down the neutrons to energy about 0.025 eV to cause fission in U235.
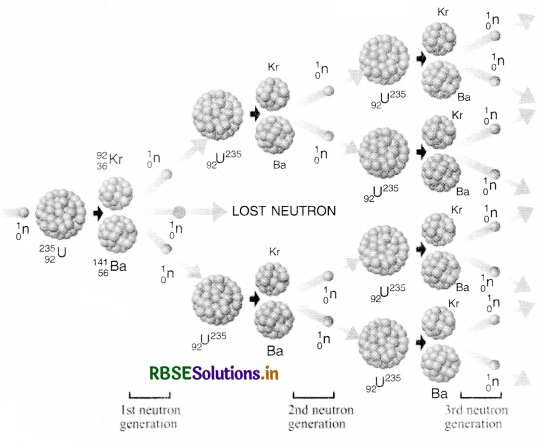

Question 19.
Explain the process of release of energy in a nuclear reactor. Draw a labelled diagram of a nuclear reactor and write down the function of each part.
Or
What is controlled nuclear chain reaction? Describe a nuclear reactor and the function of each component.
Answer:
Nuclear Reactor. Uncontrolled chain reaction causes a lot of damage. If the chain reaction is controlled in such a way that on the average, one neutron from each fission is left to excite further fission, the number of fissions occurring per second thus remains constant. In such cases, energy will not get out of control. Such a system in which the arrangement for absorption of desired number of neutrons is provided so that the chain reaction may proceed in a controlled manner, is called a nuclear reactor. Thus nuclear reactor is a device in which a controlled chain reaction produces radioactive isotopes and energy.
A nuclear reactor consists of following parts:
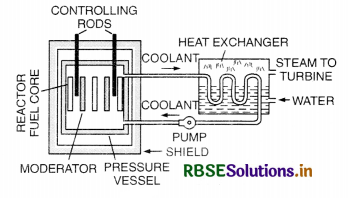
1. Nuclear Fuel. Nuclear fuel is a material such as U235, U233, Pu239 which is fissionable by thermal neutrons. Out of these materials only U235 is available in nature mixed with U238. Nuclear fuel is fabricator of the form of rods which are sealed in aluminium containers. The containing nuclear fuel are called fuel elements.
2. Moderator. The fuel elements are placed parallel to each other separated by some material known as moderator as shown in Fig. LAQ 19 (b). The purpose of a moderator is to slow down the fast neutrons emitted in fission. Most of these neutrons have energies in the range 1 MeV to 2 MeV. The energy of fast neutrons is reduced to thermal value (0.25 eV) as a result of many collisions with the atoms of the moderator. The material of the moderator should:
- have low mass number.
- not absorb neutrons
- be physically and chemically stable in the high temperature conditions in the reactor.
Graphite and heavy water fulfil these conditions and are mostly used as moderators.
3. Control rods. The chain reaction is controlled by means of control rods. The control rods are made of materials which intensely absorb neutrons. These rods can be inserted into the core partially or completely.
The part of reactor which contains fuel elements moderator and control rod is called core. The chain reaction takes place in the core and hence the energy is liberated in it.
4. Coolant. The cooling fluid which removes heat is called coolant. These are water, CO2 and liquid metal. The heat carried away by the coolant is used to generate steam which in turn operates generators.
5. Shielding. To prevent the escape of high energy radiation from the core, it is surrounded by a layer of thick concrete containing a high proportion of light and high thick elements.
- They are used to produce electric energy.
- They are used to prepare radio isotopes.
- It can generate power for propulsion of ships, submarine and aircrafts.
- It produces plutonium for explosive purpose.
Working. Neutrons produced by the action of α-particles on polonium or beryllium are slowed down and are used initially to bring about fission of say U235 nuclei. The emitted neutrons are slowed down by the passage through the moderator to split further U235 nucleus. The chain reaction is said to be steady so that effective multiplication factor Ke is 1.
Ke = \(\frac{\text { Rate of emission of neutrons }}{\text { Rate of loss of neutrons }}\)
Some lost neutrons are absorbed by 92U238 present in enriched uranium which gives rise to 92U239.
0n1 + 92U238 → 92U239 → -1ß0 + 93Np239
93Np239 → -1ß0 + 94Pu239 → 92U235 + 2He4
94Pu239 is produced as a bye product and can be used for fission purpose. India used 94Pu239 in nuclear device which was exploded on 18th May 1974 and later on May 11 & 13, 1998 at Pokhran.
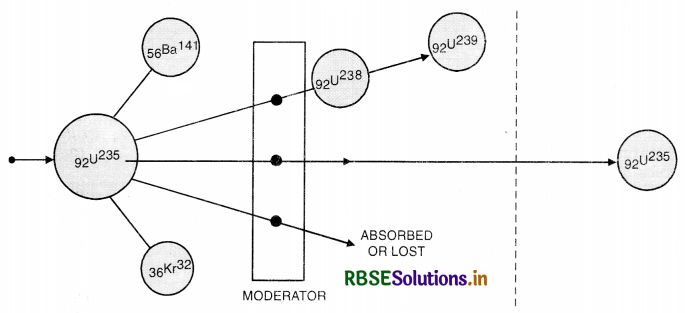
Uses. A nuclear reactor is used for generation of electricity, preparation of radio isotopes. Most of energy set free is in form of heat which through heat exchange is made to furnish steam to operate conventional turbines and electric generators.

Question 20.
(a) Explain nuclear fusion. What are the conditions for fusion?
(b) What are the sources of energy in sun and stars?
Answer:
(a) Nuclear fusion. Nuclear fusion is process in which lighter nuclei (like 1H1) are fused together into heavier atom (like 2He4), with the release of enormous amoun t of energy. The energy thus released is known as thermonuclear energy and the reaction is called thermonuclear reaction. The cause of energy is again mass defect. Nuclear fusion takes place at very high temperature and very high density.
e.g. two deuterons can fuse together to form a medium nucleus and release energy of about 24 MeV.
i.e., 1H2 + 1H2 → 2He4 + 24 MeV.
Some other examples of such reactions are
1H1 + 1H1 → 1H2 + +1e0 + 0.42 MeV
4 1H1 → 2He4 + 2 +1e0 + 26.7 MeV
1H3 + 1H2 → 2He4 + 0n1 + 17.6 MeV
3Li7 + 1H1 → 2He4 + 2He4 + 17.3 MeV.
Nuclear fusion reactions take place in the presence of very high temperature (about 107 K), so they are called thermonuclear reactions. All these reactions are exoergic and release huge energies. The energy comes from conversion of mass of final stage nucleus formed as a result of fusion is always less than that of the individual light nuclei.
The temperature of the order of 107 K is very difficult to obtain. The mechanism involved for producing high temperature is a self-sustained fission explosion. When an atom bomb containing U235 explodes, very high temperature ( ≃ 107 K) is produced. Thus atomic explosion acts as trigger and release tremendous amount of energy.
Conditions for Fusion
(1) Either the reacting nuclei should be given large amount of kinetic energy to overcome the mutual repulsion between them.
(2) Or a very higher temperature should be produced. Such high temperature exists in the territory of stars. Therefore, thermal fission is possible in stars.
(b) Source of energy in sun and stars.
The temperature of the core of the sun is about 20 million degrees. At this high temperature, proton-proton cycle takes place with the release of large amount of energy. The reactions are:
1H1 + 1H1 → 1H2 + 1e0 + 0.41 MeV
1H1 + 1H2 → 2He3 + 5.51 MeV
2He3 + 2He3 → 2He4 + 2(1H1) + 12.98 MeV
Adding these reactions, we get
4(1H1) → 2He4 + (1e0) + 24.7 MeV
Hence, fusion of 4 hydrogen nuclei results one helium nucleus, two positrons (1e0) and liberation of 24.7 M eV of energy. Estimation of hydrogen in the sun reveals that it can continue proton-proton cycle going for about 30 billion years. A similar thermonuclear reaction takes place in some stars also.
But in stars, whose core is much more hot than that of sun, another type of thermonuclear reactions take place. These are called carbon nitrogen cycle.
Carbon-Nitrogen Cycle
6C12 + 1H1 → 7N13 + hv (energy)
7N13 → 6C13 + +1e (positron)
6C13 + 1H1 → 7N14 + hv (energy)
7N14 + 1H1 → 8O15 + hv (energy)
8O16 → 7N15 + 1e (positron)
7N14 + 1H1 → 8O16 + hv (energy)
8O16 → 6C12 + 2He4
Adding, 4 1H1 → 2He4 + 2e+ + 26.7 MeV
Hydrogen bombs make use of fusion reactions. Hence, they are called fusion bombs.

Question 21.
State the laws of radioactive decay. Deduce the relation N = N0e-λt. Sketch a graph to illustrate radioactive decay. Define hlf life.
Answer:
Laws of Radioactive disintegration
- Radioactivity is spontaneous process which does not depend upon external factors.
- During disintegration either α or ß-particle is emitted. Both are never emitted simultaneously.
- Emission of α-particle decreases atomic number by two and mass number by 4.
- Emission of ß-particle increases atomic number by one but mass number remains the same.
- Emission of γ-ray does not change atomic or mass number.
- The number of atoms disintegrated per second is directly proportional to the number of radioactive atoms actually present at that instant. This law is called radioactive decay law.
Let at t = 0
N0 = number of atoms present
N = number of atom left after time t.
dN = number of atoms disintegrated in time dt.
i.e. \(-\frac{d \mathrm{~N}}{d t}\) ∝ N
or \(-\frac{d \mathrm{~N}}{d t}\) = λN, ............................(i)
where λ is a constant called disintegration constant and depends upon the nature of the radioactive substance.
Now from, (i), we have
\(\frac{1}{N}\)dN = -λ dt
or ∫\(\frac{1}{N}\) dN = -λ∫ dt
or logeN = λt + C, ................................(ii)
where C is constant of integration. To determine its value,
since N = N0 initially,
i.e. when t = 0, N = N0
logeN0 = 0 + C
Substituting the value of C in (ii), we have
logeN = -λt + logeN0
or logeN - logeN0 = -λt
loge\(\frac{\mathrm{N}}{\mathrm{N}_0}\) = -λt
or \(\frac{\mathrm{N}}{\mathrm{N}_0}\) = e-λt
or N = N0 e-λt ...........................(iii)
which is the required equation.
Eq. (iii) shows that the radioactive decay occurs according to exponential law and the number of radioactive atoms decreases exponentially. Since N shall become zero only when t becomes infinity, it shows that time taken by a radioactive element to disintegrate completely is infinitely long.
The curve in Fig. LAQ 10. shows the exponential decay radioactive substance.
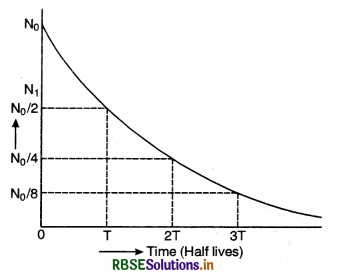
Disintegration constant
In Eq. (iii), if t = 1/λ
Then N = N0 e-λx\(\frac{1}{\lambda} \)= N0e-1 = \(\frac{\mathrm{N}_0}{e}\)
Thus disintegration constant is defined as the time after which the number of radioactive atoms reduce to 1/e times the original number of atoms.
(i) Half life period. It is the time during which the number of atoms of a radioactive material reduces to half of the original number.
Let N0 = number of atoms at t = 0 (at the start of the observation)
N = number of atoms after time t
So, N = N0e-λt ........................(1)
where λ is constant called disintegration constant
Let T = half life period
So, when t = T, N = -N0/2.
Substituting in Eq, (1), we have
\(\frac{\mathrm{N}_0}{2}\) = N0e-λt
eλt = 2
λt = loge2 = 2.303 log102
= 0.693
or T = \(\frac{0.693}{\lambda}\)
(ii) Average life. The atoms of a radioactive substance are constantly disintegrating and thus the life of every atom is different.
So average life of a radioactive substance is the ratio of sum of lives of all the atoms to the total number of atoms.
Average life =
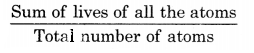
Suppose dN atoms disintegrate in a time dt, t seconds after the separation of the substance, when the actual number of atoms present is N.
Then \(\frac{d \mathrm{~N}}{d t}\) = -λN
dN = -λNdt = -λN0e-λt dt
where N0 is the number of atoms in the beginning of time. Each of these dN atoms disintegrate between the time t and t + dt i.e these atoms had a life of t seconds.
∴ Total life of dN atoms = t dN
Hence total life time of all the atoms
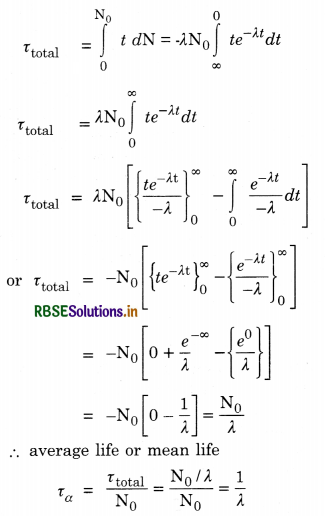
Hence average life τa of the atom is the reciprocal of the radioactive disintegration constant λ.
Relation between average life and half life
Since average life of a radioactive element is given by
τa = \(\frac{1}{\lambda}\) ...........................(i)
and half life of a radioactive element is given by
T = \(\frac{0.693}{\lambda}\)
Using Eq. (i), we get
T = 0.693 τa.

Question 22.
State three properties of nuclear forces. Show that the density of nuclear matter is independent of mass number A.
Answer:
(a) Strcture of nucleus. Rutherford's experiments on the scattering of α-particles by atoms led to the discovery of a positively charged heavy central core, called nucleus, of radius of the order of 10-15 m.
1. The entire positive charge and almost the entire mass of the atom is concentrated in the nucleus.
2. Nucleus contains protons and neutrons called nucleons. A proton carries a unit positive charge and mass 1836 times that of an electron. The neutron is electrically neutral and has mass 1836.6 times that of an electron. The number of protons in the nucleus is called atomic number (Z) whereas the total number of neutrons and protons is called mass number (A).
3. Negatively charged electrons round the nucleus revolve in various orbits. The negative charge on the electron is of the same magnitude as the positive charge on the proton. The number of electrons revolving round the nucleus is equal to that of protons so that an atom is normally electrically neutral.
(b) Nuclear charge. The positive charge of the nucleus is due to the presence of protons. The charge on a proton is equal to 1.6 x 10-19 C. If Z be the atomic number, then nuclear charge = Z x 1.6 x 10-19 C.
The mass of the nucleus is equal to the sum of the masses of all protons and neutrons minus the mass defect.
Let Z = atomic number
A = mass number
Mn = mass of neutron
and ∆m = mass defect
Then the number of neutrons = (A - Z)
∴ Mass of the nucleus = ZMp + (A - Z) Mn - ∆m.
Nuclear size. The distance of the closest approach of α-particle from. the nucleus is the measure of radius of nucleus or size of nucleus. For metals its value is about 10-14 m.
The dimensiohs of nucleus have been measured by several experiments involving the scattering of n,p,e, etc. Every time it is found that the volume of nucleus is directly proportional to the number of nucleons (A) in the nucleus.
If R is the radius of the nucleus having mass number A, then
\(\frac{4}{3}\)π R3 ∝ A
or R3 ∝ A
or R ∝ A1/3
or R = R0A1/3
where R0 = 1.2 x 10-15 m = 1.2 fm. It shows that greater the mass number, greater the nuclear radius.
Density of nucleus (ρ) It is defined as the nuclear mass per unit volume.
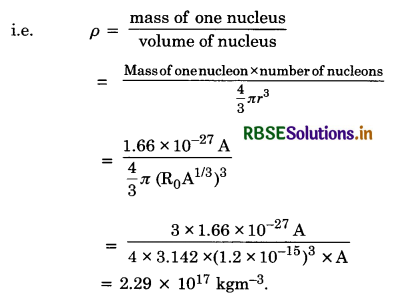
Thus the nuclear density is of the order of 1017 kgm-3 and is independent of its mass number. Therefore, all nuclei have the same approximate density.
Mass defect. It is found that the mass of a stable nucleus is always less than the sum of masses of its constituent protons and neutrons in their free state. The difference between the sum of the masses of nucleons constituting it and the rest mass of the nucleus is called mass defect.
Let
Mp = mass of protons in the nucleus
Mn = mass of neutrons in the nucleus
M = mass of nucleus
N = number of neutrons
Z = number of protons
A = total number of nucleous
∴ The mass defect
∆m = ZMp + (A - Z) Mn - M.
Packing fraction (f). Though the atomic masses are considered to be whole numbers, yet they differ from the integers by small amount. This variation of atomic masses from whole numbers is considered to be due to some deviations of the mass of the nucleus from the mass of the constituents of the nucleus (i.e. protons and neutrons). The term packing fraction is defined as the mass defect per nucleon.
∴ f = \(\frac{\text { mass defect }}{\text { mass number }}=\frac{\Delta m}{\mathrm{~A}}=\frac{\mathrm{M}-\mathrm{A}}{\mathrm{A}}\)
where M is the atomic mass, A is the mass number (i.e. number of protons and neutrons in a.m.u.)
Thus f can be +ve, -ve or zero accordingly as M > A, M < A, M = A.
Packing fraction is a fundamental characteristic of the nucleus and is directly related to the availability of the energy of the nucleus and its stability. A negative packing fraction which will also correspond to a negative mass defect will mean that the atomic mass of the nucleus is less than the nearest whole number which will correspond to some conversion of mass into energy during the formation of the nucleus. Also, negative packing fraction will imply that the nucleus is exceptionally stable because we have to supply more energy to break the nucleus into its constituent nucleons while a positive packing fraction will indicate the nucleus to be less stable.
HOTS QUESTIONS
Question 1.
How many electrons, protons and neutrons are there in 12 g of 6C12 and in 14 g of 6C14?
Answer:
12 g of 6C12 has number of atoms = 6 x 1023
∴ the number of electrons in 12 g of 6C12
= 6 x 6 x 1023 = 36 x 1023
The number of protons in 12 g of 6C12 = 36 x 1023
The number of neutrons in 12 g of 6C12 = (A - Z) x 6 x 1023 = 6 x 6 x 1023 = 36 x 1023
Similarly
No. of electrons in 14 g of 6C14 = 36 x 1023
No. of protons in 14 g of 6C14 = 36 x 1023
No. of neutrons in 14 g of 6C14 = (A - Z) x 6 x 1023
= (14 - 6) x 6 x 1023
= 48 x 1023.

Question 2.
At any instant, the ratio of the amount of radioactive substance is 2 : 1. If their half lives be respectively 12 and 16 hours, then after two days what will be the ratio of the substances?
Answer:
No. of half lives, N1 =\( \frac{48}{12}\) = 4 and N2 = \(\frac{48}{16}\) = 3 [∵ n = \(\frac{t}{\mathrm{~T}}\)]
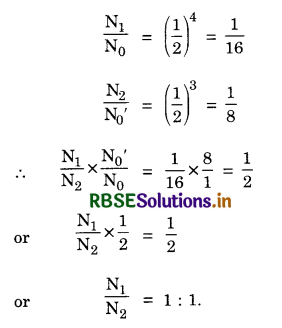
Question 3.
Radioactive element A are being produced at a cbnstant rate α. The element has a decay constant λ. At time t = 0, there are N0 nuclei of the element.
(a) Calculate the number of nuclei A at time t.
(b) If α = 2 N0λ, calculate the number of nuclei of A after one half life of A and also limiting value of N as t → ∞.
Answer:
(a) Rate of production of radioactive nuclei = λN
Rate of decay of radioactive nuclei = λN
∴ \(\frac{d \mathrm{~N}}{d t}\) = α - λN
or \(\frac{d \mathrm{~N}}{\alpha-\lambda \mathrm{N}}\) = dt ............................(i)
Since at t = 0, N = N0 and at t = 1, N = N1
So integrating eq. (i) within these limits, we get
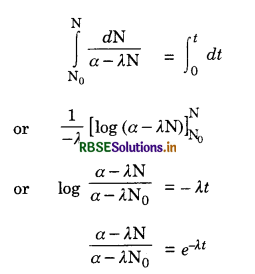
or α - λN = (α - λN0)e-λt
or λN = α - (α - λN0)e-λt
= (1 - e-λt) + λN0e-λt
or N = \(\frac{\alpha\left(1-e^{-\lambda t}\right)}{\lambda}\) + N0e-λt
(b) Given α = 2N0λ and t = \(\frac{0.693}{\lambda}\)
So N = \(\frac{2 \mathrm{~N}_0 \lambda}{\lambda}\) (1 - e-0.693) + N0e-0.693
= 2N0 - 2N0e-0.693 + N0e-0.693
or N = 2N0 - N0e-0.693
= N0 (2 - e-0.693)
= N0 [2 - \(\frac{1}{2}\)] = N0 \(\frac{3}{2}\)
or N = 1.5 N0
(c) At t → ∞
N = \(\frac{\alpha}{\lambda}=\frac{2 \mathrm{~N}_0 \lambda}{\lambda}\) = 2 N0.

Question 4.
Calculate the energy liberated in kWh,where 100 g of 3Li7 are converted into 2He4 by proton bombbardment. Given mass of 3Li7 = 7.0183 u, mass of 2He4 = 4.0040 u and mass of 1H1 = 1.0081 u.
Answer:
The given reaction is
3Li7 + 2He4 → 2(2He4)+ Q
Total mass before reaction = 7.0183 + 1.0081
= 8.0264 u
Total mass after reaction = 2 x 4.0040 = 8.0080 u
Mass defect
∆m = 8.0264 - 8.0080 = 0.0184 u
Loss of mass for 8.0183g of 3Li7 = 0.0184g
Loss of mass for 100g of 3Li7 = \(\frac{0.0184}{8.0183}\) x 100
∴ Energy librated
Q = ∆mc2 = \(\frac{0.0184 \times 100 \times 10^{-3}}{7.0183}\) x 9 x 1016 J
Q = \(\frac{0.0184 \times 1000 \times 10^{-3}}{7.0183 \times 3.6 \times 10^6}\) x 9 x 1016 kWh
= 5.53 x 105 kWh.
Question 5.
There are two radioactive substances A and B. Decay constant of B is two times that of A. Initially both have equal number of nuclei. After n half lives of A, rate of disintegration of both are equal. Find the value of n.
Answer:
Let λA = A, So λB = 2λ
If total number of atoms in A and B at t = 0 is N0
Then, initial rate of disintegration of A = λN0
Initial rate of disintegration of B = 2λN
Since λB = 2λA
∴ TB = \(\frac{\mathrm{TA}}{2}\)
So after one half life of A
\(-\frac{d \mathrm{~N}}{d t}=\frac{\lambda \mathrm{N}_0}{2}\)
And after two half lives of B
\(-\frac{d \mathrm{~N}}{d t}=\frac{2 \lambda \mathrm{N}_0}{4}=\frac{\lambda \mathrm{N}_0}{2}\)
So \(\left(-\frac{d \mathrm{~N}}{d t}\right)_{\mathrm{A}}=\left(-\frac{d \mathrm{~N}}{d t}\right)_{\mathrm{B}}\)
After n = 1 i.e. one half life of A.
