RBSE Solutions for Class 6 Science Chapter 5 Separation of Substances
Rajasthan Board RBSE Solutions for Class 6 Science Chapter 5 Separation of Substances Textbook Exercise Questions and Answers.
RBSE Class 6 Science Solutions Chapter 5 Separation of Substances
RBSE Class 6 Science Separation of Substances InText Questions and Answers
Page 35
Question 1.
But, why would we need to separate substances like this at all, is what Paheli wants to know?
Answer:
We need to separate substances in order to:
- separate harmful or non - useful substances that may be mixed with it (e g., stones or pebbles from sand), or
- pick out the useful components from a large mixture if we need to use them separately (e g., gold from mines).

Page 41
Question 2.
Paheli faced a problem while recovering salt, mixed with sand. She has mixed a packet of salt in a small amount of sand. She then tried the method suggested in Activity 7, to recover the salt. She found, however, that she could recover only a small part of the salt that she had taken. What could have gone wrong?
Answer:
When Paheli tried to recover the salt she had mixed with sand, she was able to recover only a small part of it probably because the quantity of salt that she mixed with the sand and water was much more than that required to form a saturated solution. The extra salt would have remained undissolved in the solution. During decantation, only a part of the salt that remained dissolved was removed and the remaining undissolved salt, remained with the sedimented sand. That might be the reason that she could recover only a small part of the salt that she had taken.
Page 42
Question 3.
Suppose, she did not have sufficient quantity of water to dissolve all the salt in the mixture. Is there some way that water could be made to dissolve more salt before the solution gets saturated?
Answer:
Yes, in case of shortage of water, the little water available could be made to dissolve more salt simply by heating the liquid, as heating makes the water dissolve more solutes.
RBSE Class 6 Science Separation of Substances Textbook Questions and Answers
Question 1.
Why do we need to separate different components of a mixture? Give two examples.
Answer:
It is required to separate the components of a mixture for several reasons, such as:
- For the removal of the unwanted impurities from the substance.
- Removal of those substances that are unsafe and harmful for health.
- For obtaining pure substances by removing the other unrequired substances.
- To separate two different useful components so that each of them can be used as required.
Examples:
- Rice is separated from stalks, during harvesting.
- Milk is churned to separate the butter, giving us butter as well as buttermilk.
Question 2.
What is winnowing? Where is it used?
Answer:
Winnowing is a farming method used for separating the heavier grains from the lighter chaff by either blowing or using the natural wind. This is generally used by farmers to separate lighter husk or chaff particles from the heavier seeds of grain in the farms.
Question 3.
How will you separate husk or dirt particles from a given sample of pulses before cooking?
Answer:
Husk or bigger pieces of dirt particles can be removed from a sample of pulses either by handpicking, or by washing followed by sedimentation and decantation.
Question 4.
What is sieving? Where is it used?
Answer:
Sieving is a method for the separation of substances based on differences in their sizes from a mixture. In this process, the mixture is allowed to pass through a sieve such that the finer or smaller particles are able to pass through the holes of the sieve, while the bigger impurities remain on the sieve.
Uses:
- In flour mills, to separate broken particles of grains, sticks, etc. from the refined flour.
- At construction sites, to separate pebbles and small stones from the sand.
Question 5.
How will you separate sand and water from their mixture?
Answer:
We can separate sand and water from their mixture by the following steps:
- The mixture is allowed to stand undisturbed for a few hours in a container.
- By the process of sedimentation, the sand is allowed to settle at the bottom of the container as it is insoluble and heavier than water.
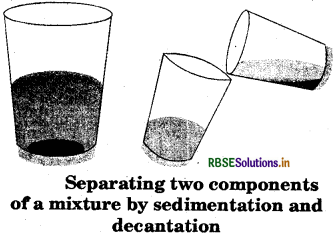
- By the process of decantation, the top layer of water is gently poured into another container.
- If fine particles of sand are still remaining then, a filter paper may also be used to remove. This is called filtration.
Question 6.
Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
Answer:
Yes, this can be achieved by sieving the mixture such that the larger sugar particles remain above the sieve while the smaller flour particles pass through it.
Question 7.
How would you obtain clear water from a sample of muddy water?
Answer:
Clear water can be obtained from muddy water:
- By allowing the muddy water to stand undisturbed in a container.
- With time, the mud settles at the bottom of the container as it is insoluble and heavier than water.
- Upper layer contains almost water free of mud.
- The clear water is gently removed in another container.
- In order to remove finer impurities, the water is again passed through a filter paper.

Question 8.
Fill up the blanks:
(a) The method of separating seeds of paddy from its stalks is called ................................
(b) When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of ................................
(c) Salt is obtained from sea water by the process of ................................
(d) Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ................................
Answer:
(a) threshing
(b) churning
(c) evaporation
(d) sedimentation and decantation.
Question 9.
True or False:
(a) A mixture of milk and water can be separated by filtration.
(b) A mixture of powdered salt and sugar can be separated by the process of winnowing.
(c) Separation of sugar from tea can be done with filtration.
(d) Grain and husk can be separated with the process of decantation.
Answer:
(a) False
(b) False
(c) False
(d) False.
Question 10.
Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar? In which case would it be possible to dissolve more sugar?
Answer:
We should add ice after adding sugar as sugar dissolves in warm water more quickly than in cold water. If we add ice before, then the water will become cold and will not allow enough sugar to dissolve in it.
