RBSE Solutions for Class 7 Science Chapter 6 Physical and Chemical Changes
Rajasthan Board RBSE Solutions for Class 7 Science Chapter 6 Physical and Chemical Changes Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 7 Science in Hindi Medium & English Medium are part of RBSE Solutions for Class 7. Students can also read RBSE Class 7 Science Important Questions for exam preparation. Students can also go through RBSE Class 7 Science Notes to understand and remember the concepts easily. The class 7 science chapter 4 heat extra questions are curated with the aim of boosting confidence among students.
RBSE Class 7 Science Solutions Chapter 6 Physical and Chemical Changes
RBSE Class 7 Science Physical and Chemical Changes InText Questions and Answers
Page 63
Question 1.
Can we call the breaking down of ozone a chemical change?
Answer:
The destruction of ozone layer is chemical change. During the reaction, ozone absorbs the radiation and breaks down into oxygen. Oxygen is different from ozone. Since, new substance is formed, we consider it as a chemical change.

Question 2.
We learnt in Chapter 1 that plants produce their food by a process called photosynthesis. Can we call photosynthesis a chemical change?
Answer:
Yes, photosynthesis is a chemical change. In photosynthesis, process water and carbon dioxide combine to form glucose and Oxygen in the presence of sunlight. Since, new chemical substances are formed. So, photosynthesis is clearly a chemical change.

Page 64
Question 3.
Paheli understood that why her friend is often complaining about iron articles rusting so fast. She lives near the coast. Comment.
Answer:
The water or moisture content is more in coastal areas, hence, iron objects get both water and air for rusting. This makes the process of rusting faster.
RBSE Class 7 Science Physical and Chemical Changes Textbook Questions and Answers
Question 1.
Classify the changes involved in the following processes as physical or chemical changes:
(a) Photosynthesis
(b) Dissolving sugar in water
(c) Burning of coal
(d) Melting of wax
(e) Beating aluminium to make aluminium foil
(f) Digestion of food
Answer:
(a) Photosynthesis - Chemical change
(b) Dissolving sugar in water - Physical change
(c) Burning of coal - Chemical change
(d) Melting of wax - Physical change
(e) Beating aluminium to make aluminium foil - Physical change
(f) Digestion of food - Chemical change
Question 2.
State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces's a chemical change. (True/False)
(b) Formation of manure from leaves is a physical change. (True/False)
(c) Iron pipes coated, with zinc do not get rusted easily. (True/False)
(d) Iron and rust are the same substances. (True/False)
(e) Condensation of steam is not a chemical change. (True/False)
Answer:
(a) False, cutting a log of wood into pieces is a physical change as no new substance is formed.
(b) False, formation of manure from leaves is a chemical change as entirely a new substance is formed by the action of decomposers. The reaction is also irreversible.
(c) True, because coating of zinc on iron is called galvanisation, i.e. prevents rusting.
(d) False, rust is hydrated iron oxide.
(e) True, in condensation process, water vapour changes to water. Change of state is reversible and no new chemical substance is formed. Hence, it is a physical change.
Question 3.
Fill in the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of ........................
(b) The chemical name of baking soda is ........................
(c) Two methods by which rusting of iron can be prevented are ........................ and ........................
(d) Changes in which only ........................ properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called ........................ changes.
Answer:
(a) calcium carbonate
(b) sodium hydrogen carbonate
(c) painting, galvanisation
(d) physical
(e) chemical.
Question 4.
When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Answer:
This is a chemical change. When baking soda is mixed with lemon juice (citric acid), a gas called carbon dioxide is evolved. Since, there is a formation of new substance in the reaction, it is called chemical change.
The reaction between baking soda and lemon juice is given below :
Lemon juice (citric add) + Baking soda (Sodium hydrogen carbonate) → Carbon dioxide (bubbles) + Other substances
Question 5.
When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Answer:
When a candle bums both physical and chemical changes take place. The melting of wax and vaporisation of melted wax is a physical change as there is no formation of new product. The burning of candle is a chemical change as new substance like carbon dioxide, carbon soot, heat and light are given off.
Another example of a change in which both physical and chemical changes occur is burning of LPG gas. Liquified petroleum gas (LPG). is filled in the cylinder in the form of liquid. When it comes out of the cylinder, it changes to vapour state. This change of state is a physical change. While the vapours are mixed with air and burn, chemical changes occur. Heat and carbon dioxide is generated as a new product.
Question 6.
How would you show that setting of curd is a chemical change?
Answer:
Setting of curd is a chemical change because the new substance is formed. Lactose present in the milk changes to lactic acid. The reaction is irreversible.
Question 7.
Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Answer:
Burning of wood is a chemical change whereas cutting it into small pieces is a physical change. When we bum wood, it turns into ash which is a new substance and cannot be reverted back to its original form. Hence, it is a chemical change. While cutting the wood into small pieces is a physical change as no new substance is formed. Only size and shape is changed.

Question 8.
Describe how crystals of copper sulphate are prepared?
Answer:
Crystals of copper sulphate are prepared by the method of crystallisation. The process is as follows:
Step 1: A cup full of water is taken in a beaker.
Step 2: Few drops of dilute sulphuric acid is added to it.
Step 3: Water is heated. When it starts boiling, copper sulphate powder is added slowly while stirring it continuously.
Step 4: Continue adding copper sulphate powder, till no more powder can be dissolved. This solution is called saturated solution.
Step 5: Filter the solution and allow it to cool undisturbed for few hours.
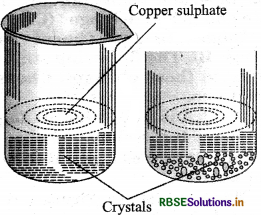
Step 6: On cooling crystal of copper sulphate is seen at the base of the solution.
Question 9.
Explain how painting of an iron gate prevents it from rusting?
Answer:
When an iron surface is painted, iron does not come in contact with air or moisture. Paint act as a protective layer and prevent exposure of iron to the atmosphere. Since, air and water are necessary for rusting. So, iron does not get rusted if painted.
Question 10.
Explain why rusting of iron objects is faster in coastal areas than in deserts?
Answer:
Percentage of moisture is high in coastal areas. Hence, iron objects get both air and, water for rusting. On deserts, the air is dry. and there is no water or moisture in the air. Hence, rusting does not take place as fast as in coastal areas.
Question 11.
The gas we use in the kitchen is called, liquified petroleum gas (LPG). In the cylinder, it exists as a liquid. When it comes out from the cylinder it becomes a gas (Change A) then it burns (Change B). The following statements pertain to these changes. Choose the correct one.
(i) Process A is a chemical change.
(ii) Process B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer:
(ii) is correct. Process B is a chemical change.
Question 12.
Anaerobic bacteria digest animal waste and produce biogas (Change A). The biogas is then burnt as fuel (Change.B). The following statements pertain to these changes. Choose the correct one.
(i) Process A is a chemical change.
(ii) Process B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer:
(iii) is correct. Both processes A and B are chemical changes.

- RBSE Class 7 Science Notes in Hindi & English Medium Pdf Download
- RBSE Class 7 Science Important Questions in Hindi Medium & English Medium
- RBSE Solutions for Class 7 Science in Hindi Medium & English Medium
- RBSE Class 7 Science Important Questions Chapter 1 Nutrition in Plants
- RBSE Class 7 Science Notes Chapter 1 Nutrition in Plants
- RBSE Class 7 Science Important Questions Chapter 14 Electric Current and its Effects
- RBSE Class 7 Science Important Questions Chapter 13 Motion and Time
- RBSE Class 7 Science Important Questions Chapter 2 Nutrition in Animals
- RBSE Class 6 Science Notes Chapter 8 शरीर में गति
- RBSE Class 7 Science Notes Chapter 18 Wastewater Story
- RBSE Class 7 Science Notes Chapter 17 Forests: Our Lifeline