RBSE Class 7 Science Notes Chapter 6 Physical and Chemical Changes
These comprehensive RBSE Class 7 Science Notes Chapter 6 Physical and Chemical Changes will give a brief overview of all the concepts.
Rajasthan Board RBSE Solutions for Class 7 Science in Hindi Medium & English Medium are part of RBSE Solutions for Class 7. Students can also read RBSE Class 7 Science Important Questions for exam preparation. Students can also go through RBSE Class 7 Science Notes to understand and remember the concepts easily. The class 7 science chapter 4 heat extra questions are curated with the aim of boosting confidence among students.
RBSE Class 7 Science Chapter 6 Notes Physical and Chemical Changes
→ Changes can be of two types: physical and chemical.
→ Change in physical properties is called a physical change. It is generally reversible and in such a change, no new substance is formed.
→ Change in which one or more new substances are formed is called a chemical change. All new substances are formed as a result of chemical changes.

→ A chemical change is also called a chemical reaction. Some chemical reactions are :
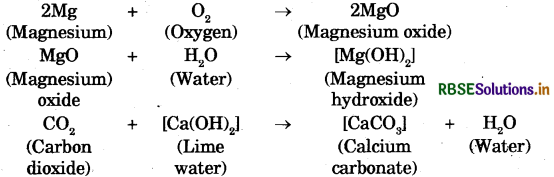
→ The process of rusting can be represented by the following equation:
Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → Rust (Iron oxide, Fe2O3)
→ We can prevent rusting by applying a coat of paint or grease. Another way is to deposit a layer of metal like chromium or zinc on iron.
→ Some substances can be obtained in a pure state from their solutions by crystallisation.

- RBSE Class 7 Science Notes in Hindi & English Medium Pdf Download
- RBSE Class 7 Science Important Questions in Hindi Medium & English Medium
- RBSE Solutions for Class 7 Science in Hindi Medium & English Medium
- RBSE Class 7 Science Important Questions Chapter 1 Nutrition in Plants
- RBSE Class 7 Science Notes Chapter 1 Nutrition in Plants
- RBSE Class 7 Science Important Questions Chapter 14 Electric Current and its Effects
- RBSE Class 7 Science Important Questions Chapter 13 Motion and Time
- RBSE Class 7 Science Important Questions Chapter 2 Nutrition in Animals
- RBSE Class 6 Science Notes Chapter 8 शरीर में गति
- RBSE Class 7 Science Notes Chapter 18 Wastewater Story
- RBSE Class 7 Science Notes Chapter 17 Forests: Our Lifeline