RBSE Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
Rajasthan Board RBSE Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties Textbook Exercise Questions and Answers.
RBSE Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
RBSE Class 11 Chemistry Classification of Elements and Periodicity in Properties InText Questions and Answers
Question 3.1.
What would be the IUPAC name and symbol for the element with atomic number 120?
Answer:
Atomic number of element = 1 2
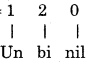
∴ IUPAC name of element = Unbinilium
Symbol = Ubn
Question 3.2.
How would you justify the presence of 18 elements in the 5th period of the peroidic table?
Answer:
For 5th period, n = 5
Electronic configuratin
= 1s2, 2s2,2p6,3s2,3p6,3d10,4s2,4p6,4d10,4f14, 5s2,5p6
According to Aufbau principle,
ast three outermost orbitals are
5s < 4d < 5p
No. of orbitals in 5s = 1
No. of orbitals in 4d = 5
No. of orbitals in 5p = 3
Total number of orbitals = 9
Since, maximum number of electrons that can be accomodated in one orbital is two. So, nine orbitals contain 18 electrons. So, there are 18 elements present n 5th period of the periodic table.

Question 3.3
The elements Z=117 and 120 have been discovered. In which family/ group would you place these elements and also give the electronic configuration in each case.
Answer:
Z = 117
Electronic configuration = [Rn] 5f14 6d10 7s2 7p5
Since there are seven outermost electrons, so this lement belongs to group 17.
Z = 120
Electronic configuratim = [Uuo] 8s2
Since, it contains two outermost electrons so, it will be laced in group 2.
Question 3.4.
Considering the atomic number an position in the periodic table, arrange th following elements in the increasing orde of metallic character: Si, Be, Mg, Na, P.
Answer:
As we know that metallic character increases dow a group and decreases along a period as we move fro left to right. Therefore, the increasing order of metalli character is: P < Si < Be < Mg < Na
Question 3.5.
Which of the following species will hav the largest and the smallest size?
Mg, Mg2+, Al, Al3+
Answer:
Mg and Al both belong to same period.
Atomic number Mg Al 12 13
Atomic size decreases from left to right across the period Thus Mg atom is larger in size than Al atom. Cation is smaller than its neutral atom. Mg2+ ion i smaller than Mg atom and Al3+ ion is smaller than Al3+ atom. Thus, Al3+ ion is smallest and Mg atom is larges in size among the given species.
Question 3.6.
The first ionization enthalpy (A; H) value of the third period elements, Na, Mg and S are respectively 496,737 and 786 kJ mol Predict whether the first (A; H) value for A will be more close to 575 or 760 kJ mol-1 Justify your answer.
Answer:
The first A; H value for Al will be more close to 57 kJ mol1.The value of ∆iH for Al should be lower tha that of Mg due to effective shielding of 3p electrons from the nucleus by 3s-electrons.
Question 3.7.
Whcih of the following will have the mos negative electron gain enthalpy and whic the least negative? P, S, CI, F. Explain you
answer.
Answer:
Electron gain enthalpy becomes more negativ along a period on moving from left to right and i becomes less negative down the group. However, when a electron is added to the 2p-orbital, then it leads t greater repulsion than adding an electron to the large 3p-orbital. Hence, chlorine (Cl) has most negative electron gai enthalpy and phosphorus (P) has least negative electro gain enthalpy.

Question 3.8.
Using the periodic table, predict th formulae of compounds which might b formed by the following pairs of elements
(a) silicon and bromine.
(b) aluminium and Sulphur.
Answer:
(a)
14 Si = 2, 8, 4
It belongs to group 14.
Valency = 4
35 Br = 2, 8, 18, 7
Valency = 1
It belongs to group 17.
∴Formula of the compound = Si Br
(b) 13 Al = 2, 8, 3
It belongs to Group 13.
Valency = 3
16S = 2, 8, 6
It belongs to group 16.
Valency = 2
∴Formula of the compound = Al2 S3
Question 3.9.
Are the oxidation state and covalency of Al in [AICI (H2O)5 ]2+ same ?
Answer:
[Al Cl (H2O)5]2+
Let oxidation state of Al = X
x - 1 + 0 = 2
or
x = 2 + 1 + 3
Covalency of Al in the complex ion = 3
Hence, the oxidation state and covalency of Al in [AlCl (H2O)]2+ are not same.
Question 3.10.
Show by a chemical reaction with water that Na2O is a basic oxide and Cl2O7 is an acidic oxide.
Answer:
Na2O reacts with water to form sodium hydroxide (strong base), which shows that Na2O is a basic oxide.
Na2O + H2O → 2NaOH
Cl2O reacts with water to form perchloric acid (strong acid), which shows that Cl2O7 is an acidic oxide.
Cl2O7 + H2O → 2HClO4
RBSE Class 11 Chemistry Classification of Elements and Periodicity in Properties Textbook Questions and Answers
Question 3.1. What is the basic theme of organisation in the periodic table?
Answer:
The systematic study of the physical and chemical r properties of all the elements and their compounds is the basic theme of organisation in the periodic table.

Question 3.2.
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
Answer:
Atomic weight was used to classify the elements in e Mendeleev's periodic table. He did stick to it and classify - elements in to periods and groups.
Question 3.3.
Answer:
What is the basic difference in approach 3 between the Mendeleev's periodic law and the Modern periodic law?
Answer:
1. Mendeleev's Periodic Law:
It states that, "the properties of the elements are a periodic function of their atomic weights."
2. Modern Periodic Law:
It states that, "the properties of the elements are a periodic function of their atomic numbers."
Question 3.4.
On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Answer:
For 6th period, n = 6
In 6th period, electrons enter in 6s, 4f, 5p and 6d subshells according to Aufbau principle.
|
Subshell |
Maximum no. of electrons in subshell |
|
6s |
2 |
|
4f |
14 |
|
5p |
6 |
|
6d |
10 |
Total maximum number of electrons in the subshells = 32
Since, the number of elements in a period corresponds to the number of electrons in the subshells. Hence, 6th period should have a maximum of 32 elements.

Question 3.5.
In terms of period and group where would you locate the element with Z = 114?
Answer:
Z = 114
Electronic configuration = [Rn]7s2,5f14,6d10,7p2
Since, last electron enters in 7th shell, so n = 7
and the element belongs to 7th period.
Since no. of outermost electrons = 4
So, it belongs to group 14 [group number = 10 + no. of electrons in the valence shell (4)]
Question 3.6.
Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
Answer:
For 17th group,
General electronic configuration = ns2 np5, for 3rd
period, it becomes 3s2 3p5.
Hence, electronic configuration of element
= Is2,2s2 2p6,3s2 3p5
Number of electrons present in element
= 2 + 2 + 6 + 2 + 5 = 17
∴ The atomic number of the element = 17
Question 3.7.
Which element do you think would have been named by:
(i) Lawrence Berkeley Laboratory
(ii) Seaborg's group?
Answer:
(i) Lawrence Berkely Laboratory has been named ement as
awrencium (Lr), Z = 103
erkelium (Bk), Z = 97
(ii) Seaborg's group has been named element as eaborgium (Sg), Z = 106
Question 3.8.
Why do elements in the same group have similar physical and chemical properties?
Answer:
The elements in the same group have similar nysical and chemical properties due to similar ectronic configuration.

Question 3.9.
What does atomic radius and ionic radius really mean to you?
Answer:
Atomic Radius : It means the distance from the entre of nucleus to the outermost shell of electrons in e atom of any element.
Ionic Radius: It means the distance between cation nd anion in ionic crystal.
Question 3.10.
How do atomic radius vary in a Period and in a group? How do you explain the variation?
Answer:
Trend of Atomic Radius along a period
Atomic radius decreases on moving from left to right in a eriod which is due to increase in effective nuclear harge from left to right in a period.
Trend of Atomic Radius in a Group:
tomic radius increases from top to bottom in a group ecause number of shells increases down the group.
Question 3.11.
What do you understand by isoelectronic species? Name a species that will be isoelectronic with each of the following atoms or ions.
(i) FTM
(ii) Ar
(iii) Mg2+
(iv) Rb+
Answer:
Isoelectronic species: The species having ame number of electrons are called isoelectronic Decies.
(i) No. of electrons in F- = 9 + 1 = 10
so electronic species = Na+ = 11 - 1 = 10
(ii) No. of electrons in Ar = 18
so electronic species = K = 19 - 1 = 18
(iii) No. of electrons in Mg2+ = 12 - 2 = 10
so electronic species = Ne = 10
(iv) No. of electrons in Rb+ = 37 - 1 = 36 soelectronic species = Kr = 36
Question 3.12.
Consider the following species:
N3-, O2-, F-‚ Na+, Mg2+ and Al3+
Arrange them in the order of increasin
(a) What is common in them?
(b) Arrange them in the order of increasing ionic radii.
Answer:
(a)
|
Species, |
No. of electrons |
|
N3- |
7 + 3 = 10 |
|
O2- |
8 + 2 = 10 |
|
F- |
9 + 1 = 10 |
|
Na+ |
11 - 1 = 10 |
|
Mg2+ |
12 - 2 = 10 |
|
Al3+ |
13 - 3 = 10 |
Since, all these species have equal number of electro i.e. 10. so, these are refered to as isoelectronic species. (b) Increasing order of ionic radii are :
Al3+ < Mg2+ < Na+ < F- < O2- < N3-

Question 3.13.
Explain why cations are smaller an anions are larger in radii than their pare atoms?
Answer:
Cations are smaller in radii than their pare atoms. This is due to the fact that effective nucle charge increases with loss of one or two electrons. As t force of attraction of nucleus för electrons increas therefore, ionic radii of cations decrease. Anions are larger in radii than their parent aton because effective nuclear charge decreases with gain one or two electrons. As the force of attraction of nucle for electrons decreases and hence, ionic radii of anio increase.
Question 3.14.
What is the significance of the term 'isolated gaseous atom' and 'ground stat while defining the ionization enthalpy an electron gain enthalpy?
Answer:
The term 'isolated gaseous atom' means that tĺ atoms are widely separated, so interatomic forces a minimum and therefore, it has been included definition of ionization enthalpy and electron ga enthalpy. The term 'ground state' means that the atom must present in the most stable state i.e. the ground state. S it is used to define ionization enthalpy and electron ga enthalpy.
Question 3.15.
Energy of an electron in the ground sta of the hydrogen atom is -2.18 × 10-18 Calculate the ionization enthalpy atomic hydrogen in terms of J mol-1.
Answer:
Ionization energy is the amount of energy required to remove the electron from the ground state to infinity. Now, energy of electron in ground state
= - 2.18 × 10-15 J
Energy of electron at infinity = 0
The energy required to remove an electron in the ground state of H-atom = 0- (its energy in ground state)
= (-2.18 × 10-18 J) = 2.18 × 10-18 J
∴ Ionization enthalpy per mole of H-atoms
\(=\frac{2.18 \times 10^{-18} \times 6.02 \times 10^{23}}{1000}\)
= 1312.36 kJ mol
Question 3.16.
Among the second period elements in the actual ionization enthalpies are in the order Li < B < Be < C < O < N < F < Ne.
Explain why,
(i) Be has higher ∆i H than B.
(ii) O has lower ∆iH than N and F?
Answer:
(i) 4 Be = 2,2 = 1s2,2s2
5 B = 2,3 = 1s2, 2s2 2p1x
In case of Be, electron has to be removed from an ne s-orbital while in boron, removal takes place from es p-orbital. Since we know that s-orbital is close to nucleus, so removal of electron is difficult which ns increases its ionization enthalpy Hence, Be has higher of ∆iH than B.
(ii) 8O = 2,6 = 1s2,2s2 2p
7N = 2,5 = 1s2,2s2 2p3
5B = 2,3 = 1s2 2s2 2p1
Comparison between O and N
Nitrogen has half filled p-orbital (2p3),so, its I.E. is greater than oxygen [As we know that half filled orbitals account for greater stability than partially filled]
Comparison between O and F
Among O and F, the size of F is small and it also has high nuclear charge so removal of electron is difficult in case of fluorine. Hence, its I.E, is greater than O. Therefore, it is concluded that O has lower ∆iH than N and F.

Question 3.17.
How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
Answer:
11 Na = 2, 8, 1 = 1s2,2s2, 2p6, 3s1
12 Mg = 2, 8, 2 = 1s2,2s2 2p6,3s2
In above cases, first electron has to be removed from 3s orbitals, but effective nuclear charge of Na is less than that of Mg. Hence first I.E. of Na is lower than that of Mg. When sodium loses one electron then it becomes Na+ (1s2, 2s2 2p6) i.e. noble gas configuratim and it is difficult to remove second electron from 2p subshell. When magnesium loses one electorn than it becomes, a Mg (1s2, 2s2 2p6, 3s1). The electron can be easily a removed from 3s subshell. Hence, second I.E. of sodium is higher than that of magnesium.
Question 3.18.
What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Answer:
Ionization enthalpy of the main group elements decreases down a group due to following factors:
- Atomic size: Atomic size increases on moving down the group so ionization enthalpy decreases.
- Shielding or Screening effect: On adding new shells, the number of inner shell electrons which shield the valence electrons from the nucleus increases i.e. shielding or screening effect increases. As a result, the force of attraction of nucleus for valence electron decreases and therefore, the ionization energy decreases.
- Nuclear charge: Since, nuclear charge increases down the group so, the force of attraction of the nucleus for the valence electrons increases and therefore ionization enthalpy increases.
Question 3.19.
The first ionization enthalpy values (in kJ mol-1) of group 13 elements are:
Be Al Ga In TI
801 577 579 558 589
How would you explain this deviation from the general trend ?
Answer:
Atomic size increases on moving down the group so ionization enthalpy decreases. The deviation is due to weak screening or shielding effect of d-orbital.
Question 3.20.
Which of the following pairs of elements would have a more negative electron gain s enthalpy ?
(i) O or F
(ii) F or Cl
Answer:
(i) F (-328 kJ mol-1) possesses more negative n electron gain enthalpy than O (-141 kJ mol-1).
(ii) Cl (-349kJ mol-1) posseses more negative electron t gain enthalpy than F (-328kJ mol-1).
Question 3.21.
Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first. Justify your answer.
Answer:
On adding one electron to oxygen atom, it forms Dion and energy is released in this process. So, the first lectron gain enthalpy of O is negative. Now, when nother electron is added to O- ion, then it forms O2-ion nd in this process, energy is needed to overcome the trong electrostatic repulsion between the negatively harged O- ion and the new electron being added so, the econd electron gain enthalpy of O is positive.
O (g) + e- → O-(g) ∆eg H1 = -141 KJ mol-1
O-(g) + e- → O2-(g) ∆eg H2 = +780 KJ mol-1
Question 3.22.
What is the basic difference between the terms electron gain enthalpy and electronegativity?
Answer:
The basic difference between the terms electron in enthalpy and electronegativity is that electron gain nthalpy is related to atoms in their isolated states while lectronegativity is a property of an atom in bonded tate i.e. in a molecule. Clectronegativity is only qualitative and is not a measurable quantity.

Question 3.23.
How would you react to the statement that the electronegativity of N on Pauling scale is 3.0 in all the nitrogen compounds?
Answer:
The electronegativity of N on Pauling scale is 3.0 which indicates that it is sufficiently electronegative. But it is not correct for all the nitrogen compounds. It epends on the type of hybridization in a particular ompound. Electronegativity of the element increases with increase in s-character.
Question 3.24.
Describe the theory associated with the radius of an atom as it :
(a) gains an electron.
(b) loses an electron.
Answer:
(a) When an atoms gains an electron, it forms an nion which has more number of electrons but its uclear charge remains same as that of parent atom. Since, the same nuclear charge attracts large number of lectrons, so, force of attraction of the nucleus on the lectrons of all shells decreases. It means that effective uclear charge decreases which causes expansion of the lectron cloud. Due to this reason, the distance between he centre of the nucleus and the outermost shell ncreases which results in increase in ionic radius.
(b) When an atom loses an electron it forms a cation which has lesser number of electrons than parent atom however, nuclear charge remains same. Since the same nuclear charge attracts lesser number of electrons, so the force of attraction of the nucleus on the electrons all shells increases. It means that effective nuclea charge increases which causes contraction of electro cloud. Due to this reason, the distance between the centre of the nucleus and the outermost shell decreases
which results in decrease in ionic radius.
Question 3.25.
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different Justify your answer.
Answer:
The first ionization enthalpies (IE) for tw isotopes of the same element are expected to be same because I.E. depends upon the electronic configuation and effective nuclear charge. As we know that isotopes o an element have same atomic number as well as same electronic configuration and therefore have same nuclear charge and same ionization enthalpies.
Question 3.26.
What are the major differences between metals and non-metals?
Answer:
Differences between Metals and Non-metals
|
Metals |
Non-metals |
|
(i) Metals are generally solids at room temperature except Hg (it is liquid). |
(i) Non-metals are generally, solids gases or liquids at room temperature. |
|
(ii) They are hard and lusturous. |
(ii) They are soft and nonlusturous. |
|
(iii) They have high melting points. |
(iii) They have generally low melting points. |
|
(iv) They are malleable and ductile. |
(iv) They are non-malleable and brittle. |
|
(v) They are good conductors of electricity. |
(v) They arè bad conductors of electricity. |
|
(vi) They are good reducing agents. |
(vi) They are good oxidising agents. |
|
(vii) Their oxides are basic in nature. |
(vii) Their oxides are acidic in nature. |
|
(viii) They usually form unstable hydrides. |
(viii) They usually form stable hydrides. |
Question 3.27.
Use the periodic table to answer the following questions:
(a) Identify an element with five electrons in the outer subshell.
(b) Identify an element that would tend to lose two electrons.
(c) Identify an element that would tend to gain two electrons.
(d) Identify group having metal, non- metal, liquid as well as gas at the room temperature.
Answer:
(a) An element with five electrons in the outer subshell i.e. 2p5
Element is fluorine (F) = 1s2 2s2 2p5
(b) An element that loses two electrons is calcium
(Ca) = 1s2,2s2 2p6,3s2 3p6,4s2
(c) An element that gains two electrons is oxygen
(0) = 1s2,2s2 2p
(d) Group 1 includes metal = Li, Na, K, Rb
Non-metal = H
Liquid = Cs
Gas = H2

Question 3.28.
The increasing order of reactivity among group 1 elements is Li < Na < Rb < Cs whereas that among group 17 elements is F > Cl > Br > I. Explain.
Answer:
The increasing order of reactivity among group 1 elements is Li < Na < Rb < Cs because ionization enthalpy decreases from top to bottom.
The decreasing order of reactivity among group 17 elements in F > Cl > Br > I. It is due to following reasons.
- Lowest bond dissociation energy of fluorine.
- Highest reduction potential of fluorine.
Question 3.29.
Write the general outer electronic configuration of s-,p-,d- and f- block elements.
Answer:
Block
s - block elements
p - block elements
d - block elements
f - block elements
General outer electronic configuration
ns1-2
ns2
Question 3.30.
Assign the position of the element having outer most electronic configuration.
(i) ns2np1 for n = 3,
(ii) (n − 1)d2ns2 for n=4 and
(iii) (n − 2)f (n − 1)d1ns2 for n = 6, in the periodic table.
Answer:
(i) ns2 np4
∵ n = 3
∴ It is 3s2 3 p1 (outermost electrons = 6)
Electronic configuration of element
= 1s2,2s2 2p6,3s23p4
Total number of electrons = 2 +2 + 6 +2 + 4 = 16
∴ Element is sulphur and it belongs to 3rd period and group 16.
(ii) (n − 1)d2ns2
n = 4
∴ It is 3d24s2 (outermost electrons = 4)
Electronic configuration of element = 1s2, 2s2 2p6,3s23p6 3d2 4s2
Total number of electrons = 2 + 2 + 6 + 2 + 6 + 2 + 2 = 22
∴ Element is titanium (Ti, Z = 22). It belongs to 4 period and 4th group.
(iii) (n − 2)ƒ2 (n − 1)d1 ns2
Electronic configuration of element = 1s2, 2s2 2p6‚3s2 3 p6 3d10, 4s2 4p6 4d10 4f7,
Total number of electrons
= 2 + 2 + 6 + 2 + 6 + 10 + 2 + 6 + 10 + 7 +2 + 6 + 1 + 2 = 64
∴Element is Gadolinium (Gd, Z = 64). It is an innertransition element and belongs to 4f series i.e. lanthanoids.

Question 3.31.
The first (∆i H1) and the second (∆i H2) ionization enthalpies (in kJ mol-1) and the (∆eg H) electron gain enthalpy (in kJ mol-1) B of a few elements are given below:
|
Elements |
∆i H1 |
∆i H2 |
∆eg H |
|
I |
520 |
7300 |
-60 |
|
II |
419 |
3051 |
-48 |
|
III |
1681 |
3374 |
-328 |
|
IV |
1008 |
1846 |
-295 |
|
V |
2372 |
5351 |
+48 |
|
VI |
738 |
1451 |
-40 |
Which of the above element is likely to be:
(a) The least reactive element.
(b) The most reactive metal.
(c) The most reactive non-metal.
(d) The least reactive non- metal.
(e) The metal which can form a stable binary
halide of the formula MX2 (X = halogen).
(f) The metal which can form a predominantly stable covalent halide of the formula MX (X = ( halogen)?
Answer:
(a) The least reactive element is V. It is helium. It as no tendency to lose electrons (as is clear from its high ∆iH1 and its higher ∆iH2], nor can it gain electrons positive value of electron gain enthalpy - energy has to e absorbed to gain or add an electron].
(b) The most reactive metal is II. It is potassium. Its ∆iH1 lowest and ∆iH2 is very high, i. e., it can lose one electron eadily from its valence shell [4th] to become K+. It cannot ose 2nd electron readily because of stable Ar core. )
(c) The most reactive non-metal is III. It is fluorine, hose ∆iH1 and ∆iH2 are very high, so it cannot lose lectrons readily. On the other hand, its electron gain nthalpy has a large negative value indicating it can ccept an electron readily.
(d) The least reactive non-metal is IV. It is iodine. Its ∆iH1 and ∆iH1⁄2 are very high, so it cannot lose electrons eadily. On the other hand, its electron gain enthalpy as a large negative value (but not as large negative alue as F above) indicating that it can accept an lectron readily.
(e) The metal which can form a stable binary halide of he formula MX2 (X = halogen) is VI. It is magnesium.
Mg + X2 → MgX 2
Its ∆iH1 and ∆iH2 are not very high but its electron gain nthalpy is very small, ∆iH2 is not very large as compared to ∆iH1. because of its electropositive nature and tendency to ose two electrons, it can form a stable binary halide 1X 2.
Question 3.32.
Predict the formulae of the stable binary compounds that would be formed by the combination of the following pairs of elements :
(a) Lithium and oxygen
(b) Magnesium and nitrogen
(c) Aluminium and iodine
(d) Silicon and oxygen
(e) Phosphorus and fluorine
(f) Element 71 and fluorine
Answer:
(a) 3 Li = 2,1 = 1s22s1 (Group = 1, Valency = 1)
8 0 = 2, 6 = 1s2,2s22p1 (Group = 16, Valency = 2)
∴Formula of compound is Li20.
(b) 12 Mg = 2, 8, 2 = 1s2,2s2 2p6,3s2
[Group = 2, Valency = 2]
7 N = 2, 5 = 1s2, 2s2 2 p3 [Group = 15, Valency = 3]
∴Formula of compound = Mg3 N
(c) 13 Al = 2,8,3 = 1s2 2s2 2p6 3s2 3p1
[Group = 13, Valency = 3]
53I = 2, 8, 18, 18, 7
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s24p6 4d10, 5s25p
[Group = 17, Valency = 1
∴Formula of compound = Al3
(d) 14 Si = 2, 8, 4 = 1s2, 2s2 2p6, 3s2 3p2
[Group = 14, Valency 4]
80 = 2, 6
= 1s2, 2s22p1 [Group = 16, Valency = 2]
∴Formula of compound = SiO2
(e) 15 P = 2, 8, 5 = 1s2, 2s2 2p6, 3s2 3 p3
[Group = 15, Valency = 5]
9F = 2, 7
1s2 2s2 2p5 [Group = 17, Valency = 1]
∴Formula of compound = PF5
(f) Element 71 i.e., Lutetium (Lu)
= [Xe] 4ƒ14 5d1 6s2 [Valency = 3]
F = 2,7 = 1s2, 2s2 2 p3 [Valency = 1]
∴Formula of compound = LuF

Question 3.33.
In the modern periodic table, the period indicates the value of
(a) atomic number
(b) atomic mass
(c) principal quantum number
(d) azimuthal quantum number
Answer:
(c) principal quantum number.
Question 3.34.
Which of the followings statements related to the modern periodic table is incorrect?
(a) The p-block has 6 columns, because a maximum of 6 electrons can occupy all the orbitals in a p-subshell.
(b) The d-block has 8 columns, because a maximum of 8 electrons can occupy all the orbitals in a d-subshell.
(c) Each block contains a number of columns equal to the number of electrons that can occupy that subshell.
(d) The block indicates value of azimuthal quantum number (1) for the last subshell that received electrons in building up the electronic configuration.
Answer:
Option (b) is incorrect.
Question 3.35.
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons.
Answer:
(c) Nuclear mass
Question 3.36.
The size of isoelectronic species: F, Ne and Na+ is affected by:
(a) nuclear charge (Z)
(b) valence principal quantum number (n)
(c) electron-electron interaction in the outer orbitals
(d) none of the factors because their size is the same.
Answer:
(a) nuclear charge (Z).
Question 3.37.
Which one of the following statements is incorrect in relation to ionization enthalpy?
(a) Ionization enthalpy increases for each successive electron.
(b) The greatest increase in ionization enthalpy is experienced on removal of electron from core noble gas configuration.
(c) End of valence electrons is marked by a big jump in ionization enthalpy.
(d) Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
Answer:
Statement (d) is incorrect.

Question 3.38.
Considering the elements B, Al, Mg and K, the correct order character is of their metallic.
(a) B > Al > Mg > K
(b) Al > Mg > B > K
(c) Mg > Al > K > B
(d) K > Mg > Al > B
Answer:
(d) K > Mg > Al > B
Question 3.39.
Considering the elements B,C,N,F and Si, the correct order of their non- metallic character is:
(a) B > C > Si > N > F
(b) Si > C > B > N > F
(c) F > N > C > B > Si
(d) F > N > C > Si > B
Answer:
(c) F > N > C > B > Si
Question 3.40.
Considering the elements F, Cl,O and N, the correct order of their chemical reactivity in terms of oxidizing property is :
(a) F > Cl > O > N
(b) FO > Cl > N
(c) CI > F > O > N
(d) O > F > N > Cl
Answer:
(b) F > b > Cl > N
