RBSE Solutions for Class 12 Chemistry Chapter 8 The d-and f-Block Elements
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 8 The d-and f-Block Elements Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 8 The d-and f-Block Elements
RBSE Class 12 Chemistry The d-and f-Block Elements InText Questions and Answers
Question 8.1.
Silver atom has completely filled d-orbitals (4d10) in its ground state. How can you say that it is a transition element?
Answer:
Silver can exhibit + 2 O.S. wherein it will have incompletely filled d-orbitals (4d9), hence silver is a transition element.
Question 8.2.
In the series Sc (Z - 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest, i.e., 126 kJ mol-1. Why?
Answer:
The enthalpy of atomisation of zinc is lowest because in the formation of metallic bond, no electrons from 3d-orbitals are involved in case of zinc, while in metals unpaired electrons of 3d-orbitals are involved in bond formation which increases the strength of bond.

Question .8.3.
Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
Answer:
Manganese (Mn) shows the highest OS as it atom has the maximum number of unpaired electrons.
Question 8.4.
The Eo (M2+/M) value for copper is positive (+0.34 V). What is possible reason for this?
Answer:
For the calculation of E° (M2+/M) values, three energies are responsible. They are ionisation energies, sublimation energies and hydration energies. In case of copper, the values of ionisation energies and sublimation energies are higher while the value of hydration energy is low hence E° (M2+/M) value for copper is positive (+0.34 V).
Question 8.5.
How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
Answer:
Irregular variation of ionisation enthalpies is mainly due to varying degree of stability of different 3dconfiguration. (eg. d10, d5, d10 are exceptionally highly stable).
Question 8.6.
Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer:
Because of small size and high electronegativity of oxygen and fluorine, the highest oxidation state of a metal is exhibited in its oxide or fluoride.
Question 8.7.
Which is a stronger reducing agent Cr2+ and Fe2+ and why?
Answer:
Cr2+ is stronger reducing agent than Fe2+
Reason: In case of Cr2+, the configuration changes from d4 to d3 and it is oxidised, into Cr3+. It is due to extra stability of (t2g)3 configuration. But in case of Fetion, the configuration changes from d6 to d5 and it changes into Fe3+ ion. (Note: In aqueous medium, d-configuration is more stable as compared to d5 configuration.)
Question 8.8.
Calculate the spin only magnetic moment of ion (Z = 27).
Answer:
M2+ = 3d7 4s°
So no. of unpaired e- = 3
Hence spin only magnetic moment (μ)
= \(\sqrt{n(n+2)}\)B.M.
= \(\sqrt{3(3+2)}\)
= \(\sqrt{15}\) B.M.
Question 8.9.
Explain why Cu+ ion is not stable in aqueous solution?
Answer:
Cu+ ion in aqueous solution undergoes disproportionation
i.e.,
2Cu+ (aq) → Cu2+(aq) + Cu (s)
Becuase the Eo value of this reaction is favourable as Cu2+ has higher hydration enthalpy which compensates L.E. and sublimation energies.

Question 8.10.
Actinoid concentration is greater from element to element than lanthanoid contraction. Why?
Answer:
The 5f electrons are more effectively shielded from nuclear charge. In other words, the 5f electrons themselves provide poor shielding from element to element in Actinoid series.
RBSE Class 12 Chemistry The d-and f-Block Elements Textbook Questions and Answers
Question 8.1
Write down the electronic configuration of:
(i) Cr3+
(ii) Cu+
(iii) CO2+
(iv) Mn2+
(v) Pm2+
(vi) Ce4+
(vii) Lu2+
(viii) Th4+
Answer:
(i) Cr3+ = [Ar] 3d3
(ii) Cu+ = [Ar] 3d10
(iii) CO2+ = [Ar] 3d7
(iv) Mn2+ = [Ar] 3d5
(v) Pm2+ = [Xe] 4f4
(vi) Ce4+ = [Xe]
(vii) Lu2+ = [Xe] 4f14 5d1
(viii) Th4+ = [Rn]
Question 8.2.
why are Mn2+ compounds more stable than Fe2+ towards oxidation to their + 3 state?
Answer:
The electronic confiugration of Mn2+ ion is 3d which is half filled and stable. So third I.E. is very high, hence, Mn cannot show + 3 O.S. while the electronic configuretion of Fe2+ ion is 3d. Thus it can lose third electron easily to get extra stable configuration 3d.
Question 8.3.
Explain briefly how + 2 oxidetion state becomes more stable in the first half of the first row of transition elements with increasing atomic number?
Answer:
As soon as the sum of IE1 & IE2 increases, the tendency to form + 2 oxidation state decreases. But the greater stability of + 2 O.S. in Mn is due to half filled (d) configuration and in Zn is due to completely filled (d) configuration.

Question 8.4.
To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements ? Illustrate your answer with examples.
Answer:
The stability of oxidation states in the first series of the transition elements is related to their electronic configurations as follows:
|
Element |
E.C |
O.S |
|
Sc |
3d1 4s2 |
+3 |
|
Ti |
3d2 4s2 |
+2, +3, +4 |
|
V |
3d3 4s2 |
+2, +3, +4, +5 |
|
Cr |
3d5 4s1 |
+2, +3, +4, +5, +6 |
The first four members of first transition series show that the O.S. do not increase from their total number of electrons present in outer most shells i.e., the maximum O.S. can be given by the sum of the outers and d-electrons.
Question 8.5.
What may be stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms: 3d3, 3d5, 3d8 and 3d4?
Answer:
Stable oxidation states:
3d3 (Vanadium) = + 5
3d5 (Chromium) = 3, + 6
3d5 (Manganese) = +2, +7
3d8 (Cobalt) = +2, +3
3d4 = There is no d4 configuration in ground stable.

Question 8.6.
Name the oxo metal anions of the first series of the transition metals exhibits the oxidation state equal to its group number?
Answer:
Cr2O72- and CrO42- (Group no. = 0.5 of Cr = 6)
- MnO4- (Group no.= 0.5 of Mn = 7)
- VO3- (Group no. = 0.5 of V = 5).
Question 8.7.
What is lanthanoid contraction? What are the consequences of lanthanoid contraction ?
Answer:
Lanthanoid contraction: To overall decrease in atomic and ionic radii from lanthanium (La) to lutetium (Lu), due to the imperfect shielding of one electron by another is the same subshell (i.e., 4f), is known as lanthanoid contraction. The decrease in atomic radii is not quite regular as it is regular to Mn3+ ions.
Consequences:
- It results in the slight variation in their chemical properties which helps in their separation by ion exchange method.
- It causes the radii of the members of the third transition series to be very similar to those of corresponding members by the second transition series. For example Zr and Hf have identical radii i.e., Zr = 100 pm, Hf = 159 pm.
- The covalent character of hydroxide of lanthanoids increases as the size decreases from La3+ to Lu3+. Hence the basic strength decreases. Thus La(OH)3 is least basic. Similarly the basic strength of oxides also decreases in the order from La3+ to Lu3+.
- Tendency to form stable complexes from La3+ to Lu3+ increases as the size decreases.
- Due to lanthanoid contraction, there is slight increase in the electronegativity of the trivalent ions from La to Lu.
Question 8.8.
What are the characteristics of the transition elements and why are they called transition elements ? Which of the d-block elements may not be regarded as the transition elements?
Answer:
General characteristics of transition elements:
- Except mercury which is liquid, all the other transition elements have typical metallic structure and show all the properties of metals like conductivity, malleability, ductility, metallic luxture, high tensile strength etc.
- They have high melting and boiling points, high enthalpies of vaporisation, high enthalpies of atomisation and high enthalpies of hydration of their ions.
- They are electropositive in nature.
- They show variable oxidation states.
- A number of transition metals and their compounds show catalytic properties.
- Most of the transition metals form coloured compounds.
- These compounds are generally paramagnetic in nature.
- They have a great tendency to form complexes.
- They form interstitial compounds with elements like H, C, B and N.
- They form alloys.
The d-block elements are called transition elements because these elments have their properties intermediate between those of s and p block elements and represent a change from the most electropositive s-block to the least electropositive p-block elements. Thus acting as transition elements.
Zn, Cd and Hg have general formula (n-1)d10 ns2 i.e., they have completely filled electronic configuration for dshell in ground state as well as in exited state. So they are not regarded as transition elements.

Question 8.9.
In what way is the electronic configuration of transition elements different from that of the non-transition elements?
Answer:
The electronic confiugration of transition metals is (n-1) d1 - 10 ns0 -2 i.e., they have incompletely filled dorbitals. While the electronic configuration of non-transition metals is ns1-2 or ns2 np1-6. i.e., they either have no d-orbitals are their d-orbitals are completely filled.
Question 8.10.
What are the different oxidation states exhibited by lanthanoids?
Answer:
Lanthanoids show + 2 + 3 and + 4 oxidation state but the most stable is +3.
Question 8.11.
Explain giving reason.
1. Transition metals and many of their compounds show paramangetic behaviour.
2. The enthalpies of atomisation of the transition metals are high.
3. The transition metals generally form coloured compounds.
4. Transition metals and their many compounds act as good catalyst.
Answer:
- Due to presence of unpaired electron, transition metals and their compounds show paramagnetic behaviour.
- Very high enthalpies of atomisation of transition metals can be explained on the basis of strong interatomic interaction due to unpaired electrons. Greater is the number of unpaired electrons, stronger is the bonding.
- Due to presence of unpaired electron, transition metals show d-d transition and hence generally form coloured compounds.
- Many transition metals like Co, Ni, Ptete, and their compounds are used as catalyst because of the following reasons:
- They have variable oxidation states.
- They easily absorb and re-emit the wide range of energies to provide the necessary activation energy.
- Because of variable oxidation state, they easily combine with one of the reactant to form an intermediate which reacts with the second reactant to form final product.
For example:
2Fe3+ + 2I- → 2Fe2+ + I2
2Fe2+ + S2O82- → 2Fe3+ + 2SO42-
Overall:
2I2 + 2S2O82- → I2 + 2SO42- + [Fe3+]
Question 8.12.
What are interstitial compounds? Why are such compounds well known for transition metals?
Answer:
Interstitial compounds: The compounds in which small atoms like H, C, N, He etc. occupy interstitial sites in the crystal lattice are called interstitial compounds. These compounds are well known for transition metals because small atoms can easily occupy the positions in the voids present in the crystal lattices of transition metals.
Question 8.13.
How is the variability in oxidation states of transition metals different from that of the non transition metals? Illustrate with examples.
Answer:
The oxidation states of transition metals differ from each other by unity due to incomplete filling of d-orbitals while oxidation state of non-transition elements normally differ by two units due to inert pair effect.
For example: Mn → +2, +3, +4, +5, +6, +7
Pb → +2, +4

Question 8.14.
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Answer:
Preparation: Potassium dichromate can be prepared from iron chromite ore with the help of the following steps:
(i) Conversion of chromite ore to sodium chromate: When ore is fused with sodium or potassium carbonate or sodium or potassium hydroxide in presence of air then sodium chromate is formed.
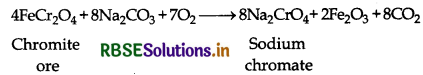
(ii) Conversion of sodium chromate into sodium dichromate: The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give a solution from which average sodium dichromate Na2Cr2O7.2H2O can be crystallised.

(iii) Conversion of sodium dichromate into potassium dichromate: Na2Cr2O7, is more soluble when it is treated with KCl then K2Cr2O7 is formed.
In basic medium:
Cr2O72- + 2OH- → 2CrO42- + H2O
In acidic medium:
2CrO42- + 2H+ → Cr2O72- + H2O
Effect of pH: Chromate and dichromate ions exist in equilibrium. Depending on pH, they can be interconverted. On increasing pH i.e., basic medium dichromate ion is converted into chromate ion. While in acidic medium i.e., on decreasing pH, chromate ion is converted into dichromate ion.
In basic medium:
Cr2O72- + 2OH- → 2CrO42- + H2O
In acidic medium:
2CrO42- + 2H+ → Cr2O72- + H2O
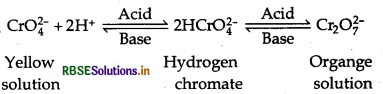
Question 8. 15.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i) Iodide,
(ii) Iron (II) solution,
(iii) H2S.
Answer:
(i) Iodide : In acidic medium, potassium dichromate oxidises iodide into iodine.
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
(2I- → I2 + 2e- ) x 3
Cr2O72- + 6I- + 14H+ → 2Cr3+ + 3I2 + 7H2O
(ii) Iron (II) Solution: In acidic medium, potassium dichromate oxidises iron (II) solution (ferrous ion) into iron (III) solution (ferric ion).
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
(Fe2+ → Fe3+ + e-) x 6
Cr2O72- + 14H+ + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+
(iii) HS: In acidic medium, potassium dichormate oxidises HS into sulphur (S).
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
(H2S → 2H+ + S + 2e-) x 3
Cr2O72- + 14H+ + 3H2S → 2Cr3+ + 6H+ + 3S + 7H2O
Question 8.16.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with (i) iron (II) solution, (ii) SO, (iii) oxalic acid ? Write the ionic equations for the reactions.
Answer:
Preparation: Potassium permanganate can be prepared from pyrolusite (MnO2) ore as follows:
Step (I): Pyrolusite ore is fused with alkali in the presence of air or an oxidising agent like KNO3, then potassium manganate is formed.
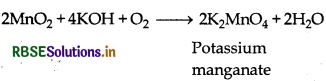
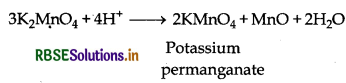
Step (II): This potassium manganate is of green coloured which disproportionate in a neutral or acidic solution to produce potassium permanganate.
Reactions of acidified permanganate solution:
(i) With iron (II) solution : Acidified permanganate oxidises the iron (II) solution into iron (III) solution.
MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
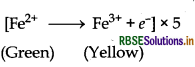
MnO4- + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O
(ii) With SO2: Acidified permanganate oxidises SO2 into sulphate ion (SO42-).
[MnO4- + 8H+ + 5e- → Mn2+ + 4H2O] x 2
[SO2 + 2H2O → SO42- + 4H+ + 2e-] x 5
2MnO4- + 16H+ + 5SO2 + 10H2O → 2Mn2+ + 8H2O → 2Mn2+ + 8H2O + 5SO42- + 2OH-
OR
2MnO4- + 2SO2 + 2H2O → 2Mn2+ + 5SO42- + 4H+
(iii) With oxlaic acid: Acidified permanganate oxidises oxlate ion into carbon dioxide.
(MnO4- + 8H+ + 5e- → Mn2+ + 4H2O) x 2
(C2O42- → 2CO2 + 2e-) x 5
2MnO4- + 16H+ + 5C2O42- → 2Mn2+ + 8H2O + 10CO2

Question 8.17.
For M2+/M and M3+/M2+ systems, the E° values for some metals are as follows:
|
Cr2+/Cr |
-0.9 V |
Cr3+/Cr2+ |
-0.4 V |
|
Mn2+/Mn |
-1.2 V |
Mn3+/Mn2+ |
- 1.5 V |
|
Fe2+ /Fe |
-0.4 V |
Fe3+/Fe2+ |
+ 0.8 V |
Use this data to comment upon:
1. The stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ and
2. The ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer:
(i) Cr3+/Cr+ has a negative reduction potential. So, Cr3+ cannot be reduced to Cr2+ i.e., Cr3+ is most stable. Mn +/Mn2+ has larger positive Evalues. Thus Mn3+ can be easily reduced into Mn2+ i.e., Mn3+ is least stable. E' value for Fe3+/Fe2+ is +ve but small so it is more stable than Mn3+ but less stable than Cr3+
Cr3+ > Fe3+ > Mn3+
2. Oxidation potential for the given pairs will be +0.9 V, +1.2 V and +0.4 V hence the order of the metal getting oxidised into divalent cation is Mn > Cr>Fe.
Question 8.18.
Predict which of the following will be coloured in aqueous solution? Ti, V3+, Cu, se Mn2+ Fe3+ and CO2+.Give reasons for each.
Answer:
The ions having unpaired are coloured while fully filled and the metals having empty d-orbitals are coloureless. Sc2+, Ti2+, V2+, Mn2+, Fe3+, CO2+ and MnO4-, are coloured while Sc3+ is colourless.
Question 8.19.
Compare the stability of +2 oxidation state for the elements of the first transition series.
Answer:
Elements (+ 2 state) Sc2+, Ti2+, V2+, Cr2+, Mn2+ have more stable + 2 oxidation state and their electronic configurations are 3d1, 3d2, 3d3, 3d4 and 3d5 respectively. In all the elements listed, the removal of two 4s electrons, the 3d-orbitals get gradually occupied. Since, the number of empty d-orbitals decreases or the number of unpaired electrons in 3d-orbitals increases with increase in atomic number of cations, so the stability of the actions (M ) increases from Sc? to Mn?
Question 8.20.
Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(i) electronic configuration,
(ii) oxidation state,
(iii) atomic and ionic sizes and
(iv) chemical reactivity.
Answer:
(i) Electronic configuration: Their general electronic configuration is (n-2)f1-14 (n-1)d0-1 nisor 5f1 -14 6d0-1 7s2. All the actinides have 7s2 only the configuration of 5f and 6d is variable. Actually the differentiating electron enters in 5f-sub energy level. The fourteen electrons are formally added to 5f, though not in thorium (Z = 90) but from Pa onwards the 5f orbitals are complete at element 103. The electronic configuration of actinides show irregularities because the energies of 5f and 6d subshell, are almost equal. Due to extra stability off configuration, the electronic configuration of Amand Cm are [Rn]5f7 7s2 and [Rn] 5f7 6d1 7s2.
Some important points related to electronic configuration are given below:
(1) All the actinides have 7s confiugration while 5 and 6d-subshell are filled variably.
(2) The filling of 5f-subshell starts from protactinium not from thorium
(3) Irregularities in configuration is due to extra stability of ff' and configuration. For example:
Am: [Rn]5f7 7s2
Cm: [Rn]5f7 6d1 7s2
No: [Rn] 5f14 7s2
Lr : [Rn] 5414 6d1 7s2
(ii) Oxidation states: They show variable oxidation states this is because in these elements the energies of 5f and 7s orbitals are almost similar and electrons from all the energy levels may be used for bond formation. There is a greater range of oxidation states. But the actinides show in general + 3 oxidation state. The elements in the first half of the series frequently show higher oxidation states because the energy required for the conversion of 5f → 6d is lesser than that required for the conversion of 4f 5d. Hence they show higher oxidation states for example +4, +5, +6 and + 7 as compared to lanthanides.
In last half of the actinide series, the energy required for the conversion 5f 6d is more than that required for the conversion 4f 5d, these actinides, therefore show more lower oxidation states. The most stable oxidation state of these elements is + 3 and their stability increases with increase in atomic number. In addition they also show +2, +4, +5, +6 and +7 oxidation states. The actinides resemble the lanthanides in having more compounds in +3 state than in the + 4 state. However + 3 and + 4 ions tend to hydrolyse. Because the distribution of oxidation states among the actinides is so uneven and so different for the earlier and latter elements. It is unsatisfactory to review their chemistry in terms of oxidation states.
(iii) lonic size:
The general trend in lanthanoids is observable in the actinoids as well there is a gradual decrease in the size of atoms or M ions across the series. This may be referred to as the actinoid contraction (like lanthanoid contraction). The contraction is however, greater from element to element in this series resulting from poor shielding by 5f electrons.
(iv) In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.

Question 8.21.
How would you account for the following:
1. of the species, Cr2+ is strongly reducing while managnese (TTT) is strongly oxidising.
2. Cobalt (IT) is stable is aquoues solution but in the presence of complexing reagents it is easily oxidised.
3. lons having d' configuration are very unstable.
Answer:
- Cr2+ is readucing as its configuration changes from de to dy the latter having a half-filled tyg level. On the other hand, the change from Mn2+ to Mn+ results in the half-filled (ds) configuration which has extra stability.
- CO (III) has greater tendency to form coordination complexes than CO (II). So in presence of ligand, CO (II) changes into CO (III) i.e., easily oxidised
- The ions with d configuration have tendency to lose one electron to acequire stable de configuration. So they are ustable in aqueous state.
Question 8.22.
What is meant by disproportionation? Give two examples of disproportionation reaction in aqueous solution.
Answer:
Disproportionation reaction: When a particular oxidation state becomes less stable relative to other oxidation state, one lower, one higher, it is said to undergo disproportionation
Exanple:
- 2Cu+ → Cu2+ + Cu
- 2Sn2+ → Sn4+ + Sn
Question 8.23.
Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Answer:
(Cu), Because it can easily lose one electron to acequire stable 3d10 configuration.
Question 8.24.
Calculate the number of unpaired electrons in the following gaseous ions:
Mn3+, Cr3+ V3+ and Ti3+. Which one of these in the most stable in aequeous solution?
Answer:
Mn3+ = 3d4 4s0 (unpaired e = 4)
Cr3+ = 3d3 4s0 (unpaired é = 3)
v3+ = 3d2 4s0 (unpaired e = 2)
Ti3+ = 3d1 4s0 (unpaired = 1)
Question 8.25.
Give example and suggest reasons for the following features:
- The lower oxide of the transition metal are basic and the highest oxides are amphoteric/acidic.
- A transition metal exhibits highest oxidation state in oxides and fluorides.
- The highest oxidation state is exhibited in oxoanins of a metal.
Answer:
- The lower oxides of transition metal are basic because of presence of low oixdation state whereas highest oxides are acidic due to high oxidation state. Example : MnO is basic while Mn2O7 is acidic oxidation state.
- Due to small size and high electronegativity of oxygen and fluorine, A transition metal exhibits highest oxidation state in oxides and fluorides.
- Oxometal anions have highest oxidation state for example, In Cr2O72- the oxidation state of Cr is + 6. It is due to the combination of metal with oxygen which is highly electronegative element.
Question 8.26.
Indicate the steps in the preparation of:
(i) K2Cr2O7 from chromite ore
(ii) KMnO4 from pyrolusite ore.
Answer:
(i) In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
Potassium dichromate can be prepared from chromite ore in three steps.
Step 1: Conversion of chromite ore into sodium chromate:
4FeCr2O4(s) + 8Na2CO3(s) + 7O2(g) 8Na2CrO4(aq) + 2Fe2O3(aq) + 8CO2(g)
Step 2: Conversion of Sodium chromate into sodium dichromate:
2NaCrO4(aq) + 2H+(aq) → Na2Cr2O7(aq) + 2Na+(aq) + H2O(l)
Step 3: Conversion of Sodium dichromate into potassium dichromate:
Na2Cr2O7(aq) + 2KCl(s) → K2Cr2O7(aq) + 2NaCl(aq)
(ii) Preparation of KMnO4 from pyrolusite ore:
The chemical equation for the preparation of KMnO4 from pyrolusite ore is depicted below.
Step 1: Conversion of pyrolusite ore into Potassium manganate
2MnO2(aq) + 4NaOH(aq) + O2(g) → 2K2MnO4(aq) + 2H2O(l)
Step 2: Potassium manganate is dissolved into acid to obtain Potassium permanganate
3MnO42-(aq) + 4H+(aq) → 2MnO4(aq) + MnO2(S) + 2H2O(l)
Question 8.27.
What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention it uses.
Answer:
- Alloys: An alloy is a homogeneous mixture of two or more metals or non-metals. It is prepared by blending the metals (and/or non-metals) in molten state.
- Misch metal: It is an important alloy containing lanthanoids metal (95%), iron (5%) and traces of S, C, Ca and Al.
- Uses of Misch metal: It is used in Mg based alloy to produce bullets, shell and lighter flint.
Question 8.28.
What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
Answer:
The f-block elements are inner transition elements. They are lanthanoids (58 - 71) and actinoids (90 - 103). Thus the elements having atomic number 59,95 and 102 are inner transition elements.

Question 8.29.
The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Answer:
Lanthanolds show a limited number of oxidation states (+2, +3 and +4) due to large energy gap between 4f, 5d and 6s subshells. While actinoids show +3, +4, +5, +6, +7 i.e., a large number of oxidation state due to small energy gap between 5f, 6d and 7s sub-shells. On the other hand, all the actinoids are radioactive in nature, so the chemistry of the actinoid elements is not so smooth as that of the lanthanoids.
Question 8.30.
Which is the last elements in the series of the actionids? Write the electronic configuration of this element. Comment of the possible oxidation state of this element.
Answer:
Last actinoid = Lawrencium (Z = 103)
Electronic configuration = [Rn] 5f14 6d1 7s2 oxidation state = + 3
Question 8.31.
Use Hund's rule to derive the electronic configuration of Ce3+ ion, and calculate its magnetic moment on the basis of 'spin only formula.
Answer:
Ce = [Xe] 4f1 5d1 6s2
Ce3+ = [Xe] 4f1 (i.e., no. of unpaired electron=1)
Magnetic moment (u) = \(\sqrt{n(n+2)} \) B.M
= \(\sqrt{1(1+2)}\) B.M
= \(\sqrt{3}\) B.M
= 1.73 B.M.
Question 8.32.
Name the members of the lanthanoid series which exhibit + 4 oxidation states and those which exhibit +2 oxidation states. Try to correlates this type of behaviour with the electronic configuration of these elements.
Answer:
+4 oxidation state = Ce, Pr, Nd, Tb, Dy
+2 oxidation state = Nd, Sm, Eu, Tm, Yb
+2 oxidation state is exhibited when the lanthanoid has the configuration 5d0 6s2 so that 2e- can easily be removed. +4 oxidation state is exhibited when configuration is close to 4f0, 4f1, 4f2 or close to 4f7.
Question 8.33.
Compare the chemistry of the actinoids with that of lanthanoids with reference to:
(i) Electronic configuration
(ii) Oxidation states and
(iii) Chemical reactivity.
Answer:
(i) Electronic configuration: Their general electronic configuration is (n-2)f1-14 (n-1)d0-1 nisor 5f1 -14 6d0-1 7s2. All the actinides have 7s2 only the configuration of 5f and 6d is variable. Actually the differentiating electron enters in 5f-sub energy level. The fourteen electrons are formally added to 5f, though not in thorium (Z = 90) but from Pa onwards the 5f orbitals are complete at element 103. The electronic configuration of actinides show irregularities because the energies of 5f and 6d subshell, are almost equal. Due to extra stability off configuration, the electronic configuration of Amand Cm are [Rn]5f7 7s2 and [Rn] 5f7 6d1 7s2.
Some important points related to electronic configuration are given below:
(1) All the actinides have 7s confiugration while 5 and 6d-subshell are filled variably.
(2) The filling of 5f-subshell starts from protactinium not from thorium
(3) Irregularities in configuration is due to extra stability of ff' and configuration. For example:
Am: [Rn]5f7 7s2
Cm: [Rn]5f7 6d1 7s2
No: [Rn] 5f14 7s2
Lr : [Rn] 5414 6d1 7s2
(ii) Oxidation states: They show variable oxidation states this is because in these elements the energies of 5f and 7s orbitals are almost similar and electrons from all the energy levels may be used for bond formation. There is a greater range of oxidation states. But the actinides show in general + 3 oxidation state. The elements in the first half of the series frequently show higher oxidation states because the energy required for the conversion of 5f → 6d is lesser than that required for the conversion of 4f 5d. Hence they show higher oxidation states for example +4, +5, +6 and + 7 as compared to lanthanides.
In last half of the actinide series, the energy required for the conversion 5f 6d is more than that required for the conversion 4f 5d, these actinides, therefore show more lower oxidation states. The most stable oxidation state of these elements is + 3 and their stability increases with increase in atomic number. In addition they also show +2, +4, +5, +6 and +7 oxidation states. The actinides resemble the lanthanides in having more compounds in +3 state than in the + 4 state. However + 3 and + 4 ions tend to hydrolyse. Because the distribution of oxidation states among the actinides is so uneven and so different for the earlier and latter elements. It is unsatisfactory to review their chemistry in terms of oxidation states.
(iii) In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.

Question 8.34.
Write the electronic configuration of the elements with atomic numbers 61, 91, 101 and 109.
Answer:
61 = [Xe] 4f8 5d0 6s2
91 = [Rn] 5f2 6d1 7s2
101 = [Rn] 4f13 6d0 7s2
109 = [Rn] 5f14 6d7 7s2
Question 8.35.
Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
1. Electronic configuration,
2. Oxidation states,
3. Ionisation enthalpies and
4. Atomic sizes.
Answer:
|
Properities |
First Transition series |
Second and Third Transition series |
|
1. electronic configuration |
3d1-10 4s0-2 |
Second = 4d1-10 5s0-2 |
|
2. Oxidation state |
Show variable oxidation |
Third = 5d1-10 6s1-2 |
|
3. Ionistion enthalpy |
On moving left to right Increases regularly with an exception of half filled and full filled configuration. |
Same as first series |
|
4. Atomic size |
On moving left to right first decreases then become constant and after this increases regularly. |
The I.E. of second & third transition series is approximate equal due to Lanthanoid contraction (in vertical column). The size of second and third transition series is equal due to Lanthanoid contraction |
Question 8.36.
Write down the number of 3d electrons in each of the following ions: Ti2+ v2+, Cr3+, Mn2+, Fe2+, Fe3+, CO2+ Ni2+ and Cu2+ Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral)
Answer:
(i) Ti2+ = 3d2 → 2e- in 3d series → (t2g)2 (eg)0
(ii) V2+ = 3d3 → 3e- in 3d series → (t2g)3 (eg)0
(iii) Cr3+ = 3d3 → 3e- in 3d series → (t2g)3 (eg)0
(iv) Mn2+ = 3d5 → 5e- in 3d series → (t2g)5 (eg)0
(v) Fe2+ = 3d6 → 6e- in 3d series → (t2g)6 (eg)0
(vi) Fe3+ = 3d5 → 5e- in 3d series → (t2g)5 (eg)0
(vii) CO2+ = 3d7 → 7e- in 3d series → (t2g)6 (eg)1
(viii) Ni2+ = 3d8 → 8e- in 3d series → (t2g)6 (eg)2
Question 8.37.
Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements?
Answer:
The heavier transition elements belong to fourth (4d), fifth (5d) and sixth (60) transition series. Many proeperties of these heavier transition elements are expected to be different from those of first transition (34) series due to the following reasons:
- Atomic radii of the elements of 4d and 5d series are more due to greater number of electron shells. However, the atomic radii of the 3d and 4d series are comparatively equal due to lanthanoid contraction.
- The melting and boiling points at the elements of 4d and 5d series are higher due to stronger interatomic interactions.
- Ionisation enthalpies are expected to decrease as we move from one series to the another. However, the values for the elements of 5d series are higher as compared to the elements belonging to the another two series due to lanthanoid contraction. Actually as the atomic size decreases, effective nuclear charge increases, as a result, ionisation energy in case of 3d elements also increases.
Question 8.38.
What can be inferred form the magnetic moment values of the following complex species?
(i) K4 [Mn(CN)6]
(ii) [Fe (H2O)6]2+
(iii) K2[MnCl4]
Answer:
The magnetic moment can be calculated with the help of spin only formula given below:
\(\mu=\sqrt{n(n+2)} \text { B.M. }\)
Wheren = number of unpaired electrons For one unpaired electron (n = 1);
\(\mu=\sqrt{1(1+2)}=\sqrt{3}\) = 1.73 B.M
For two unpaired electrons (n = 2);
\(\mu=\sqrt{2(2+2)}=\sqrt{8}\) = 2.83 B.M
For three unpaired electrons (n = 3);
\(\mu=\sqrt{3(3+2)}=\sqrt{15}\) = 3.87 B.M
For four unpaired electrons (n = 4);
\(\mu=\sqrt{4(4+2)}=\sqrt{24}\) = 4.9 B.M
For five unpaired electrons (n = 5);
\(\mu=\sqrt{5(5+2)}=\sqrt{35}\) = 5.92 B.M
With the help of above calcutated values, let us study the complex given below.
(i) K4 [Mn(CN)6]
Oxidation state of Mn:
K4[Mn(CN)6]
4 x (+1) + x + 6 x (-1) = 0
4 + 1 - 6 = 0
x = +2
The magnetic moment of this complex is 173 B.M. which indicates the presence of one unpaired electron in this complex. Mn (25) = 3d5 4s2 4p0
The above explanation confirms the presence of one unpaired electron in the complex. So, the complex is paramagnetic and octahedral in geometry due to d2sp3 hybridisation.
(ii) [Fe (H2O)6]2+
Oxidation state of Fe:
[Fe (H2O)6]2+
x + 6 x 0 = + 2
x = +2
The magnetic moment of this complex is 5 3 B.M., which indicates the presence of four unpaired electrons in this complex.
Fe (26) = 3d6 4s2 4p0 4d0
Fe (II) = 3d6 4s0 4p0 4d0
The aboue explanation confirms the presence of four unpaired electrons in the complex. So the complex is paramagnetic and have octahedral geometry (due to sp3d2 hybridisation)
(iii) K2[MnCl4]
Oxidation state of Mn:
K2 [MnCl4]
2 x (+1) + x + 4 x (-1) = 0
+ 2 + x - 4 = 0
x = + 2
The magnetic moment of this complex is 5-9 B.M., which indicates the presence of five unpaired electrons in the complex
Mn (25) = 3d5 4s2 4po
Mn(II) = 3d5 4s0 4po
The above explanation confirms the presence of five unpaired electrons in the complex. So the complex is paramagnetic and has tetrahedral geometry (due to sp3 hybridisation).

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम