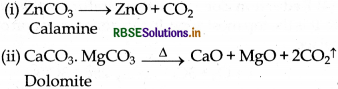RBSE Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 6 General Principles and Processes of Isolation of Elements
RBSE Class 12 Chemistry General Principles and Processes of Isolation of Elements InText Questions and Answers
Question 6.1.
Which of the ores mentioned in Table 6.2 of this book can be concentrated by magnetic separation method?
Answer:
Ores in which one of the components, either the impurity or the actual ore, is magnetic can be concentrated by magnetic separation method. e.g., ores containing iron (haematite, magnetite, siderite and iron pyrites).
Question 6 2.
What is the significance of leaching in the extraction of aluminium?
Answer:
Leaching is significant in the extraction of aluminium as it helps in removing the impurities like SiO2, Fe2O3, TiO2 etc from the bauxite ore.

Question 6 3.
The reaction, Cr2O2 + 2Al → Al2O3 + 2Cr (∆G° = - 42 kJ) is thermodynamically feasible as is appartent from the Gibbs energy value. Why does it not take place at room temperature?
Answer:
Certain amount of activation energy is essential even for such reactions which are thermodynamically feasible.
Question 6 4.
It is true that under certain conditions, Mgcan reduce Al2O3 and Al can reduce MgO?
Answer:
Yes, below 1623K Mg can reduce Al2O3 and above 1623 K temperature, Al can reduce MgO. It can be inferred from ∆G vs T plots i.e., from Ellingham diagram.
RBSE Class 12 Chemistry General Principles and Processes of Isolation of Elements Textbook Questions and Answers
Question 6.1.
Copper can be extracted by hydrometallurgy but not zinc explain?
Answer:
In electrochemicall series, the position of zinc is above hydrogen i.e., the value of standard electrode potential of zinc is E°Zn2+/Zn = -0.76V. While the position of copper is belo hydrogen i.e., the value of standard electrode potential of copper is E°Cn2+/Cn = +0.34V. Hence zinc metal reacts with water and produces Zn2+ ion and release H2 gas. But copper metal can not react with water. Due to above given reason copper can be extracted by hydrometallurgy but not zinc. In other words we can say that zinc is highly reactive metal, it may not be possible to replace it from a solution of ZnSO4 so easily.
Question 6.2.
What is the the role of depressant in forth floatation process?
Answer:
When more than one sulphides ore to be concentrated them depressents are used. They changes the oil to water tendency of ore or gangue for example: NaCN is used as depressant in concentration of PbS and ZnS. NaCN reacts with ZnS and converted into Na, [Zn(CN)] which is soluble in water. In this way wetting tendency of Pbs with oil increases while with water decreases. On the other hand wetting tendency of ZnS with water increases.
Question 6.3.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Answer:
The Gibbs energies of formation of most sulphides are greater than that for CS. In fact, CS, is an endothermic compound hence it is common practice to roast sulphide ores to corresphonding oxides prior to reduction.

Question 6.4.
Explain (i) Zone refining (ii) Column chromatography.
Answer:
(B) Reduction of Oxide to the metal
Now calcined or roasted ore is reduced into crude metal by using suitable reducing agents. The reducing agent depends upon the reactivity of the metal. The following methods are used for the reduction of oxides into crude metal.
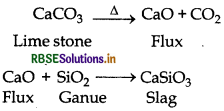
(a) Smelting or Reduction by carbon: The process of extraction of metal by heating the metal oxide with suitable reducing agent above its melting point is called smelting. In smelting, the roasted or calcined ore is mixed with suitable quantity of coke or charcoal and heated strongly at high temperature above its melting point. The process of smelting is done in blast furnace. Here coke or charcoal behave as reducing agent and converts oxide ore into molten metal. Some times during reduction, an additional reagent is also added to the ore to remove the impurities still present with ore. This additional reagent is known as flux. This flux combines with infusion impurities present with ore and converts it into fusible product. This fusible product is known as slag. In this way during smelting ore is converted into crude metal.
In smelting, carbon monoxide can also be used as reducing agent. Smelting process can be used to reduce oxides of metals like Zn, Cu, Sn, Pb, Fe etc. Some reactions involved in reduction by smelting using carbon or carbon mono oxide (produced by incomplete combustion of carbon) as reducing agent is as follows:
example:
(i) Mx Oy + yC → xM + yCO
(ii) PbO + C → Pb + CO
(iii) PbO + CO → Pb + CO2
(iv) Fe2O3 + 3C → 2Fe + 3CO
(v) 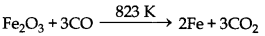
(vi) 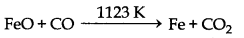
(vii) 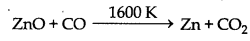
(viii) MnO2 + 2C → Mn + 2CO
(ix) Mn2O3 + 3C → 2Mn + 3CO
(x) 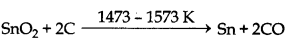
Note: The process of smelting is done in blast furnace.
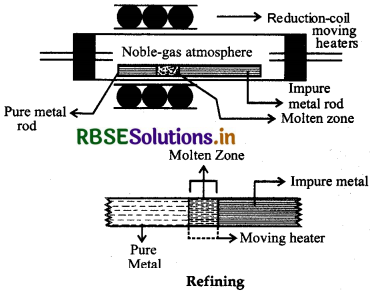
(b) Zone refining: Elements like Silicion (St), Germanium (Ge), Gallium (Ga) etc. which are used as semiconductors can be refined by this method. It is used when the metals are required in high degree of purity. This method is based on the principle that the impurities are more soluble in the molten form than in solid state of the metal. In this method an impure metal rod is taken and a circular movable heater of high freqeuncy is fitted at one end of a rod. The heater is slowly moved across the rod. The metal melts at melting point during heating. As the heater moves on from one end to other end of rod the pure metal crystallises while the impurities goes on in the adjacent molten zone. The process is repeated several times. The end of the rod where the impurities have collected, is cut off.
(ii) Vapour phase refining: This method is based on the principle that the metal can be converted into their volatile compounds by reacting with suitable reagent while the impurities remain uneffected during the reaction. Now this volatile compound can be decomposed easily on heating into metal. Thus for this type of refining, following two requirements should be fullfilled by the metal:
(i) Metal can form a volatile compound with appropriate reagent.
(ii) The volatile compound should be easily decomposes so that pure metal can be recovered.
The following examples illustrate this method :
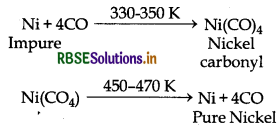
(i) Mond Process: This method is used to refine nickel. In this method impure nickel is treated with CO at 330 - 350 K temperature then volatile nickel carbonyl is formed. This nickel carbonyl is heated to still higher temperature i.e., 450 - 470K then it decomposes giving pure nickel.
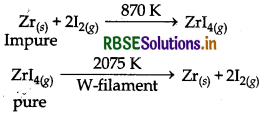
(ii) Van-Arkel Method : This method is applied for getting very pure metal. In this method, first of all metal is converted into a volatile compound, while impurities remain uneffected. The compound thus obtained is decomposed to get the pure metal. For the decomposition of gaseous compound a white hot tungsten fialment is used. This method is used to refine metals like Titanium and Zirconium. The involved reactions are as follows:
Refining of Titanium:
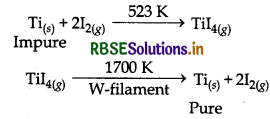
Refiining of Zirconium:
Question 6.5.
Out of C and CO, which is a better reducing agent at 673K?
Answer:
According to Ellingham diagram, at temperature 983 K, the curves intersect, AGR value for the oxidation of CO into CO2 is more than the ∆G" value for the oxidatio of C into CO2. It means that at this temperature or above it, Cis better reducing agent. However, below this temperature carbon monooxide is better reducing agent. Hence CO is a better reducing agent at 673K.

Question 6.6.
Name the common elements present in the anode mud in electrolytic refining of Copper. Why are they so present?
Answer:
Anode mud have selenium, tellusium, silver, gold metals. Because these are less reactive than copper.
Question 67.
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Answer:
During the extraction of iron in blast furrade, the various reactions taking place in different zones are as follows:
(1) Combustion Zone: It is the lowermost zone in blast furnace. Its temperature is 2170 K. In this zope the combustion of carbon takes place.
C + O2 → CO2 + energy
(2) Fusion Zone: The temperature of this zone is 1570 K. This zone is above combusion zone.
In this zone, formation of carbon mono oxide takes place. If any iron oxide come in this zone then it also reduce
CO2 + C → 2CO - energy
FeO + CO → Fe + CO2
(3) Slag formation Zone: The temperature of this zone is 1270 K. Formation of slag takes place in this zone.
(4) Reduction Zone: The temperature of this zone is 1070 K. It is the top most zone. In this zone formation of iron takes place.

FeO + CO → Fe + CO2
Fe2O3 + FeO → Fe3O4
Fe3O4 + CO → 3FeO + CO2
FeO + CO → Fe + CO2
Question 6.8.
Write chemical reactions taking place in the extraction of zinc from zinc blende.
Answer:
The reactions taking place in the extraction of zinc from zinc blende are as follow:
(a) Concentraction: Zinc blende are is concentrated by froth floatation process.
(b) Roasting: Concentrated ore is heated strongly upto about 1200 K temperature in presence of air. During roasting zinc sulphide (zinc blende) is converted into zinc oxide.
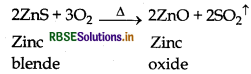
(c) Reduction: Now zinc oxide is mixed with powdered coke and heated upto 1673 K temperature in a fire clay retort. Here zinc oxide is reduced into zinc.

At 1673 K temperature zinc metal vapourises as its boiling point is 1180 K.
(d) Electrolytic refining: During electrolytic refining impure zinc is made anode while thin strip of pure zinc is made cathode. An aqueous solution of ZnSO4 acidified with dil H2SO4 is used as electrolyte. On passing electric current pure Zn is deposited over cathode anode dissolves. The reactions are as follow:
At cathode:
Zn2+ + 2e- → Zn (reduction)
At anode:
Zn → Zn2+ + 2e-
Question 6.9.
State the role of silica in the metallurgy of Copper.
Answer:
In the metallurgy of copper silica (SiO2) acts as an acidic flux. This flux combines with FeO, a gangue, and forms slug
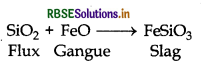
This slag is lighter than molten metal and can be topped off.
Question 6.10.
What is meant by the term chromatography?
Answer:
The term chromatography means colour writing (In Greek: chroma means colour:graphy means writing). As chromatography, initially, was used to separate only the coloured components. But now a days any type compounds/components/constituents can be separated by chromatography. It is also useful to separate those components/ elements which exist in traces. e.g. lanthanoids.

Question 6.11.
What criterion is followed for the selection of the stationary phase in chromatography ?
Answer:
Criteria for the selection of stationary phase in chromatography are as follows:
- Impurities should be strongly adsorbed or are more soluble in stationary phase.
- Stationary phase should have high but selective adsorption power.
- The adsorbent should not react chemically with the solvent used for elution or with the components of the mixture under investigation.
- The impurities will be retained by stationary phase whereas the pure component should be easily removed.
Question 6.12.
Describe the method for refining nickel.
Answer:
Mond's Process: Nickel can be refined by mond's process. In this process nickel is first heated with carbon mono oxide at about 330 - 350 K temperature then it is converted into volatile complex leaving behind non volatile impurities. This volatile complex is further heated at high temperature then it decomposes into pure nickel metal and carbon mono oxide gas.
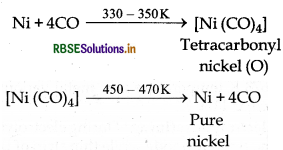
Question 6 13.
How can you separate alumina from silica in a bauxite ore associated with silica? Give equation, if any.
Answer:
Baeyer's Process for the purification of Bauxite : Bauxite ore has the impurities of FeO3, Silica and TiO2. The pulverised ore is digested with concentrated NaOH solution at 200 to 280°C temperature and 35 to 36 bar pressure. During digestion bauxite is conveted into solution of sodium meta aluminate and silica into soluble sodium silicate leaving behind the impurities of TiO, and Fe2O, as red mud, which is filtered off.
Now soluble aluminate and silicate is diluted with water in presence of traces of freshly prepared aluminium hydroxide (Al(OH)3) which initiate the precipitation of aluminium hydroxide. Aluminium hydroxide is filtered and ignited at 1473 K temperature then pure aluming is obtained.
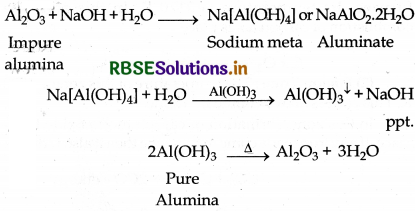
Question 6.14.
Giving examples, differentiate between 'roasting' and 'calcination'.
Answer:
Differences between Roasting and Calcination:
|
Roasting |
Calcination |
|
(1) In Roasting, concentrated ore is heated in presence of air below its melting point. |
(1) In calcination, concetrated ore is heated in absence of air below its melting point. |
|
(2) Sulphide ores are roasted.
|
(2) Carbonate and hydroxide ores are calcined. |
Question 6.15.
How is 'cast iron' different from 'Pig iron'?
Answer:
Cast iron is different from Pig iron with respect to the carbon contents. Pig iron is obtained from blast furnance and have about 4% carbon and other impurities like S, P, As, Mn etc. It can be mould into different shapes. Cast iron is obtained from Pig iron when Pig iron is mixed with scrap of carbon and oxidised by passing hot air through it. Cast iron has 3% impurities of carbon and other elements.

Question 6.16.
Differentiate between 'minerals' and 'ores'.
Answer:
- Minerals: These are the combined states of metal in which metal accurs naturally in the earth's crust. For example: Clay, Bauxite, mica, dolomite, Zinc blende etc.
- Ores: The minerals from which a metal can profitably and conveniently extracted is called ores. All the ores are minerals but all the minerals are not ores.
- For example: Bauxite is an ore but clay and mica are not because aluminium metal can be extracted economically of profitably from bauxite not from clay or mica.
Question 6.17.
Why copper matte is put in silica lined converter?
Answer:
Copper matte consists of a mixture of Cu Sand Cu,O. It also have small amount of FeS and FeO. Copper matte is put in a silica lined converter which is known as. Bessemer converter in order to remove impurities of FeS and FeO. Because silica (SiO)2 acts as flux and reacts with gangue (FeO) and forms slag. This slag can be removed from the ore. The related reactions are as follow:
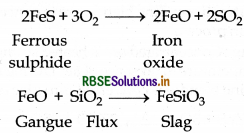
When all the impurities of FeS and FeO is removed in the from of slag then Cu2S is oxidised into CuO which is reduced into Cu.
2CU2S + 3O2 → 2CU2O + 2SO2
2CU2O + CU2S → 6CU + SO2
Question 6.18.
What is the role of cryolite in the metallurgy of aluminium?
Answer:
The role of cryolite in the metallurgy of aluminium is as follow:
- It mainly reduces the melting point of electrolyte alumina.
- It increases the conductivity of electrolyte mixture.
Question 6.19.
How is leaching corried out in case of low grade copper ores?
Answer:
The leaching of low grade copper ores is carried out with acid in presence of air. Then copper goes into the solution as Cu2+ ions.
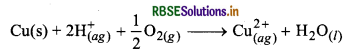

Question 6.20.
Why is zinc not extracted from zinc oxide through reduction using CO?
Answer:
According to Ellingham diagram the value of ∆G for the oxidation of CO into CO, is higher than the value of ∆Go for the oxidation of Zn into ZnO. Hence CO can not reduce ZnO into Zn. Further more the reduction may require very high temperature if CO is used as a reducing agent in this case.
Question 6.21.
The value of ∆Go for the formation of Cr2O3 is - 540 KJ mol and that of Al2O3 is - 827 KJ moll. Is the reduction of Cr2O3 possible with Al?
Answer:
Yes, the reduction of Cr2O3 is possible with Al. It can be proved by the coupling of reaction. As after coupling the value of ∆Go for the reduction of Cr2O3 comes -ve.
2Al + 3/2O2 → Al2O3, ∆G° = - 827 KJ/mol
Cr2O3 → 2Cr + 3/2 O2, ∆G° = + 540 KJ/mol
Cr2O3 + 2Al → Al2O3 + 2Cr
∆G° = -287 KJ/mol
Question 6.22.
Out of Cand CO, which is a better reducing agent for ZnO?
Answer:
C is better reducing agent for ZnO.
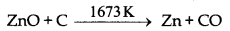
Because according to Ellinghan diagram the value of ∆Go for the oxidation of Cinto CO is lower at temperature above 1180 K while the value of ∆Go for the oxidation of C into CO is lower at temperature above 1270 K than the value of ∆G for the oxidation of Zn into ZnO. Thus above 1270 K, ZnO can be reduced into Zn by C. While the ∆G' value of CO2 from CO is always higher than the value of ∆GR for ZnO from Zn. Thus CO cannot be used for the reduction of ZnO.
Question 6.23.
The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Answer:
The thermodynamic factor helps in choosing a suitable reducing agent for reduction. It can be proved with following examples. According to Elligham diagram, metal having higher-ve value for free energy of formation of oxide, can reduce that metal which have lower - ve value for ∆G of oxide. It can be understood as:
- Above 1600 K, Al metal can reduce MgO and below 1600 K Mg can reduce Al2O3.
- CO cannot reduce ZnO but C can reduce ZnO because there is hardly any change in the free energy as a result of the reaction.
Thus the curve of metal oxide which is placed lower is Ellingham diagram cannot be reduced by the metal involved in formation of that oxide whose curve is placed higher in the diagram.

Question 6.24.
Name the process from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Answer:
Chlorine can be obtained as by-product in Down's process, - In Down's process molten NaCl is subjected to electrolysis.

At Cathode: Na+ + e- → Na
At Anode: Cl- → 1/2 Cl2 + e-
Here sodium metal is obtained as main product while chlorine is obtained as by product
Electrolysis of aqueous NaCl: Chlorine can also be obtained by the electrolysis of aqueous NaCl in Nelson's Cell. The related reactions are as follow:
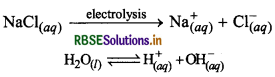
At Cathode: Na+ and H+ both ions migrate towards cathode but H+ ions are dicharged preferenlially,
At cathode:
H+ + e- → 1/2H2(g)
At anode: both Cl and OH- ions migrate towards the anode but Cl ions are discharged preferentially.
At cathode:
Cl- → 1/2Cl2 + e-
Thus remaining ions i.e. Na+ & OH combines with each other and forms NaOH.
Na+ + OH- → NaOH
Question 6.25.
What is the role of graphite rod in the electro metallurgy of aluminium?
Answer:
In electrometallurgy of aluminium, oxygen gas is evolved åt anode. This oxygen gas reacts with anode which is made up of graphite and form carbon monooxide and carbon dioxide. If we use another metal electrodes act anode, then oxygen gas will react with aluminium metal formed during electrolysis. Hence to save aluminium which is costly than graphite, we use graphite electrode as its wastage or consumption can be tolerated.
Question 6.26.
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Answer:
(i) Zone refining: This method is used to refine those metals which are needed in high grade purity and other methods are not applicable. Example-In refining of silicon, germanium. The principle of this method is that the impurities are more soluble in molten form than in crystal form. In this method a mobile heater is fitted with a log of metal which is to be purify. Heater moves from one end to another end. When the concentration of impurities become highest in liquid form of metal in heater, then it is removed and thus we get pure metal.
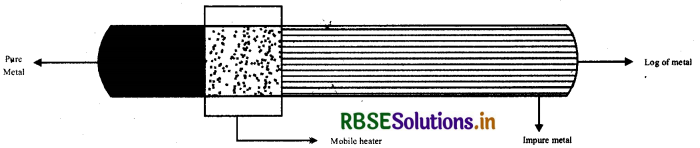
(ii) Electrolytic refining: In this method, the impure metal is made to act as anode. A strip of same metal in pure form is used as cathode. They are put in a suitable electrolytic both containing soluble salt of the same metal. The more basic metal remains in the solution and less basic ones go to the anode mud.
For example: Electrolytic refining of copper: Anode: Impure copper rod Cathode: a strip of pure copper Electrolyte: An aqueous solution of CuSO with small amount of H2SO4
On electrolysis anode dissolves leaving behind impurities of Sb, Se, Te, Ag, Au and Pt as anode mud while copper is transferred on cathode in pure form.
At anode:
Cu → Cu2+ + 2e-
At cathode:
Cu2+ + 2e- → Cu
(iii) Vapour phase refining: In this method, the metal is converted into its volatile compound and collected elsewhere. It is then decomposed to give pure metal. So, the two requirements are:
- The metal should form a volatile compound with an available reagent.
- The volatile compound should be easily decomposable, so that the recovery is easy.
Example: Van-Arkel Method : It is very useful method for removing all the oxygen and nitrogen present in the form of impirity in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises.
Zr + 2I2 → ZrI4
It is now decomposed on a tungsten filament electrically heated to about 1800 K. Then pure metal is formed.
ZrI4 → Zr + 2I2

Question 6.27.
Predict the conditions under which Al might be expected to reduce Mgo.
Answer:
According to Ellingham diagram, Mg can reduce Al2O, above 1665 K temperature because the value of ∆G for the oxidation of Mg into MgO is more negative than the value of ∆G for the oxidation of Al into Al2O, above 1665 K. Hence above 1665 K temperature Mg can reduce Al2O. On the other hand, below 1665 K temperature the value of ∆Go for the oxidation of Mg into MgO is less negative than the value of ∆Go for the oxidation of Al into Al2O3. Hence at this condition, Al can reduce MgO into Mg.

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम