RBSE Solutions for Class 12 Chemistry Chapter 14 Biomolecules
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 14 Biomolecules Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 14 Biomolecules
RBSE Class 12 Chemistry Biomolecules InText Questions and Answers
Question 14.1.
Glucose and sucrose are soluble in water but cyclohexane and benzene (simple six membered compounds) are insoluble in water. Explain.
Answer:
Glucose (CH2O) and sucrose (C2H2O11) have a number of polar - OH groups (five in case of glucose and eight in sucrose) which form hydrogen bonds with water molecules and hence easily dissolve in water. On the other hand, cyclohexane and benzene are non-polar and do not form any hydrogen bonds with water molecules and therefore these are insoluble in water.

Question 14.2.
What are the expected products of hydrolysis of lactose?
Answer:
Lactose is a disaccharide. On hydrolysis, gives two molecules of monosaccharides i e. one molecule of each of D-(+)- glucose and D-(+)-galactose.
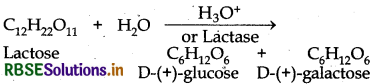
Question 14.3.
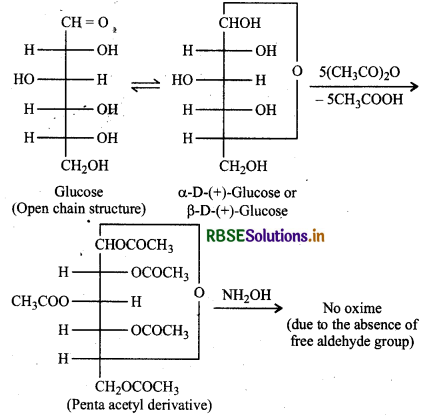
How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Answer:
When glucose (a and B) is treated with acetic anhydride, it forms a pentaacetyl derivatives which does not contain a free hydroxyl (-OH) group at C-1, it cannot be hydrolysed in aqueous solution to give the open chain aldehyde group and hence glucose pentaacetate does not react with NH2OH to form glucose oxime.
Question 14.4.
The melting points and solubility in water of a-amino acids are higher than those of the corresponding haloacids Explain?
Answer:
α-Amino acids are dipolar in nature as they exist as Switter ions 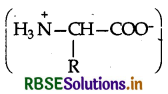 Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are quite higher than haloacids which do not have salt like character. These are also involved in intermolecular H-bonding with the water molecules and are therefore, water soluble. On the contrary, the haloacids RCH(X) COOH are not dipolar like aminoacids. Moreover, only the carboxyl groups of haloacids are involved in Hbonding with the water molecules and not the halogen atoms. These have therefore, comparatively less melting points and are also water soluble to smaller extent.
Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are quite higher than haloacids which do not have salt like character. These are also involved in intermolecular H-bonding with the water molecules and are therefore, water soluble. On the contrary, the haloacids RCH(X) COOH are not dipolar like aminoacids. Moreover, only the carboxyl groups of haloacids are involved in Hbonding with the water molecules and not the halogen atoms. These have therefore, comparatively less melting points and are also water soluble to smaller extent.
Question 14.5.
Where does water in the egg go after boiling the egg?
Answer:
When the egg is boiled, denaturation and then coagulation of globular proteins present in it occurs. The water present in the egg either gets absorbed or adsorbed in the coagulated proteins probably through hydrogen bonding.
Question 14.6.
Why cannot vitamin C be stored in our body?
Answer:
Vitamin Cisa water soluble vitamin, therefore, it is readily excreted through urine, hence cannot be stored in our body.

Question 14.7.
Which products should be formed when a nucleotide from DNA containing thymine is hydrolysed?
Answer:
Upon hydrolysis besides thymine, the two other components are: 2-deoxy-D-ribose and phosphoric acid are formed.
Question 14.8.
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained ? What does this fact suggest about the structure of RNA?
Answer:
When RNA is hydrolysed, there is no relation between the quantities of four bases: cytosine (C), guanine (G), adenine (A) and uracil (U) obtained therefore, the base pairing principle te(A) pairs with (T) and (C)pairs with (G) is not followed as like in DNA. This suggest that unlike DNA (a double stranded structure), the RNA has single stranded structure.
RBSE Class 12 Chemistry Biomolecules Textbook Questions and Answers
Question 14.1.
What are monosaccharides?
Answer:
Those carbohydrates which cannot be hydrolysed to smaller molecules are called monosaccharides. Their general formula is (CH2O)n where n-3-7. These are of two types : Those which contain an aldehyde (- CHO) group are called aldoses and those which contain a keto (>C = 0) are called ketoses. The number of carbon atoms in the molecule is indicated by a Greek Prefix.
|
No of carbon atoms in the molecule |
General term |
Aldose |
Ketose |
|
3 |
Trisoe |
Aldotose |
Ketotiose |
|
4 |
Tetrose |
Aldotretose |
Ketotetrose |
|
5 |
Pentose |
Aldopentose |
Ketopentose |
|
6 |
Hexose |
Aldohexose |
Ketohexose |
|
7 |
Heptose |
Aldoheptose |
Ketoheptose |
Question 14.2.
What are reducing sugars?
Answer:
Carbohydrates which reduce Fehling's solution to red precipitate of Cu2O or Tollen's reagent to shining silver mirror are called reducing sugars. All monosaccharides and disaccharides except sucrose are reducing sugars.

Question 14.3.
Write two major functions of carbohydrates in plants.
Answer:
- Cellulose (Polysaccharide) acts as the chief structural material of the plant cell walls.
- Starch (Polysaccharide) is the major reserve food material in plants mainly stored in seeds.
Question 14.4.
Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ansswer:
- Monosaccharides : Ribose, 2-deoxyribose, galactose and fructose.
- Disaccharides: Maltose and lactose.
Question 14.5.
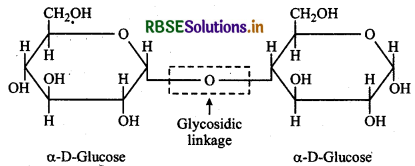
What do you understand by glycosidic linkage?
Answer:
The oxygen (etheral) linkage through which two monosaccharide units are joined together by the elimination of a water molecule to form a molecule of disaccharide is called a glycosidic linkage. The glycosidic linkage in maltose molecule is shown below:

Question 14.6.
What is glycogen? How is it different from starch?
Answer:
Ans. Starch is not a single compound but a mixture of two components, an amylose (15 - 20%) soluble component and amylopectin (80 - 85%) water insoluble component, Amylose is a linear polymer of α-D - glucose. But glycogen and amylopectin both are branched polymers of α - D-glucose. Glycogen is more highly branched polymer than amylopectin. Glycogenchains consist of 10 - 14 glucose units where as amylopectin chain consist of 20 - 25 glucose units.
Question 14.7.
What are the hydrolysis products of (i) Sucrose (ii) Lactose?
Answer:
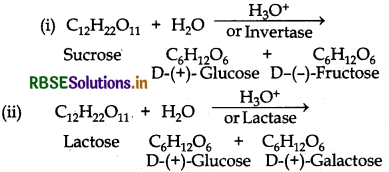
Question 14.8.
What is the basic structural difference between starch and cellulose?
Answer:
Starch is not a single compound. It consists of amylose (15 - 20%) and amylopectin (80 - 85%). In contrast cellulose is a single compound. Amylose is a linear polymer of α-D-glucose while cellulose is a condensation polymer of β-D-glucose. In amylose, C-1 of one glucose unit is joined to C-4 of the other unit through glycosidic linkage. In cellulose, C-1 of one glucose unit is joined to C-4 of the other unit through B - glycosidic linkage. Amylopectin has highly branched structure.
Question 14.9.
What happens when D-glucose is treated with the following reagents:
(i) HI
(ii) Bromine water
(iii) HNO3?
Answer:
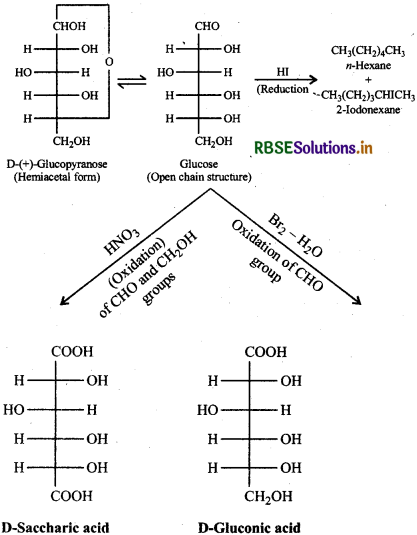

Question 14.10.
Enumerate the reactions of D-glucose which cannot be explained by its open structure.
Answer:
Following reactions cannot be explained by the open chainstructure of D-glucose.
- Although aldehyde group is present in glucose, it does not give2,4-DNP test, Schiff's test and it does not react with.
- The pentaacetate of glucose does not react with hydroxyl amine.
Question 14.11.
What are essential and non-essential amino acids? Give two examples of each type.
Answer:
Essential Amino Acids: Amino acids which can't be created in our body but can only be received from proper food or diet are called Essential Amino Acids. Examples: Histidine, Isoleucine, Lysine.
Non- essential amino acids: Amino acids which can be created in our body itself are Non- essential amino acids. Examples-alanine, arginine, asparagine, aspartic acid. Examples: Histidine, Isoleucine, Lysine.
Question 14.12
Define the following as related to proteins.
(i) peptide linkage,
(ii) primary structure,
(iii) Denaturation
Answer:
(i) Peptide Linkage: A peptide bond is an amide type of covalent chemical bond sinking two consecutive alpha-amino acids from C of one alpha-amino acid and N2 of another along a peptide chain as shown in the diagram.
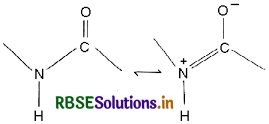
(ii) Primary Structure: The sequence of amino acids describe the primary structure of the protein.
(iii) Denaturation: Destroying the structure and activity of a protein by some actions like boiling is called Denaturation. An example is Boiling of eggs.
Question 14.13
What are the common types of secondary structure of proteins?
Answer:
The conformation which the polypeptide chains assume as a result of H - bonding is called the secondary structure of proteins. The two types of secondary structures are:
- α - helical structure and
- β - pleated sheet structure.
Question 14.14
what type of bonding helps in stabilising the α - helix strucutre of proteins?
Answer:
The α - helix structure of proteins is stabilized by intramolecular hydrogen bonding between C = O group of one amino acid residue and the N - H of the fourth amino acid resiude in the chain.

Question 14.15
Differentiate between globular and fibrous proteins.
Answer:
The main point of difference between globular and fibrous proteins are given below:
|
Globular proteins |
Fibrous proteins |
|
These are cross linked condensation polymers of amino acids. |
These are linear condensation polymers of amino acids. |
|
Protein molecules give three dimensional shape in which peptide chain is stabilised by intramolecular H - bonding. |
Long linear chains are held together by intermolecular H - bonding. |
|
Globular proteins have almost spheroidal shape due to the folding of the polypeptide chain. |
Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres. |
|
These are soluble in water, acids, bases and salts. |
These are soluble in strong acids and bases and insoluble in water. |
|
Globular proteins are sensitive to small changes of temperature and pH. Therefore, they undergo denaturation on heating or on treatment with acids or bases. |
These are stable to moderate changes of temperature and pH. |
|
Globular proteins possess biological activity. For example: enzymes, hormones, antibodies and haemoglobin. |
Fibrous proteins do not have any biological activity. They serve as a cheif structural material of animal tissues. For example: Keratin, collagen, myosin and fibroin. |
Question 14.16.
How do you explain the amphoteric behaviour of amino acids?
Answer:
Amino acids contain an acidic carboxyl (-COOH) group and a basic amino (-NH2) group with in a same molecule. In aqueous solution, they neutralize each other. The -COOH group loses a H+ (proton) while the amino group accepts it. As a result, a dipolar ion or zwitter ion is formed as shown below:
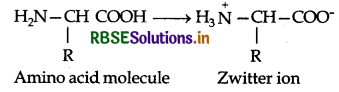
Due to zwitter ion structure, α-amino acids react with both acids and bases and hence shows amphoteric behaviour. In the acidic medium, COO-ion of the zwitter ion accepts a proton to form the cation (I) while in the basic medium, NH3 ion loses a proton to form an anion (II)
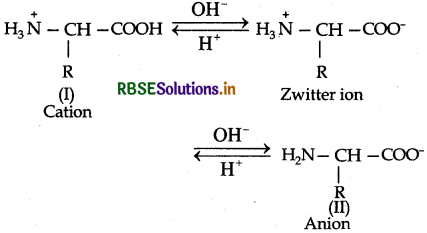
Question 14.17.
What are enzymes?
Answer:
Enzymes are proteins that help speed up metabolism, or the chemical reactions in our bodies. They build some substances and break others down. All living things have enzymes. Our bodies naturally produce enzymes. But enzymes are also in manufactured products and food.
They break down fats, proteins and carbohydrates. Enzymes use these nutrients for growth and cell repair.
Enzymes also help with:
- Breathing.
- Building muscle.
- Nerve function.
- Ridding our bodies of toxins.
Some of the most common digestive enzymes are:
- Carbohydrase breaks down carbohydrates into sugars.
- Lipase breaks down fats into fatty acids.
- Protease breaks down protein into amino acids.
Each enzyme has an ideal temperature and pH:
- pH: Enzymes are sensitive to acidity and alkalinity. They don’t work properly if an environment is too acidic or basic. For example, an enzyme in the stomach called pepsin breaks down proteins. If your stomach doesn’t have enough acid, pepsin can’t function optimally.
- Temperature: Enzymes work best when your body temperature is normal, about 98.6°F (37°C). As temperature increases, enzyme reactions increase. But if the temperature gets too high, the enzyme stops working. That’s why a high fever can disrupt bodily functions.
Disease cause of enzyme:
- Fabry disease prevents body from making enzymes (alpha-galactosidase A) that break down fat (lipids).
- Krabbe disease (globoid cell leukodystrophy) affects enzymes needed for the protective covering (myelin) on nerve cells (Central Nervous System).
- Maple syrup urine disease affects enzymes needed to break down certain branch chain amino acids.
Question 14.18.
What is the effect of denaturation on the stucture of proteins?
Answer:
Denaturation of proteins is done either by change in temperature or by bringing a change in the pH of the medium. As a result, the H-bonding is disturbed and the proteins lose their biological activity. During the denaturation, both the tertiary and secondary structures of proteins are destroyed while the nary structures remain intact.

Question 14.19.
How are vitamins classified? Name the vitamin responsible for coagulation of blood?
Answer:
Vitamins are classified into two groups on the basis of their solubility in water and fats.
- Fat soluble vitamins: These include vitamin A, D, E and K. They are stored in liver and adipose tissues.
- Water soluble vitamins : These include vitamin B-complex (B1 B2, B3, B5, B6, B12 and folic acid) and vitamin -C.
However, vitamin - H (biotin) is neither soluble in water nor in fats and oils. Vitamin K is responsible for coagulation of blood.
Question 14.20.
Why are vitamin A and vitamin C essential tous? Give their important sources.
Answer:
Functions of Vitamin A
- It helps in the maintenance of healthy, glowing and soft skin.
- It helps in maintaining proper eye sight particularly night vision
- It is essential for healhy teeth and is essential during pregnancy and loctation period.
Deficiency diseases: Deficiency of vitamin A can causes the following diseases:
(a) retarted growth, (b) night blindness, (c) xerophthalmia (Hardening of cornea of eye)
Important sources : Fish liver oil, carrots, butter, milk, egg yolk and green leafy vegetables.
Functions of Vitamin C:
- It helps the growth of good teeth, gums and joints.
- It helps in healing of cuts and wounds.
- It improves appetite and helps in the curing of cold.
- It provides resistance against diseases and infections.
Deficiency diseases: Vitamin C causes scurvy (bleeding gums) and pyorrhea (loosening and bleeding of teeth).
Important sources: Citrus fruits like lemon, amla, green leafy vegetables, tomatoes etc.
Question 14.21.
What are nucleic acids? Mention their two important functions.
Answer:
Nucleic acids are important biomolecules which are found in the nuclei of all living cells in the form of nucleoproteins or chromosomes. Chemically they are poly nucleotides.
Nucleiec acids are of two types:
- Deoxyribonucleic acid (DNA) and
- Ribonucleic acid (RNA)
The main two functions of nucleic acids are:
- DNA is responsible for transmission of hereditary characters from one generation to other. This function is due to the unique property of replication of DNA molecules during cell division as a result of which two indentical DNA molecules are transfered to daughter cells.
- DNA and RNA are responsible for synthesis of all proteins needed for growth and maintenance of our body.
Question 14.22.
What is the difference between a nucleoside and a nucleotide?
Answer:
Nucleic acids are the polymers of nucleotides. Nucleotide is formed by condensation of a phosphoric acid with a nucleoside.
Nucleoside is a condensation product of a pentose sugar and a heterocyclic base i,e purine or pyrimidine.
Thus the nucleotide is a bigger component than nucleoside and it consists of three chemical constituents:
- A nitrogenous heterocyclic base i,e purine or pyrimidine
- A pentose sugar containing 5 carbon atoms
- phosphoric acid.
For example, structure (I) represents a nucleoside and structure (II) represents a nucleotide.
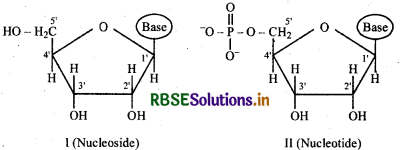

Question 14.23.
The two strands in DNA are not identical but are complemantary. Explain.
Answer:
The two strands in DNA molecule are held together by H - bonding between purine base of one strand and pyridine base of the other stand and vice-versa. Because of different sizes and geometries of the purine and pyrimidine bases, the only possible pairing in DNA are guanine (G) and cytosine (C) through three hydrogen bonds ( C = G) and between adenine (A) and thymine (T) through two hydrogen bonds (A = T). Due to the base pairing principle, the sequence of bases in one strand of DNA automatically fixes the sequence of bases in other strand. Thus, the two strands are complementary and not identical as shown below:

Question 14. 24.
Write the important structural and functional differences between DNA and RNA.
Answer:
Structural differences between DNA and RNA:
|
DNA |
RNA |
|
1. In DNA, 2- deoxy - D - (-) ribose sugar is present. |
In RNA, D (-) ribose sugar is present. |
|
2. It contains cytosine and thymine as pyrimidine bases. |
It contains cytosine and uracil as pyrimidine bases. |
|
3. It has double stranded α - helical structure. |
It has single stranded ?- helical structure. |
|
4. DNA molecule is relatively large with high molecular masses ranging from 6 x 10-6 - 16 x 106 u. |
RNA molecule is relativoly much smaller with molecular masses ranging from 20000 – 40000U. |
Functional differences between DNA and RNA:
|
DNA |
RNA |
|
1. DNA has a unique property of replication. |
RNA usually does not replicate. |
|
2. DNA is responsible for the transmission of hereditary characters. |
RNA controls the protein synthesis. |
Question 14.25.
What are the different types of RNA found in the cells?
Answer:
Three types of RNA are found in the living cells:
- Ribosomal RNA (r RNA)
- Messenger RNA (m RNA)
- Transfer RNA (t RNA)

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम