RBSE Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Rajasthan Board RBSE Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids Textbook Exercise Questions and Answers.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
RBSE Class 12 Chemistry Aldehydes, Ketones and Carboxylic Acids InText Questions and Answers
Question 12.1.
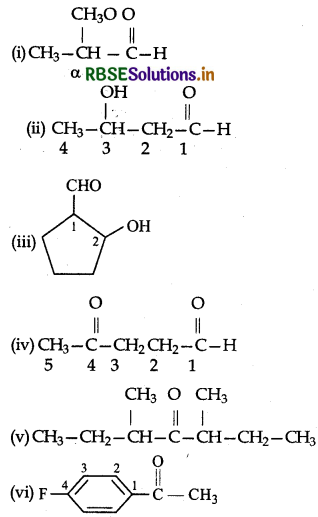
Write the structures of the following compounds:
(i) α-Methoxypropionaldehyde
(ii) α-Hydroxybutanal
(iii) 2-Hydroxy cyclopentane carbaldehyde
(iv) 4-oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoro acetophenone
Answer:
Question 12.2.
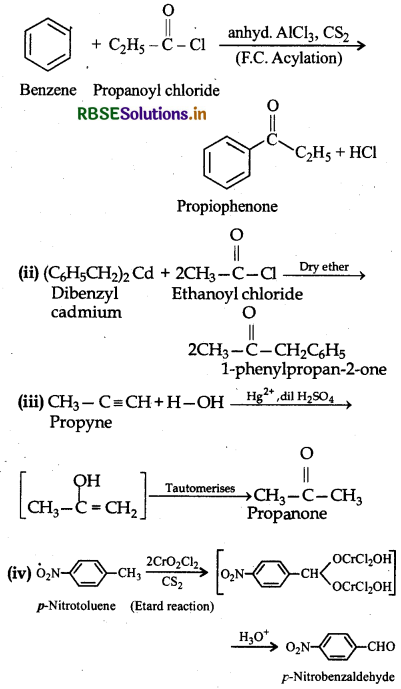
Write the structures of products of the following reactions:
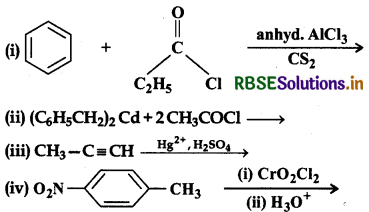
Answer:

Question 12.3.
Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OH, CH3CH2CH3
Answer:
The molecular masses of all given compounds are comparable:
CH3CHO (44), CH3CH2OH (46), CH3COCH3 (46), CH3CH2CH3 (44).
Among these, CH3CH2OH undergoes extensive intermolecular hydrogen bonding and it exists as associated molecule and hence its boiling point is highest (351 K). CH3CHO is more polar than CH3OCH3 and therefore dipoledipole interactions are stronger in CH3CHO than in CH3OCH3. Hence the boiling point of CH3CHO is higher than that of CH3OCH3. In CH3CH2CH3, there are only weak van der Waals forces and hence its boiling point is lowest. The increasing order of boiling points of all these compounds is:
CH3CH2CH3 < CH3OCH3 < CH3CHO < CH3CH2OH
(213 K) (249 K) (293 K) (351 K)
Question 124.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
(i) Ethanal, Propanal, Propanone, Butanone
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone
Answer:
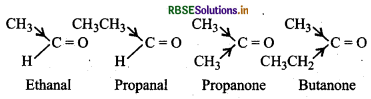
Ethanal Propana! Propanone Butanone As we move from ethanal → propanalpropanone → butanone, the +I effect of the alkyl group increases. As a result, electron density on the C-otom of the carbonyl group progressively increases and hence attack by the nucleophile becomes slower and slower. Thus, the increasing order of reactivity of the carbonyl compounds towards nucleophilic addition reaction is:
Butanone < Propanone < Propanal < Ethanal
(ii) Among these given compounds, acetophenone is ketone while all others are aldehydes, therefore acetophenone is least reactive. In p-tolualdehyde, there is -CH3 group at the p-position w.r.t. the carbonyl group which increases electron density or decreases positive charge on the C-atom of carbonyl group by hyperconjugation, there by making it less reactive than berzaldehyde.
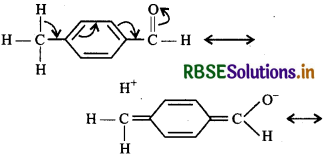
In contrast, p-nitrobenzaldehyde, the NO2 group is a powerful electron-withdrawing group. It withdraws electrons both by inductive and resonance effect thereby
decreasing electron density or increasing positive charge on the C-otom of the carbonyl group. This facilitates the nucleophilic attack and hence makes it more reactive than benzaldehyde.
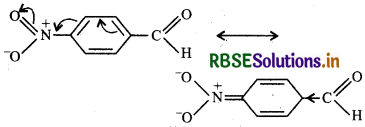
Therefore the overall reactivity increases in the following order:
Acetophenone < p-Tolualdehyde < Benzaldehyde < p Nitrobenzaldehyde
Question 12.5.
Predict the products of the following reactions:
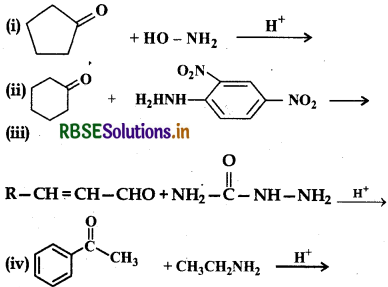
Answer:
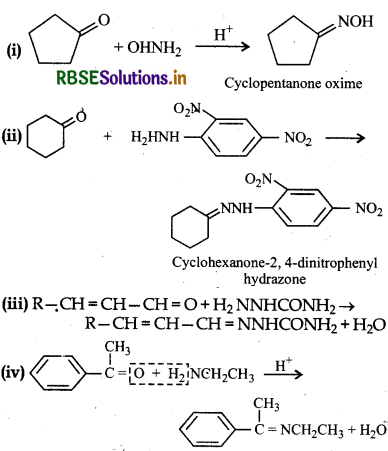
Question 12.6.
Give the IUPAC names of the following compounds:
(i) PhCH2CH2COOH
(ii) (CH3)-C = CHCOOH.
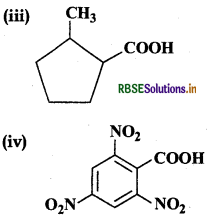
Answer:
(i) 3-Phenyl propanoic acid
(ii) 3-Methylbut-2-enoic acid
(iii) 2-Methylcyclopentane carboxylic acid
(iv) 2, 4, 6-Trinitrobenzoic acid or 2, 4, 6-Trinitrobenzene carboxylic acid

Question 12.7.
Show how each of the following compounds could be converted to benzoic acid.
(i) Ethylbenzene
(ii) Acetophenone
(iii) Bromobenzene
(iv) Phenylethene (styrene)
Answer:
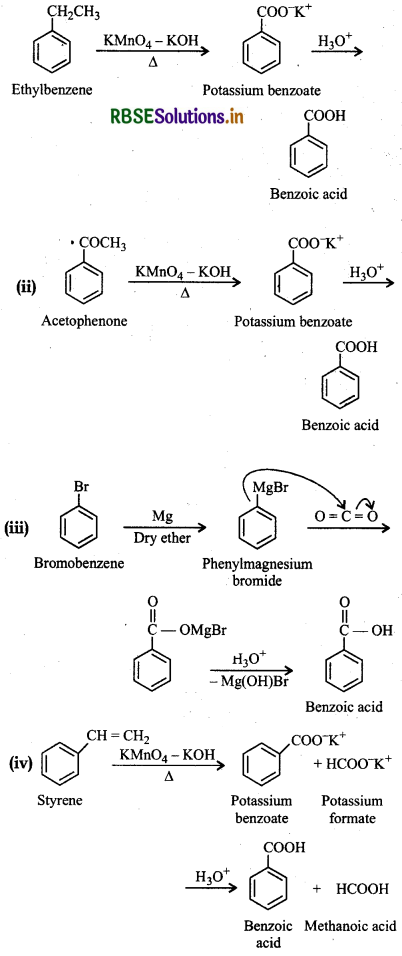
Question 12.8.
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or FCH2COH
(ii) FCH2CO2H or CICH2CO2H
(iii) FCH2CH2CH2CO2H or CH3CHFCH2CO2H
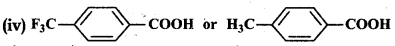
Answer:
CH3 group with +I effect increases the electron density on O-atom of O-H bond in the carboxyl group and cleavage of bond becomes difficult. Therefore, it decreases the acid strength. F has very strong -I effect i.e., electron withdrawing effect. It decreases the electron density on the O atom and cleavage of the bond becomes easy. Therefore acid strength increases. It is further related to the (i) number of Fatoms present in the molecule and (ii) relative position of the Fatom in the carbon atom chain.
In the light of given discussion:
(i) FCH2CO2H is a stronger acid.
(ii) FCH2CO2H is a stronger acid.
(iii) CH3CHFCH2COOH is a stronger acid.
(iv) 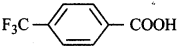 is a stronger acid.
is a stronger acid.
RBSE Class 12 Chemistry Aldehydes, Ketones and Carboxylic Acids Textbook Questions and Answers
Question 12.1.
What is meant by following terms ? Give an example of the reaction in each case.
(i) Cyanohydrin
(ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(viii) Imine
(ix) 2,4-DNP derivative
(x) Schiff's base
Answer:
(i) Cyanohydrin: The compounds containing hydroxyl and cyano groups on the same carbon atom are known as cyanohydrins (i.e., gem-hydroxy nitriles). These are obtained by addition of HCN to aldehydes or ketones in weakly acidic medium.
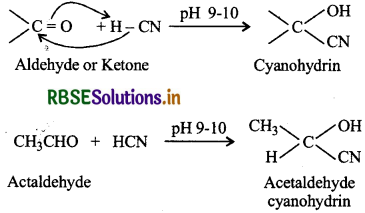
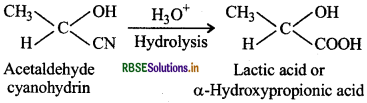
Cyanohydrins are useful synthetic agents because they are used to prepare a-hydroxy acids. For example:
(ii) Acetal: Gem-Dialkoxyalkanes in which the two alkoxy groups are present on the terminal C-atom are known as acetals. These are obtained by the action of an aldehyde with two equivalents of a monohydric alcohol in the presence of dry HCl gas.
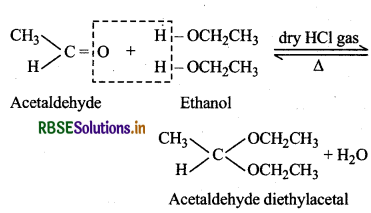
These are easily hydrolysed by dilute mineral acids to regenarate the parent aldehydes. Therefore, these are used for the protection of aldehyde group in organic synthesis.
(iii) Semicarbazone: These are the derivatives of aldehydes and ketones produced by the action of semicarbazide on them in weakly acidic medium.
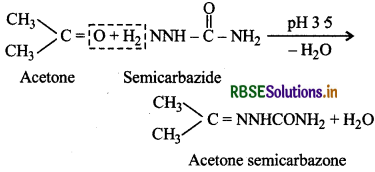
These are used for identification and characterization of carbonyl compounds (aldehydes and ketones).
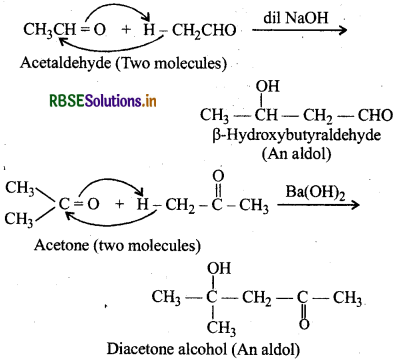
(iv) Aldol: Aldols are B-hydroxyaldehydes or ketones. They are produced by the condensation of two molecules of same or one molecule each of two different aldehydes or ketones in presence of a dilute aqueous alkali.
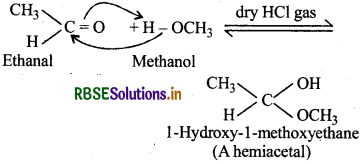
(v) Hemiacetal: These are a-alkoxyalcohols and are obtained by addition of one molecule of a monohydric alcohol to an aldehyde in presence of dry HCl gas.
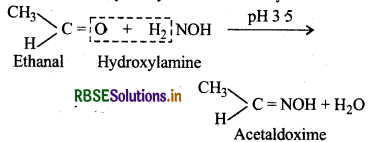
(vi) Oxime: These are obtained when aldehydes or ketones react with hydroxylamine in weakly acidic medium.
(vii) Ketals: Gem-Dialkoxyalkanes in which the two alkoxy groups are present on the same C-atom are called ketals. These are obtained when a ketone is heated with ethylene glycol in presence of dry HCl gas or ptoluenesulphonic acid (PTS).
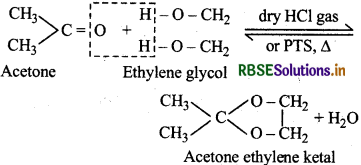
These are easily hydrolysed by dilute mineral acids to regenerate the original ketones. Therefore, ketals are used for the protection of keto groups in organic synthesis.
(viii) Imines: Compounds containing > = C-N-group are known as imines. These are produced when aldehydes and ketones react with ammonia and its derivatives.
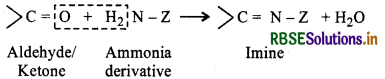
(ix) 2, 4-DNP Derivatives: These are 2, 4-dinitrophenylhydrazones and are obtained when aldehydes or ketones react with 2,4-dinitrphenylhydrazine in weakly acidic medium.
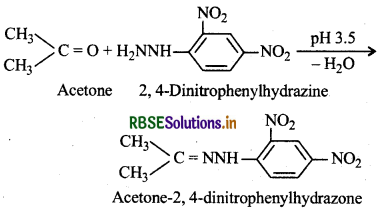
These derivatives are used for indentification and characterisation of aldehydes and ketones.
(x) Schiff's base: Aldehydes and ketones react with 1° aliphatic or aromatic amines in presence of an acid to form azomethines or Schiff's base.
These bases are also regarded as imines.


Question 12.2.
Name the following compounds according to IUPAC system.
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH = CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3 CCH2COOH
(vii) OCHC6H4CHO-p
Answer:
(i) 4-Methylpentanal
(ii) 6-Chloro-4-ethylhexan-3-one
(iii) But-2-en-1-al
(iv) Pentane-2, 4-dione
(v) 3,3,5-Trimethyl hexan-2-one
(vi) 3,3-Dimethylbutanoic acid
(vii) Benzene-1, 4-dicarbaldehyde
Question 12.3.
Draw the structures of the following compounds:
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) p-p'-Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic acid.
Answer:
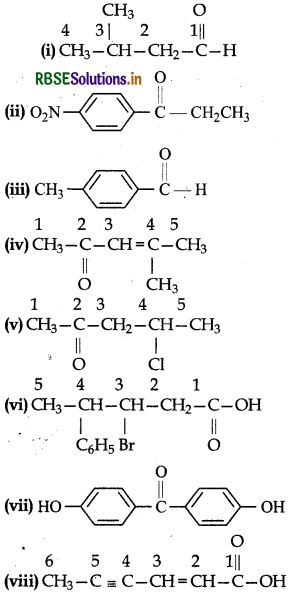
Question 12.4.
Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names:
(i) CH3CO (CH2)4 CH3
(ii) CH3CH2CHBrCH2CH(CH3) CHO
(iii) CH3(CH2)5 CHO
(iv) Ph-CH=CH-CHÓ
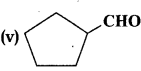
(vi) phCOph
Answer:
|
IUPAC Name |
Common Name |
|
(i) Heptan-2-one |
Methyl n-pentyl ketone |
|
(ii) 4-Bromo-2-Methylhexanal |
γ-Bromo- α methyl caproaldehyde |
|
(iii) Heptanal |
β-Phenylacrolein |
|
(iv) 3-Phenylprop-2-enal |
Cyclopentane carbaldehyde |
|
(v) Cyclopentane carbaldehyde |
Benzophenone |
|
(vi) Diphenylmethanone |
Methyl n-pentyl ketone |
Question 12.5.
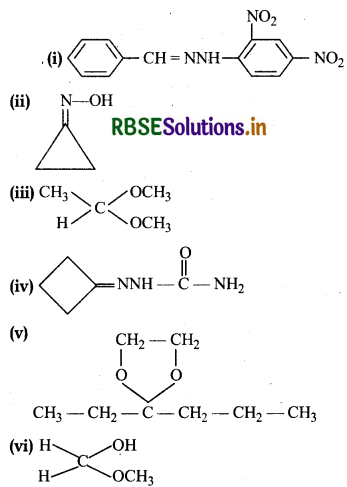
Draw structures of the following derivatives:
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cyclopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Answer:

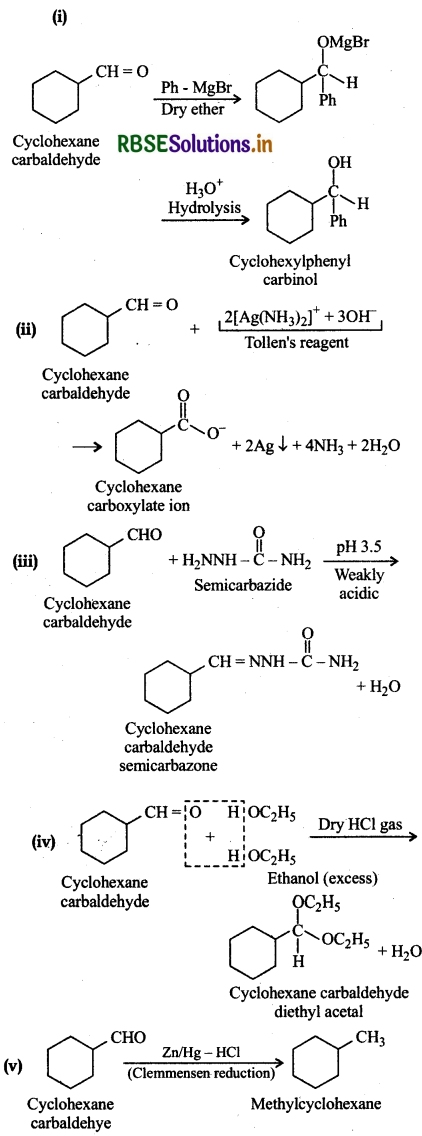
Question 12.6.
Predict the products formed when cyclothexanecarbaldehyde reacts with following reagents:
(i) PhMgBrand then H3O+
(ii) Tollen's reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Answer:
Question 12.7.
Which of the following compounds will undergo aldol condensation, which the annizzaro reaction and which neither ? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vi) 1-Phenyl propanone
(vii) Phenylacetaldehyde
(viii) Butan-1-ol
(ix) 2,2-Dimethylbutanal
Answer:
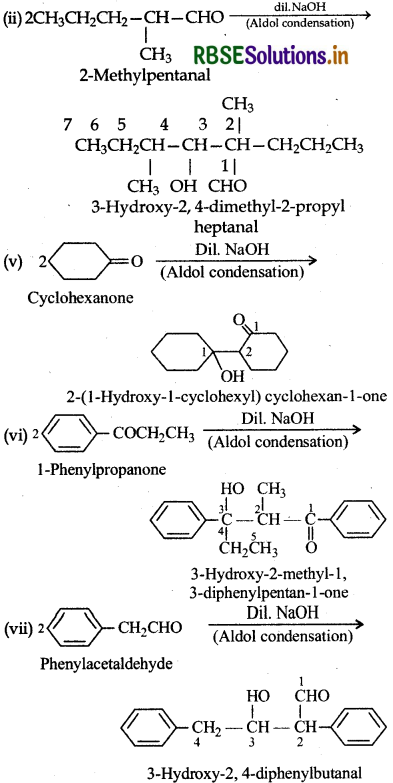
I. (ii) 2-Methylpentanal, (v) Cyclohexanone, (vi) 1-Phenylpropanone and (vii) Phenylacetaldehyde contain one or more α-hydrogen atoms hence undergo aldol condensation. The reactions are given below:
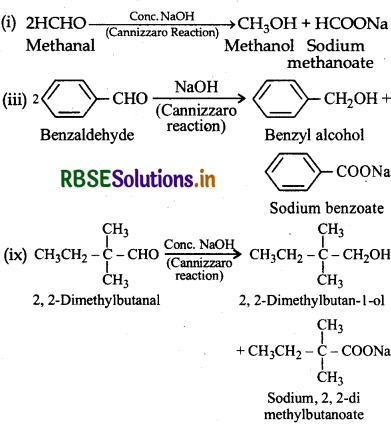
II. (i) Methanal, (ii) benzaldehyde and (ix) 2, 2dimethylbutanal do not contain α-hydrogen atoms and undergo Cannizzaro reaction.
The reactions are given below:
(III) (iv) Benzophenone is a ketone which does not have a-hygrogen and (viii) butan-1-ol is an alcohol. Both of these compounds neither undergo aldol condensation nor Cannizzaro reaction.

Question 12.8.
How will you convert ethanal into the following compounds:
(i) Butane-1-3-diol,
(ii) But-2-enal
(iii) But-2-enoic acid
Answer:
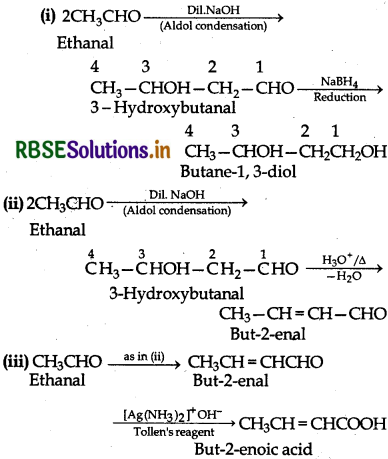
Question.12.9.
Write structural formulae and names of four possible aldol condensation products from propanal and
butanal. In each case, indicate which aldehyde act as nucleophile and which as electrophile.
Answer:
Both propanal and butanal contain a-hydrogens. These can undergo self aldol condensation as well as cross aldol condensation to give four coumpounds as given below:
(i) Condensation involving propanal: It is a self condensation reaction.
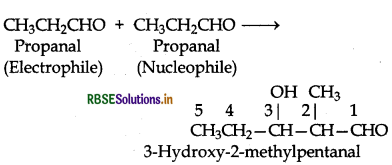
(ii) Condensation involving butanal: It is also self condensation reaction.
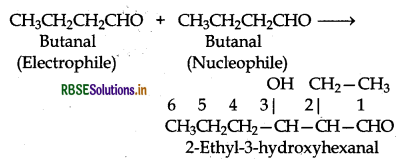
(iii) Condensation involving butanal and propanal: It is a cross-aldol condensation reaction.
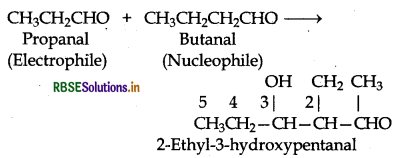
(iv) Condensation involving propanal and but is also a cross aldol condensation.
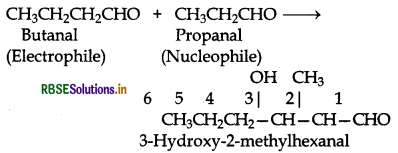
Question 12.10.
An organic compound with the molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen's reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
Answer:
(i) Since the given compound (M.F. = C9H10O) forms 2,4-DNP derivative on reacting with 2,4-DNP, it is a carbonyl compound. As the compound feduces Tollen's reagent and undergoes Cannizzaro's reaction, it is an aldehyde nota ketone and -CHO group is directly attached to the benzene ring.
(ii) Since on vigorous oxidation, it gives 1, 2benzenedicarboxylic acid, therefore, it must be an orthosubstituted benzaldehyde. The only o-substituted aromatic aldehyde having molecular formula C9H10O is 2ethylbenzaldehyde. The reactions involved are given below:
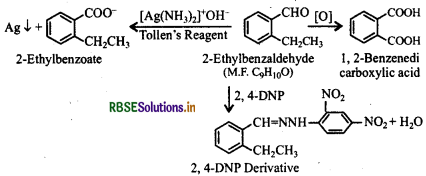
Question 12.11.
An organic compound A (molecular formula C8H6O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene as the major product. Write equations for the reaction involved.
Answer:
(i) The given informations show that the compound [A] upon hydrolysis gives a carboxylic acid (B) and an alcohol (C). It must be anester.
(ii) Since the alcohol (C] upon oxidation with chromic acid gave back the carboxylic acid [B]., both the carboxylic acid and alcohol must have the same number of C-atom i.e., four each.
(iii) On dehydration, the alcohol (CI gave an alkene.
The relevant equations for all the reactions involved may be explained as follows:
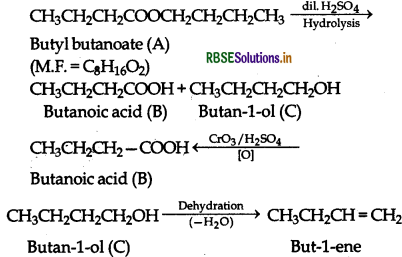
Question 12.12.
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butylketone, Methyl tert-butylketone (Reactivity towards HCN).
(ii) CH3CH2CH (Br) COOH, CH3CH (Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (Acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-methoxybenzoic acid (Acid strength).
Answer:
(i) The reactivity towards HCN addition decreases as +I effect of the alkyl groups increases and/or the steric hindrance to the nucleophilic attack by CN- at the carbonyl carbon atom increases. Thus the increasing order of reactivity is:
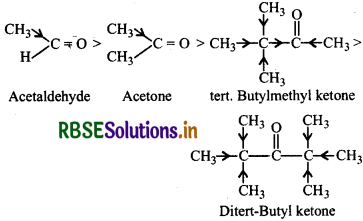
(ii) As we already know that +I effect decreases while -I effect increases the acidic strength of carboxylic acids. The+I effect of isopropyl group is more than that of n-propyl group, therefore, (CH3)2 CHCOOH is a weaker acid than CH3CH2CH2COOH.
Since -I-effect decreases with distance therefore CH3CH2CH (Br) COOH is a stronger acid than CH3CH (Br) CH2COOH. Thus, the overall increasing order of acid strength is.

(iii) As electron donating groups decrease the acid strength, therefore 4-methoxybenzoic acid is a weaker acid the benzoic acid. Since electron withdrawing groups increase the acidic strength, therefore, both 4-nitrobenzoic acid and 3, 4. dinitrobenzoic acids are stronger acids than benzoic acid. Further due to presence of - NO2 group (additional) at - position w.r.t. -COOH group, 3, 4-dinitrobenzoic acid is little stronger acid than 4-nitrobenzoic acid. Thus the increasing order of acid strength is:
4-methoxy benzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3 ,4-Dinitrobenzoic acid.

Question 12.13.
Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
Answer:
(i) Propanal and Propanone:
(a) Propanal reduces Tollen's reagent to metallic silver while propanone does not.
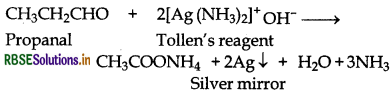
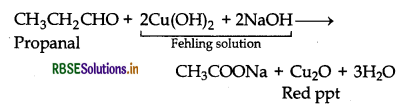
(b) Propanal gives red precipitate with Fehling solution, while propanone does not.
(ii) Acetophenone and Benzophenone:
Acetophenone gives iodoform test while benzophenone does not
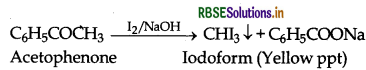
(iii) Phenol and Benzoic acid:
(a) Benzoic acid decomposes NaHCO3 to evolve CO2 but phenol does not
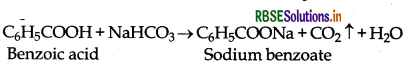
(b) Phenol gives violet colouration with neutral FeCl3 solution while benzoic acid gives buff coloured precipitate of ferric benzoate.
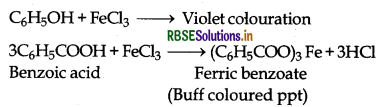
(iv) Benzoic acid and Ethyl benzoate:
(a) Benzoic acid decomposes NaHCO3 to evolve CO2 while ethyl benzoate does not.
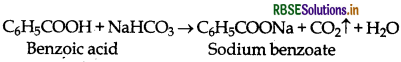
(b) Ethyl benzoate gives iodoform test while benzoic acid does not.
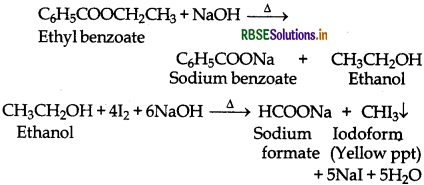
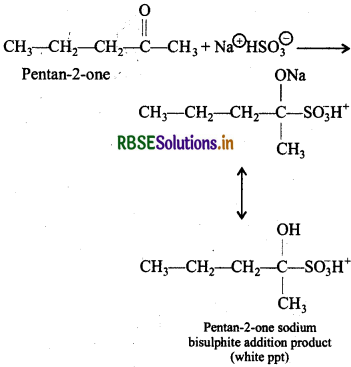
(v) Pentan-2-one and pentan-3-one:
Pentan-2-one being a methyl ketone when treated with a saturated solution of NaHSO3 gives a white precipitate of pentan-2-one sodium bisulphite addition product while pentan-3-one does not
(vi) Benzaaldehyde or Acetophenone:
(a) Acetophenone being a methyl ketone gives positive iodofrom test but benzaldehyde does not.
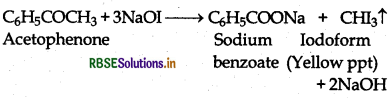
(b) Benzaldehyde reduces Tollen's reagent to give metallic silver but acetophenone does not.
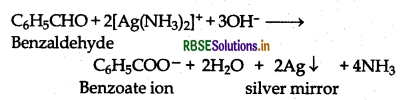
(vii) Ethanal and Propanal:
Ethanal contains the grouping CH3CO- linked to H and hence gives positive iodoform test but propanal does not contain the grouping CH3CO-linked to H or C and hence does not give positive iodoform test.
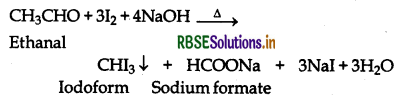
Question 12.14.
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
(i) Methyl benzoate
(ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid
(iv) Phenylacetic acid
(v) p-Nitrobenzaldehyde
Answer:
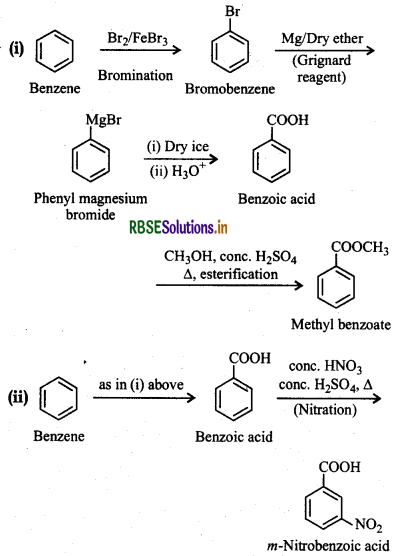
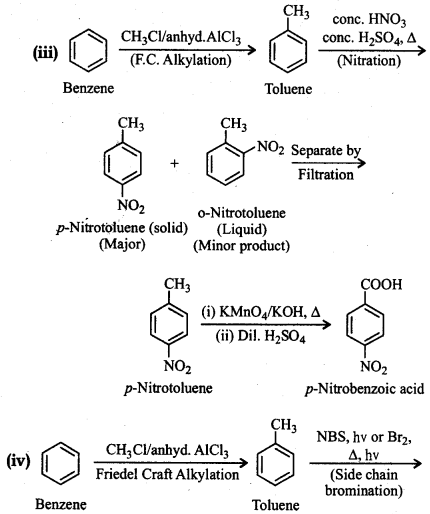
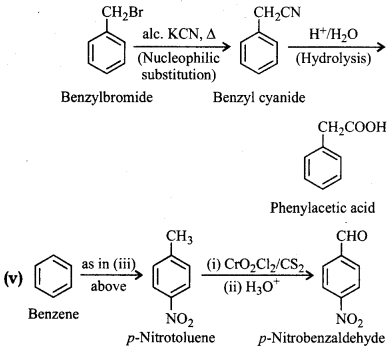
Question 12.15.
How will you bring about the following conversions in not more than two steps?
(i) Propanone to Propene
(ii) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(iv) Benzene to m-Nitroacetophenone
(v) Benzaldehyde to Benzophenone
(vi) Bromobenzene to l-phenyl ethanol
(vii) Benzaldehyde to 3-phenylpropan-1-ol
(viii) Benzaldehyde to a-Hydroxyphenylacetic acid
(ix) Benzoic acid to m-Nitrobenzyl alcohol.
Answer:
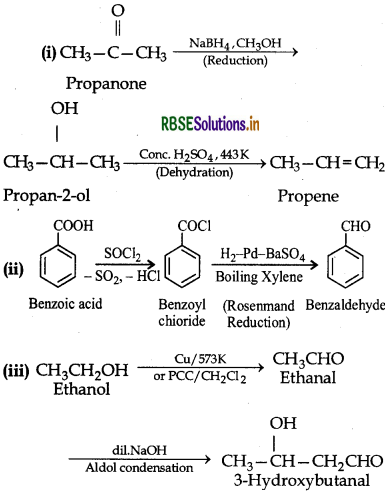
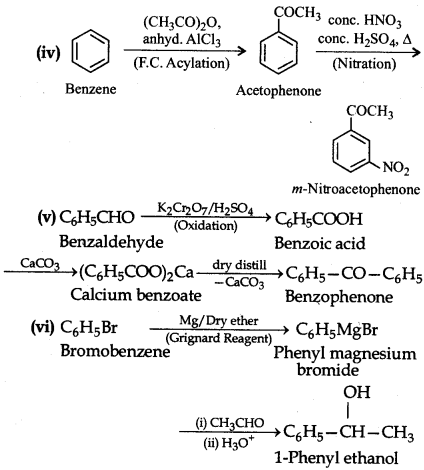
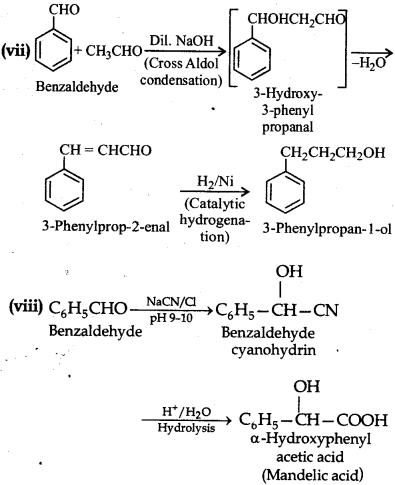
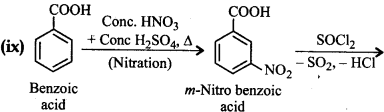
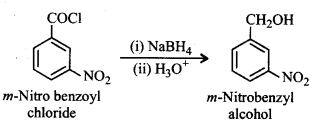
Question 12.16.
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
Answer:
(i) Acetylation:
Acetyl group is introduced in an organic compound. Reagent is acetyl chloride or acetic anhydride. Bases such as pyridine and dimethylaniline are used to neutralize acid (HCl or acetic acid).
(ii) Cannizzaro reaction:
Aldehydes (not having alpha hydrogen atoms) undergo self oxidation-reduction (disproportionation) reaction. One molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Concentrated alkali is the reagent.
(iii) Cross aldol condensation:
Aldol condensation between different carbonyl compounds (aldehdyes and ketones). If both reactants contain alpha hydrogen atom, four different products can be obtained.
(iv) Decarboxylation
Loss of CO2 from carboxylic acids to form hydrocarbons. For this, sodium salts of carboxylic acids are heated with soda lime.

Question 12.17.
Complete each synthesis by giving missing starting material, reagent or products:
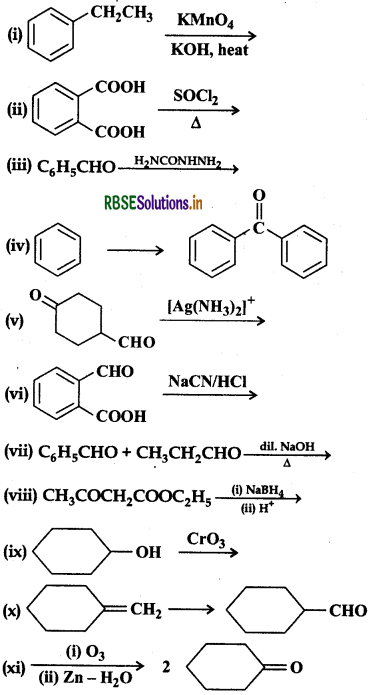
Answer:
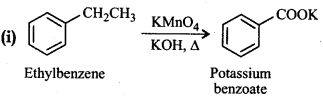
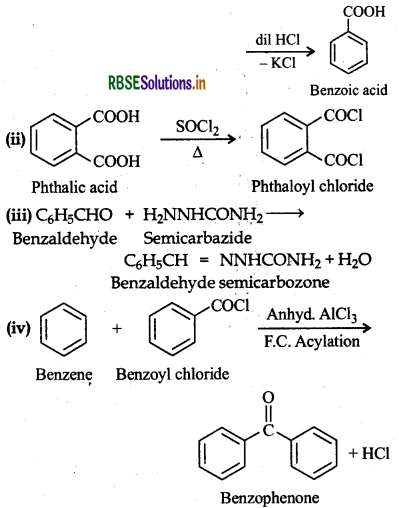
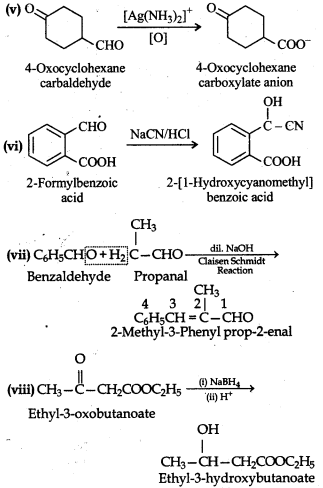
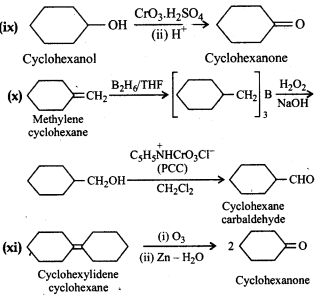
Question 12.18.
Give possible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2.2, 6-Trimethyl cyclohexanone does not.
(ii) There are two-NH2 groups in semicarbazide. However, one is involved in the formation of semicarbazone.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Answer:
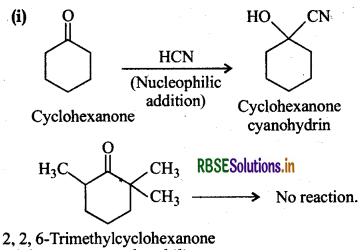
2,2, 6-Trimethylcyclohexanone Incyclohexanone, nucleophilic attack of CN ion takes place at the carbonyl carbon atom. However, in 2,4,6trimethyl cyclohexanone, the three-CH3 groups being electron releasing in nature (+I effect) will considerably increase the electron density on the carbonyl C-atom and the nucleophilic attack does not seem to be feasible. Moreover, the two-CH3 substituents at the o-positions will also hinder the attack of nucleophile CN-ion on the carbonyl group.
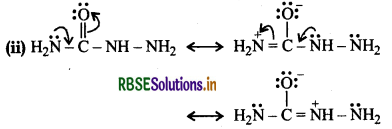
Although semicarbazide has two-NH2 groups but one of them which is directly attached to C = O is involved in resonance as shown above. As a result, electron density on this -NH2 group decreases and hence it does not act as a nucleophile. On the other hand, the lone pair of electrons on the other -NH2 group does not take part in resonance and hence it is available for nucleophilic attack on the C = 0 group of aldehydes and ketones.
(iii) The formation of esters from a carboxylic acid and an alcohol in presence of an acid catalyst is a reversible reaction.
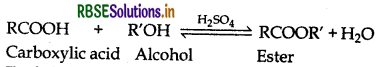
To shift the equilibrium in the forward direction, H2O or the ester formed should be removed immediately as it is formed.
Question 12.19.
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen's reagent but forms addition compound with sodium hydrogensulphite and gives positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Write the possible structure of the compound.
Answer:
Step I: Calculation of molecular formula of the compound.
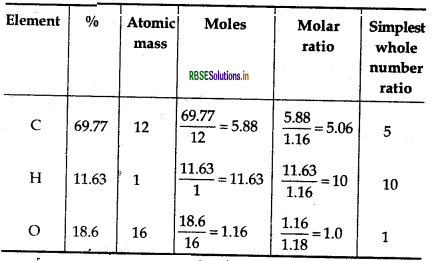
Thus, empirical formula of the compound
= C5H10O
∴ Empirical formula mass
= 5 x 12 + 10 x 1 + 1 x 16
= 86
∴ Molecular formula = n x Empirical formula
= C5H10O
Step II: To determine the structure of the compound
(1) Since the given compound forms addition compound with NaHSO3 therefore it must be either an aldehyde or methyl ketone.
(ii) As the compound does not reduce Tollen's reagent therefore it can not be an aldehyde and hence it must be a methyl ketone.
(iii) Since the given compound gives positive iodoform test, therefore the given compound is a methyl ketone.
(iv) Since the given compound on vigorous oxidation gives a mixture of ethanoic acid and propanoic acid therefore the methyl ketone is pentan-2-one.
Step II : To explain the reactions involved in the given problem:
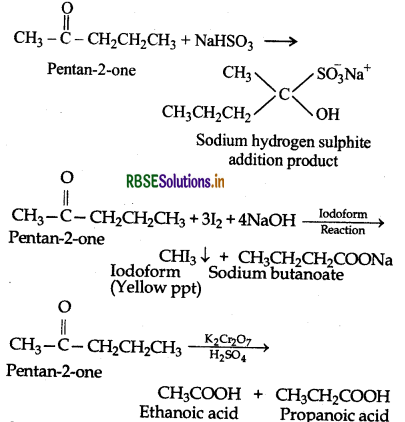

Question 12.20.
Although phenoxide has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Answer:
The structure that collectively represents all resonating structures is believed to be most stable and is called the resonating hybrid.
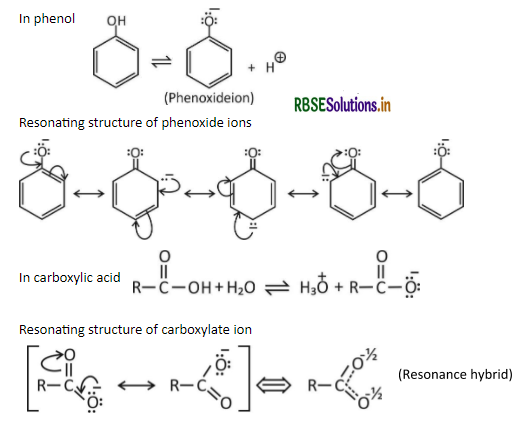
Carboxylic acid is more acidic than phenol. This can be explained on the basis of resonance, phenoxide ion has more number of resonating structures as compared to carboxylate ion. In resonating structures of phenoxide ion, the negative charge is present on one electronegative oxygen atom and the less electronegative carbon atom. The contribution of resonance structures towards resonance stabilization of phenoxide ion is less.
In resonating structures of carboxylate ions, the negative charge is present on two electronegative oxygen atoms. The contribution of resonance structures towards resonance stabilization of carboxylate ion is more. Hence carboxylate ions are more resonance stabilized than the phenoxide ions. Due to this the carboxylic acid is more acidic than phenol (higher the stability leads to greater acidic strength).

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम