RBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics
These comprehensive RBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics will give a brief overview of all the concepts.
Rajasthan Board RBSE Solutions for Class 12 Chemistry in Hindi Medium & English Medium are part of RBSE Solutions for Class 12. Students can also read RBSE Class 12 Chemistry Important Questions for exam preparation. Students can also go through RBSE Class 12 Chemistry Notes to understand and remember the concepts easily.
RBSE Class 12 Chemistry Chapter 4 Notes Chemical Kinetics
→ Ionic or Instantaneous Reactions: The reactions which take place between ions and possess high rate of reaction, are called ionic reactions.
Example: NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
→ Very Slow Reactions: These reactions occur between molecules. Their rate is very slow. So, there is no measurable change in these reactions.
Example: Formation of water by combination of hydrogen and oxygen in the absence of catalyst.
→ Molecular or Slow Reactions: Those reactions which take place between molecules, are called molecular reactions. Their rates can be determined.
Example:
(i) Formation of ester by reaction of acid and alcohol
CH3COOH(aq) + C2H5OH(aq) ⇌ CH3COO2H5(aq) + H2O(l)
(ii) Oxidation of chloroform by light.
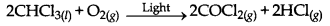

→ Rate of Reaction: The change in concentration of a reactant or product in unit time is called rare of reaction. Its unit is mol L-1 min-1.
→ Average Rate: The change in concetration of reactant or product present in a chemical reaction per unit time is called average rate of reaction.
→ Instantaneous Rate: The actual rate of reaction at any time is called instantaneous rate.
→ Temperature Coefficient: The ratio of rate constants of reaction at 25°C and 35°C temperature Or difference of 10°C temperature is called temperature coefficient. Its value is approximate 2 to 3.
Temperature coefficient =\(\frac{\text { Rate constant of reaction at } 35^{\circ} \mathrm{C}}{\text { Rate constant to reaction at } 25^{\circ} \mathrm{C}}\)
→ Rate Law or Rate Expression: The equation which establishes relationship between rate of reaction and concentration of reactants, is called rate law
For example: a A + bB → cC + dD
According to rate law for reacton,
Reaction rate (r) = k[A]a [B]b
→ Molecularity of Reaction: The total number of molecules of reactants taking part in elementary step of reaction is called molecularity of reaction.
→ Simple Reactions: The reactions whose molecularity is equal to number of reactants, are called simple reactions.
Example: O2F2 → O2 + F2
(Molecularity = 1)
→ Complex Reactions: The reactions, in which number of reactants is more than three, takes place generaly in two or more steps. The slowest step in these reactions is called rate determining step. These reactions are called complex reactions.
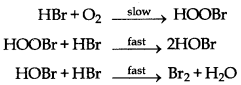
Or
Example: 4HBr + O2 →2H2O + 2Br2
Rate = k [HBr] [O2]
→ Order of Reaction: The number of reactant molecules taking part in a reaction which shows measurable change in concentration and determines the rate of reaction, is called order of reaction or the sum of power of concentration of reactants in rate law expression is known as order of reaction.
→ Half-life Period: The time required to change the half part of reactant in to product in a reaction is called half-life period (t½) of that reaction.
→ Pseudo tmimolecular Reactions: The reactions whose molecularity is two or more but order is one, are called pseudo unimolecular reactions.
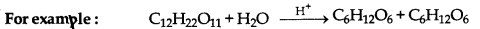
→ Activation Energy: The minimum extra amount of energy which is essentially provided to molecules for effective collision is Vailed activation energy.
Ea = ET - ER
Where, Ea = Activation energy
ET = Threshold energy
ER = Average energy of reactants.
→ Collision Frequency: The number of collisions taking place in a reaction mixture per unit volume per second is called collision frequency.
→ Average rate = \(\frac{\text { Decrease in cncentration of reactant }}{\text { Time taken }} = \frac{\text { Increase in concentration of product }}{\text { Time taken }}\)

→ For a general reaction aA + bB → cC + dD
Rate of reaction = \(\frac{1}{a}\left(\frac{-\Delta[\mathrm{A}]}{\Delta t}\right)=\frac{1}{b}\left(\frac{-\Delta[\mathrm{B}]}{\Delta t}\right)\)
= \(\frac{1}{c}\left(\frac{+\Delta[C]}{\Delta t}\right)=\frac{1}{d}\left(\frac{+\Delta[\mathrm{D}]}{\Delta t}\right)\)
Rate law expression
Rata= [A]x[B]y where order of reaction
→ For Zero Order eaction
(a) Rate law expression
Rate k[A]° or \(\frac{-d[\mathrm{~A}]}{d t}\) = k[A]°
(b) Integrated rate equation
k = \(\frac{[\mathrm{A}]_0-[\mathrm{A}]}{t}\)
(c) Half life period
t½ = \(\frac{[\mathrm{A}]_0}{2 k}\)
i.e., t½ ∝ a (initial concentration of reactant)
(d) Unit of rate constant = mol L-1 s-1
(e) Unit of rate constant for gaseous reactions = atm s-1.
→ For first order reaction
(a) Rate law expression
Rate = k[A] or \(\frac{-d[\mathrm{~A}]}{d t}\) = k[A]
(b) Integrated rate equation
k = \(\frac{2 \cdot 303}{t} \)log \(\frac{[\mathrm{A}]_0}{[\mathrm{~A}]}\)
(c) Half life (t½) = \(\frac{0.693}{k}\)
(d) Unit of rate constant = s-1
(e) Unit of rate constant for gaseous reaction = s-1.
→ For nth order reaction
(a) Integrated rate equation
kn = \(\frac{1}{t_{(n-1)}}\left[\frac{1}{[\mathrm{~A}]^{n-1}}-\frac{1}{[\mathrm{~A}]_0^{n-1}}\right]\)
where, [A] = final cocentration after time' t'
[A]0 = initial concentration
(b) t½ ∝ \(\frac{1}{[\mathrm{~A}]_0^{n-1}}\)
(c) Unit of rate constant = (mol L-1)1-ns-1
(d) Unit of rate constant for gaseous reaction = (atm)1-n s-1.
→ Integrated rate equation for a typical first order gas phase reaction
A(g) → B(g) + C(g)
k = \(\frac{2 \cdot 303}{t}\) log \(\frac{p_i}{2 p_i-p t}\)
→ Relation between partial pressure and number of moles
Partial Pressure of A = \(\frac{\text { Number of moles of gas A }}{\text { Total number of moles }}\) × Total pressure
→ Amount of reactant left after n half life
= \(\frac{a}{(2)^n}\)
→ Time required to complete nth fraction of the reaction
t½ = \(\frac{2 \cdot 303}{k}\) log \(\frac{1}{\left(1-\frac{1}{n}\right)}\)
→ Arrhenius equation
(a) k = Ae-Ea/RT
(b) ln k = ln A - \(\frac{Ea}{RT}\)
(c) log k = log A - \(\frac{\mathrm{Ea}}{2303 \mathrm{RT}}\)
(d) log\(\frac{k_2}{k_1}=\frac{\mathrm{E} a}{2303 \mathrm{R}}\left[\frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{~T}_1 \mathrm{~T}_2}\right]\)
Here, k1 and k2 are the rate constants at the temperature t1 and t2 respectively.

→ For the reaction,
a A + bB → cC + dD.
Instantaneous rate
= \(\frac{1}{a}\left(\frac{-d[\mathrm{~A}]}{d t}\right)=\frac{1}{b}\left(\frac{-d[B]}{d t}\right)\)
= \(\frac{1}{c}\left(\frac{d[C]}{d t}\right)=\frac{1}{d}\left(\frac{d[D]}{d t}\right)\)

- RBSE Class 12 Chemistry Notes Chapter 16 दैनिक जीवन में रसायन
- RBSE Class 12 Chemistry Notes Chapter 15 बहुलक
- RBSE Class 12 Chemistry Notes Chapter 14 जैव-अणु
- RBSE Class 12 Chemistry Notes Chapter 13 ऐमीन
- RBSE Class 12 Chemistry Notes Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल
- RBSE Class 12 Chemistry Notes Chapter 11 ऐल्कोहॉल, फीनॉल एवं ईथर
- RBSE Class 12 Chemistry Notes Chapter 10 हैलोऐल्केन तथा हैलोऐरीन
- RBSE Class 12 Chemistry Notes Chapter 9 उपसहसंयोजन यौगिक
- RBSE Class 12 Chemistry Notes Chapter 8 d- एवं f-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 7 p-ब्लॉक के तत्व
- RBSE Class 12 Chemistry Notes Chapter 6 तत्वों के निष्कर्षण के सिद्धांत एवं प्रक्रम